Manuscript accepted on :March 14, 2009
Published online on: 11-11-2015
Plagiarism Check: Yes
B. M. Dinesh¹ and R. K. Mohamed Mutahar²*
1Department of Pharmaceutics. K.L.E.S. College of Pharmacy. II Block. Rajajinagar. Bangalore - 560 010 (India).
²Department of Pharmaceutics. Dr. H.L.T.College of Pharmacy, Kengal, Channapatna, Bangalore (Rural) - 571 502 (India).
Abstract
The purpose of the present research was to compare the characteristics of different lubricants like Talc, Magnesium stearate (MGS), Polyethylene glycol 8000 (PEG), and Sodium benzoate (SBZ) used in Effervescent Tablets (ETs). Another main aim of the present work was also study the dissolution or scumming problems of lubricants in formulation of ETs. The formulated powder blends were evaluated for angle of repose, bulk density, tapped density, compressibility index and hausner ratio (Table: 2) were showed satisfactory flow property.The ETs were subjected to weight variation, thickness, hardness, friability, and drug content tests were found to be within limits (Table: 3). The disintegration time of all formulations was in between 17 to 22- Sec (Table: 3) (Fig: 2) and were slightly acidic (pH 4.2 to 4.4) (Table: 3) to augment the taste of the solution.The dissolution of F4 and F10 was 98.6% and 98.4% for 03 hrs in 6.8 pH, (Fig: 1) was excellent as compared to all other formulations (Fig: 1a). The present study concluded that, the single SBZ used in Formulation F4 (Table: 1) for ETs was the best lubricant. Moreover the combination of SBZ, with MGS and PEG, Formulation F10 (Table: 1) in the ratio of 6:1:1 which enhances the plasticity and external appearance of the final ETs. The selected Formulation F10 was further subjected to infrared interpretation (Fig: 4&5) there was no interaction between lubricants and Drug, and stability studies showed they were quite stable at different RT and RH (Fig: 6).
Keywords
Effervescent Tablets; Lubricants; Scumming; Talc; Sodium benzoate; Nicotinic acid
Download this article as:| Copy the following to cite this article: Dinesh B. M, Mutahar R. K. M.Compatibility Studies of Intrinsic lubricants for Effervescent Tablets: Formulation and In Vitro Evaluation. Biomed. Pharmacol. J.2009;2(1) |
| Copy the following to cite this URL: Dinesh B. M, Mutahar R. K. M.Compatibility Studies of Intrinsic lubricants for Effervescent Tablets: Formulation and In Vitro Evaluation. Biomed. Pharmacol. J.2009;2(1). Available from: http://biomedpharmajournal.org/?p=562 |
Introduction
The term “lubricant” is used to describe three different functions, glidant anti-adherent and lubrication.1 Lubricants are classified according to their Water- Solubility, (water-soluble lubricants (WSLs) and water-insoluble lubricants (WISLs). WISLs were more effective than WSLs, but generally WSLs are used only when tablets must be completely water-soluble ETs or when unique disintegration or, more commonly dissolution characteristics are desired. Lubricants may be Intrinsic Lubricants (ILs) or extrinsic Lubricants (ELs). A lubricant applied directly to the tableting tool surface in the form of a film, as by spraying onto the die cavity and/or punch surfaces, is known as ELs. The ILs are incorporated in the material to be tableted. The Intrinsic lubrications were provided by those materials that were compounded directly into the tablets as the granulation was prepared. 2
The main objective of the present research work was, to study the dissolution or scumming problems of lubricants in formulation of ETs. At the time of dissolution of ETs, the lubricants may cause “scumming” and / or agglomeration. (Leave an objectionable “scum” when an ET is placed in a glass of water). “Scum” reduces the aesthetic appeal of the solution made from an effervescent dosage form. Therefore, there were continued efforts to improve the effervescent dosage form in order to achive an optimal formulation. These efforts mainly focus on the lubricants used for ETs to solve the dissolution and scumming problems.
In the present research, different lubricants- Talc, MGS, PEG, and SBZ, are used in the formulation of ETs with Drug (Nicotinic acid). (Table: 1).Talc which is widely used in oral solid dosage forms as a lubricant is practically insoluble in dilute acids and alkalis, organic solvents and water.3 MGS is widely used as a lubricant in tablets manufactured at a concentration of 0.25% and 5.0% w/w. The PEG is widely used in a variety of pharmaceutical formulations. PEG is water-soluble and generally regarded as nontoxic and non-irritant material. 4 In solid dosage formulations, higher- molecular weight PEGs (PEG 8000) can enhance the effectiveness of tablet binders and impart plasticity to granules. 5 The WHO acceptable daily intake of total PEGs was estimated up to 10mg/ Kg of body-weight.6 The WHO acceptable daily intake of total benzoates (SBZ) was estimated up to 5mg/ Kg of body-weight. 7 Polyvinylpyrrolidine (PVP) is widely used as an excipient, particularly in oral tablets and solutions. 8 In tableting PVP solution is used as a binder in Wet-granulation process.9
The present study was conducted to formulate and evaluate ETs of nicotinic acid (NA) using different lubricants and effervescent mixture like the basic excipients (a carbonate) and the acid excipients (an organic acid) will react with each other, liberating carbon dioxide. Due to the dynamics of this process, turbulence will be created and the active ingredient will dissolve more rapidly in water. 10 Here we have taken NA as a model drug, which is better known as niacin or vitamin B3. Since it is very good at normalizing blood lipid levels it is declared the treatment of choice for reducing cholesterol by Expert Panel of the National Cholesterol Education Program (NCEP). 11
Materials and Methods
Materials
Nicotinic acid, Tartaric Acid, Citric Acid (anhydrous), Sod Bicarbonate, Talc, SBZ, MGS and PEG, Aspartame, and Sod Saccharine, provided by Jagdale Scientific Research Foundation, Bangalore. All other Chemicals used AR grade have been purchased from Vasa chemicals,Bangalore.
Processing
The processing of Effervescent Granules (EGs) and ETs require special environmental conditions: In the present process a maximum of 25% RH at a controlled RT of 250 or less, was found to be satisfactory to avoid problems related to atmospheric moisture.
Preparation of EGs
EGs were prepared by Wet Granulation Method with PVP. First sieve all the ingredients with a # 60 sieve. Weigh and collect the required quantity of sieved ingredients (Table: 1), (except Flavor and the Lubricants). Weigh the clinically relevant dosage strength of 500 mg NA. Both were placed in a tray and mixed with liquid binder PVP (5% in Anhydrous Ethanol) to make a coherent mass. This mass was immediately passed through a # 16 sieve, which was superimposed on # 44 sieve, then spread out on a tray, and dried in an Oven at 500 for 1 hour. These EGs were immediately transferred to suitable Dessicator and tightly closed.
Addition of lubricants
Lubricants were passed through a 100-Screen (Nylon Cloth), and then it was added to dried granules at a point where the other components were in a homogeneous state and mixed for a period of only 2 to 5 minutes.
Preparation of ETs:
Blend the EGs thoroughly with the weighed and sieved flavor and lubricants (Table: 1). Now the ETs can be prepared by compressing the lubricated EGs on Single punch Tablets machine at a RH of 25% and RT of 250, using 13 mm in diameter and flat faced, beveled edge die and punch set, making 3.350mg ET. The thus prepared ETs pass through curing oven; cool; and were packed.
Evaluation of Pre compression Parameters
All prepared EGs were evaluated for the official tests. The Angle of Repose: ETs were determined by using Funnel Method. Apparent Bulk Density: ETs were determined by placing presieved EGs into a graduated cylinder and measuring the volume and weight “as is.” Tapped density: ETs were determined by placing a graduated cylinder, containing a known mass of EGs on mechanical tapping apparatus. Compressibility index and Hausner ratio: was measured for the property of EGs to be compressed. As such they are measured for relative importance of interparticulate interactions.
Evaluation of Post compression Parameters
Physical Parameters
Weight variation: Twenty ETs were selected at random and weighed individually. The average weight and standard deviation of all twenty ETs was calculated. Thickness: ETs were measured by using a Sliding Caliper Scale, Twenty ETs were selected at random in a holding tray and total crown thickness was measured. Hardness: ETs were measured by using Monsanto Hardness Tester. Friability: ETs were determined by using Roche Friabilator.
Chemical Parameters
Solution pH
Solution pH was measured with pH Digital Meter, by placing ET in a beaker containing standardized volume of 200ml of water at a standardized temperature of 150 to 250. The pH was measured after complete disintegration of the ET.
Drug content Determination
Drug content was determined by dissolving the ETs in 200 ml of water. Concomitantly determine the absorbance of this solution and a solution of NA in the same medium, at a concentration of about 20 mcg/ml, in 1-cm cells at a wavelength of maximum absorbance at about 262 nm, with UV/ Visible Spectrophotometer, (Shimadzu 1700).
Disintegration time
Place ET in a beaker containing 200 ml of water at 150 to 250; numerous bubbles of gas were evolved. When the evolution of gas around the ET or its fragments ceases, the ET has disintegrated in the water. No agglomerate of particles remains.
In-Vitro Release Study
In- Vitro dissolution studies were carried out using six stage dissolution rate test USP Type 1 apparatus at 100 rpm, 0.1N Hydrochloric acid (pH 1.2) for first 2 hrs and phosphate buffer (pH 6.8) for 01 hrs. A quantity of 900 ml of the dissolution fluid was maintained at 37 ± 1° with a stirring speed of 100 ± 2 rpm used for the study. Aliquots of 5 ml were withdrawn at predetermined time intervals and an equivalent amount of fresh buffer maintained in the same temperature was replaced. Then absorbance was measured at about 262 nm with UV/ Visible Spectrophotometer. The content of drug was calculated using the equation generated from standard curve of NA. All dissolution studies were performed in triplicate.
Drug-excipients interaction
Drug-excipients interaction was studied by taking FTIR, by KBr pellet Method. The solvent used was Nujol and the Spectra were taken directly on KBr window.
Stability Studies
Stability Studies as per ICH guidelines was carried out at ( 290, RH 60 ± 2 %), and at ( 450, RH 70 ± 1%), for three months.
Result and Discussion
The ETs of NA was successfully prepared in the form of “pleasantly flavored effervescent drink” by compressing the EGs, which was prepared by Wet-Granulation Method with PVP as binder that produced a granulation of good compressibility. The vigorous effervescence with rapid disintegration of the resulting ETs was within 20 Seconds {(Fig:-2a), immediately when ET was placed in 200ml of water} and {(Fig:-2b), after 14 Sec} was obtained by using the different proportion of effervescent mixture in Formulations (Table: 1).
Compatibility studies of different lubricants like Talc, MGS, PEG and SBZ, were undertaken in the present research. Talc used in Formulations F1 (Table: 1), acts as both Lubricant and Glidant. Glidency of granules was well facilitated during material flow, eliminating binding in the die and minimizing picking and sticking to punch-face surface on compression. Compared to other lubricants the disintegration time was good- 20 Sec (Table: 3) and dissolution of ETs was 92.8% for 03 hrs in 6.8 pH, (Fig:1).But when single ET of Formulation F1(Table:1) was dropped in a glass of water it disintegrated leaving a thin separate layer of talc at the bottom of the glass (Fig.3a.), this was because of the practical insolubility of talc in dilute acids and alkalis, organic solvents and water. It formed “scumming” and / or agglomeration. This “scum” reduced the aesthetic appeal of the solution made from an effervescent dosage form. The present research thus concludes that single talc in large quantity (as lubricant) in ETs was not suitable.
Table 1: Formulation of ETs.
| INGREDIENTS | Formulation codes | |||||||||
| Quantity / Tablet (mg) | ||||||||||
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | |
NA |
500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 |
Tartaric acid |
900 | 900 | 900 | 900 | 900 | 900 | 900 | 900 | 900 | 900 |
| Citric acid | 850 | 850 | 850 | 850 | 850 | 850 | 850 | 850 | 850 | 850 |
| Sod Bicarbonate | 1000 | 1005 | 1005 | 1000 | 1000 | 1000 | 1000 | 1020 | 1000 | 1020 |
| PVP | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 |
Talc |
35 | — | — | — | — | — | 15 | — | — | — |
| MGS | — | 30 | — | — | — | 15 | — | 7.5 | 7.5 | 2.5 |
PEG 8000 |
— | — | 30 | — | 15 | — | — | 7.5 | 7.5 | 2.5 |
| SBZ | — | — | — | 35 | 20 | 20 | 20 | 20 | 20 | 30 |
| Sod Chloride | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Aspartame | — | — | — | — | — | — | — | 20 | — | 20 |
| Sod Saccharine | 40 | 40 | 40 | 40 | 40 | 40 | 40 | — | 40 | — |
| Color | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Flavor | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
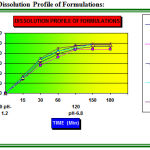 |
Figure 1: Dissolution Profile of Formulations.
|
MGS used in the Formulations F2 (Table: 1), acts both as Lubricant and Glidant.MGS well facilitated the glidency of granules during material flow, and there was no picking and sticking problems to punch-face surface during compression. The disintegration time was- 22 Sec (Table: 3), but the dissolution of this formulation 87.6 % for 03 hrs in 6.8 pH, (Fig: 1), was not as good as that compared to other formulations. When single ET of Formulation F2 (Table: 1) was dropped in a glass of water it dissolved completely without any scum but it forms floating upper layer (Fig: 3b) which was due to the practical insolubility of MGS in water. That floating upper layer can reduce the aesthetic appeal of the solution made from an effervescent dosage form. The present research thus concludes that single MGS in large quantity (as lubricant) in ETs was not suitable.
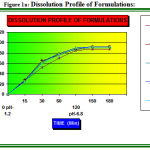 |
Figure 1a: Dissolution Profile of Formulations.
|
Table 2: Evaluation of Pre Compression Parameters of ETs.
| Parameters | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 |
| Thickness(mm)* | 4.97 | 5.0 | 4.98 | 5.0 | 5.0 | 4.99 | 5.0 | 5.0 | 5.0 | 5.0 |
| Diameter(mm)* | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 |
| Weight (mg)* | 3349 | 3350 | 3349 | 3350 | 3350 | 3349 | 3350 | 3350 | 3350 | 3350 |
| Hardness (Kg/cm2 )* | 5.7 | 5.9 | 5.7 | 5.9 | 5.2 | 5.9 | 6.1 | 5.1 | 5.1 | 5.8 |
| Friability(%)* | 0.37 | 0.31 | 0.36 | 0.32 | 0.29 | 0.30 | 0.31 | 0.29 | 0.27 | 0.33 |
| Disintegration Time (Sec)* | 20 | 22 | 20 | 18 | 18 | 19 | 17 | 17 | 22 | 18 |
| Solution pH | 4.4 | 4.2 | 4.3 | 4.2 | 4.4 | 4.4 | 4.4 | 4.2 | 4.2 | 4.3 |
| Assay (%Drug Content) * | 96.7 | 91.9 | 97.8 | 99.1 | 93.4 | 97.8 | 94.0 | 98.9 | 98.7 | 98.5 |
| % Drug Release* | 92.8 | 87.6 | 96.9 | 98.6 | 87.2 | 92.1 | 89.2 | 92.2 | 91.2 | 98.4 |
* Mean ± SD, n =3(All values are the average of three Determination)
PEG 8000 was used as the water-soluble lubricant in Formulation F3 (Table: 1). It was having little sticking to punch face surface on compression. The disintegration time was 20-Sec (Table: 3), and the dissolution of this formulation was 96.9 % for 03 hrs in 6.8 pH, (Fig.1). It imparts the plasticity to the ETs and was shiny, and also the final ETs were very good in appearance. When single ET of Formulation F3 (Table: 1), was dropped in a glass of water it dissolved completely without any scumming and / or agglomeration or sediment and the final solution was crystal clear (Fig: 3c). But at the end of every sip of the drink it gave a bad flavor of PEG even in the presence of adequate Flavoring agent and was unfit for drinking. The present research thus concludes that single PEG in large quantity (as lubricant) in ETs was not suitable.
 |
Figure 2: Disintegration of ETs: Time in Seconds.
|
Table 3: Evaluation of Post Compression Parameters of ETs.
| Parameters | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 |
| Bulk density
(gm/cm3 )* |
1.267 | 1.301 | 1.245 | 1.198 | 1.231 | 1.276 | 1.290 | 1.238 | 1.233 | 1.298 |
| Tapped density
(gm/cm3 )* |
1.439 | 1.540 | 1.461 | 1.369 | 1.475 | 1.441 | 1.468 | 1.412 | 1.452 | 1.498 |
| Hausners ratio* | 1.1358 | 1.1837 | 1.1735 | 1.1427 | 1.1982 | 1.1293 | 1.1379 | 1.1405 | 1.1776 | 1.1540 |
| Compressibility
Index(% )* |
17.2 | 23.9 | 21.6 | 17.1 | 24.4 | 16.5 | 17.8 | 17.4 | 21.9 | 20 |
| Angle of
Repose ( 0 )* |
27.34 | 27.05 | 26.46 | 26.33 | 27.13 | 26.79 | 26.98 | 27.15 | 27.16 | 27.12 |
* Mean ± SD, n =3(All values are the average of three Determination)
SBZ was used as the water-soluble lubricant in Formulation F4 (Table: 1), SBZ is used as both Lubricant and glidant. Glidency of granules was well facilitated during material flow, eliminating binding in the die and minimizing picking and sticking to punch-face surface on compression. Compared to other lubricants the disintegration time was good- 18 Sec (Table: 3) and dissolution of ETs was 98.6% for 03 hrs in 6.8 pH, (Fig.1).When single ET of Formulation F4 was dropped in a glass of water it dissolved completely without any scumming and / or agglomeration or sediment and the final solution was crystal clear (Fig: 3d). The aesthetic appeal of the solution was good and the final drink of this effervescent dosage forms was pleasantly flavored.
 |
Figure 3: Compatibility studies of different lubricants.
|
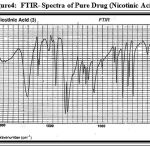 |
Figure 4: FTIR- Spectra of Pure Drug (Nicotinic Acid).
|
 |
Figure 5: FTIR- Spectra of Formulation F10.
|
Combination of SBZ, MGS and PEG in the ratio of 6:1:1 in Formulation F10 (Table: 1).The present research finally concluded that SBZ used in Formulation F4 (Table: 1) for ETs was the best lubricant among the other lubricants used like Talc, MGS, and PEG. Further more to improve lubrication, gilidant, anti adherent property and plasticity of ETs, so as to make best among the best, the Formulation F10 (Table: 1) was prepared with the combination of SBZ, MGS and PEG in the ratio of 6:1:1. Disintegration time was 18- Sec (Table: 3) and dissolution was 98.4% for 03 hrs in 6.8 pH, (Fig.1). There was no any compression problem and results were fair in both pre and post compression tests; no scumming and / or agglomeration or sediment and the final solution were crystal clear. (Fig: 3e); moreover the liquid of ETs gives pleasantly flavored drink.
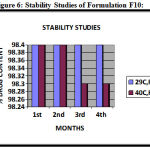 |
Figure 6: Stability Studies of Formulation F10.
|
Other Combination of lubricants (Formulation F5, F6, F7, F8, and F9) (Table: 1). Disintegration and dissolution results were fair (Table: 3) (Fig.1a). there was no any compression problem. But the aesthetic appeal of the solution of all above said formulations was not good because of scumming problems.
All the formulations were evaluated for the official tests like, Angle of Repose, Bulk Density, Tapped Density, Compressibility, & Hausner Ratio (Table: 2) for EGs and Weight Variation, Thickness, Hardness, Friability, and Drug content for ETs (Table: 3) were found to be within limits. All the formulations were slightly acidic (pH 4.2 to 4.4) (Table: 3) to augment the taste of the solution and disintegration time was between 17 to 22 minutes. (Table: 3) (Fig: 2).
In-Vitro release studies were evaluated using six stage dissolution rate test USP Type 1 apparatus. The release of NA significantly varies with the use of different lubricants, the Formulation F4 and F10 (Table: 1) (Fig: 1) shows good release as compared to the rest of the formulations. Formulation F5, F6, F7, F8, and F9 (Fig: 1a) shows the release of drug with combination of lubricants. Assay of ETs shows that all formulations are of required purity and match the USP specifications (Table: 3)
Drug-excipients interaction was studied by taking FTIR-Spectra (Fig: 4) and (Fig: 5). From the Spectra it was clear that there was no significant shift in the major peaks, which indicates that there may not be any interaction between the excipients and the NA used.
Stability Studies were carried out as per ICH guidelines on selected Formulations F10 (Table: 1) (Fig: 6) at ( 290, RH 60 ± 2 %), and at (450, RH 70 ± 1%), for three months. There was no change in the appearance and negligible amount of drug 0.2 % was degraded at (450, RH 70 ± 1%), but it was quite stable at ( 290, RH 60 ± 2 %), for three months.
Conclusion
In the present investigation of this research work we finally conclude that, the best lubricant for ETs was SBZ {Formulation F4 (Table: 1)}. Combination of SBZ with negligible amount of other lubricants like MGS and PEG in the ratio of 6:1:1 was taken in the Formulation F10, which enhances the plasticity and external appearance of the final ETs. This was considered to be the best formulation of ETs {Formulation F10 (Table: 1)}.This study also confirms that NA can be successfully prepared in the form of “pleasantly flavored effervescent drink” from ETs by compressing EGs. Finally we suggest this technique as most applicable and feasible to industrial practice and production.
Acknowledgements
The Authors are grateful to the Chairman, Prof.T.V.Narayana, and the Principal. Prof R.Ramesh Dr.H.L.T.College of Pharmacy. Bangalore (Rural), for providing all necessary facilities for the project and constant encouragement. Also thankful to JSRF, Bangalore, for providing gift Sample.
References
- Larry, L. Augsburger., Stephen, W.Hoag. Pharmaceutical Dosage Forms: Tablets. Vol. II, 3rd By Informa Healthcare, USA, 251 (2008).
- Herbert, A., Liberman, Leon lachman, and Joseph B.,Schwart. Pharmaceutical Dosage Forms: Tablets. Vol. II, 2nd edn, by Marcel Dekker Inc, New York, 111-114.292-293 (1989).
- Raymond, C. Rowe. Paul, J. Sheskey and Paul, J. Weller, Handbook of Pharmaceutical Excipients, A Joint Publication of the American Pharmaceutical Association and Royal Pharmaceutical Society of Great Britain, Britain, 4th 455.641 (2003).
- Smyth, H.F.,Carpenter, C.P., Weil, C.S., The chronic oral toxicology of the polyethylene glycols. J Am Pharm Assoc (Sci), 44, 27-30 (1955).
- Wells, J.I., Bhatt, D.A., Khan, K.A., Improved wet massed tableting using plasticized binder, J Pharm Pharmacol (Suppl.) 46, 34 (1982).
- FAO/WHO. Evaluation of certain food additives, Twenty-third report of the joint FAO/WHO expert committee on food additives. World Health Organ Tech Rep Ser, 648 (1980).
- FAO/WHO. Evaluation of certain food additives. Twenty-seventh report of the joint FAO/WHO expert committee on food additives. World Health Organ Tech Rep Ser, 696 (1983).
- Wessel, W., Schoog, M., Winkler, E., Polyvinylpyrrolidone (PVP) its diagnostic, therapeutic and technical application and consequences thereof, Arzneimittelforschung, 2,1468-1482(1971).
- Becker, D., Rigassi, T., Bauer-Brandl, A., Effectiveness of binders in wet granulation: comparison using model formulations of different tabletability. Drug Dev Ind Pharm, 23(8), 791-808(1997).
- Ansel, H.C., Povovich, N.G., and Allen, L.V., Pharmaceutical Dosage Forms and Drug Delivery Systems, 8th edn, 92, 149,162-171,302.
- The Expert Panel. Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection. JAMA, 269, 3015-3023(1993).
- Roaymond, M., In Lachman, Leon, Liberman, H. A., Joseph, B., Schwartz. Pharmaceutical Dosage Forms: Tablets, Vol. II, 2nd edn, by Marcel Dekker Inc, New York, 294,295,304,322 (1989).
- Marshall, K., In Lachman, Leon, Liberman, H. A., Knig, J.L. Eds., The theory and Practice of industrial pharmacy, Varghese Publishing House, Mumbai, 3rd edn, 67 (1987).
- Fiese, E.F., and Hagen, T.A., In Lachman, Leon, Liberman, H. A., Knig J.L. Eds., The theory and Practice of industrial pharmacy, Varghese Publishing House, Mumbai, 3rd edn, 183 (1987).
- Sameer,H.L., Formulation development and evaluation of mouth dissolving tablets of ondansteron hydrochloride. Asian Journal of Pharmaceutics, Vol-1,151(2007).
- Banker,G.S., Anderson, N.R., In Lachman, Leon, Liberman, H.A., Knig J.L Eds., The theory and Practice of industrial pharmacy, Varghese Publishing House, Mumbai, 3rd edn, 297-300(1987).
- The United States Pharmacopoeia 27 / National formulary 22, Asian Edition, United States Pharmacopoeial convention, Inc, Rockville, MD, 1313(2004).
- British pharmacopoeia, by the stationary office on behalf of the department of health and social services for Northern Ireland, Crown Copy-right, First published, Vol-II, 1452 (1998).
- Indian Pharmacopoeia, Government of India, Minister of Health and Family Welfare,Vol-II, by controller of publications, Delhi, Appendices, S-66 (1996).







