Manuscript accepted on :12-10-2022
Published online on: 04-01-2023
Plagiarism Check: Yes
Reviewed by: Dr. Mario Vincenzo Russo, Dr. Tejaswi Chavan
Second Review by: Dr. Daya Shankar Gautam
Final Approval by: Dr. Ziyad S. Haidar
Nisha Patel , Ram Prakash Saran*
, Ram Prakash Saran* , Nisha Kanwar
, Nisha Kanwar and Priyanka Riyad
and Priyanka Riyad
Department of Zoology, Jai Narain Vyas University, Jodhpur-342005, Rajasthan, India
Corresponding Author E-mail: saranrp@live.com
DOI : https://dx.doi.org/10.13005/bpj/2594
Abstract
The main objective of the study was to investigate long term use of meloxicam on avian species especially on Gallus domesticus. Animals were divided into 4 groups each having 5 animals. Group I was control and was fed normal diet (maize, grains and millet). Group II was treated with low dose of meloxicam for 15 days. Group III was treated with low dose of meloxicam for 30 days. Group IV was treated with low dose of meloxicam for 60 days. The effect was studied specially on hepatic and renal functions. Meloxicam was given at low dose (0.1 mg/kg body weight) to treatment groups for 15, 30 and 60 days. For biochemical estimation blood and serum were collected by sacrificing animals. Tissues of vital organs were fixed for histological examination. Long term administration of meloxicam cause variation in liver and kidney functioning. No significant change was observed in organ weight of treated groups as compared with control. No significant changes were observed in treated groups as compared to control in hematological parameters, biochemical parameters and lipid profile except SGOT, SGPT and alkaline phosphate in these parameters significant increase was observed. Significant changes were also observed in antioxidant analysis of treated groups as significant increase was observed in LPO and significant decrease in FRAP concentration was observed which signifies the results of histological changes especially in kidney and liver. Changes in histology of treated groups showed effect on kidney and liver as reduction (shrinkage) in glomerulus size and changes in histoarchitecture of central vein in liver of treated group was observed as compared to control group other histological examinations were normal. From results this can be concluded that long term low dose treatment of meloxicam has altered the histoarchitecture of liver and kidney of Gallus domesticus.
Keywords
Gallus domesticus; Meloxicam; NSAIDs (Non steroidal anti-inflammatory drugs)
Download this article as:| Copy the following to cite this article: Patel N, Saran R. P, Kanwar N, Riyad R. Assessment of Dose Dependent Toxicity of Meloxicam in Gallus Domesticus. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Patel N, Saran R. P, Kanwar N, Riyad R. Assessment of Dose Dependent Toxicity of Meloxicam in Gallus Domesticus. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3WQFfIw |
Introduction
Anti-inflammatory, analgesic, and antipyretic properties are beneficial properties of NSAIDs (Non steroidal anti-inflammatory drugs). Besides these important benefits these are associated with various adverse effects such as increased complications in gastrointestinal functions, high renal failure incidences i.e. renal adverse drug reactions (ADRs), Interstitial nephritis, acute renal failure, Nephritic syndrome, tubular necrosis, inflammatory bowel disease, heart failure risks. In totality the NSAIDs are associated with major effects on kidney and liver. Therapeutic activities of majorly used Non-steroidal anti-inflammatory drugs (NSAIDs) are associated with the inhibition of cyclooxygenase (COX) enzyme, which is involved in the production of prostaglandin1. The inflammatory drugs are following their mechanism of action through inhibition of COX-1(cyclooxygenase-1), COX-2 and linked pathways2.
Anti-inflammatory properties of NSAIDs by which they derive their mechanisms of action such as – reduction of superoxide radicals, induction of apoptosis, inhibition of cell adhesion molecule expression, reduction in pro-inflammatory cytokine levels, decrease of nitric oxide synthase, (tumor necrosis factor-α, interleukin-1), alteration of cellular membrane functions and modification of lymphocyte activity3. Recently, reported in several studies and claims that a major threat to avian fauna is the toxicity caused by inflammatory drugs and one such drug which is responsible for the declining population of vultures is diclofenac 4, 5. Several evidences support that the veterinary drug diclofenac is major reason behind the collapsing population of Gyps vulture species which is endemic to south Asia6-8. For managing inflammation in injury, diseased livestock and managing pain in India, Pakistan and Nepal a non-steroidal anti-inflammatory drug (NSAID) is routinely used i.e. Diclofenac7. Livestock carcasses which are shortly before death treatment with drug when such carcasses are consumed by vultures than they are exposed to diclofenac and consumption of such contaminated tissues with diclofenac cause the death of vulture within few days of their exposure to the drug. Extensive visceral gout is the clinical sign which is observed in the dead bodies of vultures. In experimentally dosed and wild vulture population high proportion of diclofenac residues and clinical signs have been found7-9. A safer candidate to replace diclofenac is meloxicam10. Nowadays most commonly used NSAIDs are certainly meloxicam because of its lesser side effects and greater COX-2 selective activity for example-gastrointestinal ulceration, renal function impairment and inhibition of platelet function11.
The current study was assigned to evaluate comparative sub-chronic toxicity of different days of treatment of meloxicam against a specific dose in Gallus domesticus animal model for vulture. The current experimentations were designed to evaluate comparative toxicity or adverse effects of meloxicam drug which is routinely used for pain relaxation or post-operative activities. The assessments were made based on body-weight alteration, serum biochemical changes, lipid profile, hematological parameters, antioxidant tests and histopathology. The main adverse effects were reported that NSAIDS primarily target the renal and hepatic tissues through degenerative changes and apoptic activities. Therefore, the serum biochemical parameters were also emphasized to assess alteration of renal and hepatic function assessment parameters along with enzymatic antioxidant status.
Materials and Method
Experimental animals and Dose regime
The six-week broilers (Gallus domesticus), weighing approximately 1.5± 0.2 kg were used as an experimental animal model, were purchased from Sagar poultry farm Jodhpur, Rajasthan and were acclimatized for a week in spacious cages under controlled conditions of an environment (i.e. 12 hours light/dark cycle with 230C temperature). Animals were supplemented with grain food and water. All the experimental work was confirmed by the Institutional Animal Ethical Committee (IAEC NO. 1646/GO/ERe/S/19/CPCSEA).
Meloxicam was administered intra-muscular for the different duration and the dose of meloxicam was estimated by the LD5030 test i.e. 0.1mg/kg body weight.
Experimental Design
Healthy and adult broilers were randomly divided into four groups each having five broilers.
Group 1: Vehicle control treated with distilled water.
Group 2: Meloxicam intra-muscular treatment at the dose of 0.1 mg/kg body weight for 15 days.
Group 3: Meloxicam intra-muscular treatment at the dose of 0.1 mg/kg body weight for 30 days.
Group 4: Meloxicam intra-muscular treatment at the dose of 0.1 mg/kg body weight for 60 days.
Criteria of Observation
After the completion of the experimental period, 24 hours fasted experimental broilers were autopsied under prolonged ether anesthesia. Through direct heart puncture blood sample was collected, some amount of blood sample was stored in EDTA vials for the estimation of hematological parameters and the rest of the blood sample was used for serum separation. Serum was separated by using a centrifuge and for biochemical assessments; serum sample was stored at -200C. For histological study organs like the heart, kidney, liver, and small intestine were removed, rinsed, weighed, and fixed in 10% formalin.
Estimation of Hematological Parameters
Hematological parameters such as HB, TRBC, HCT, MCV, MCH, MCHC, RDW, TLC, PLT, MPV, and PDW were determined by the standard protocols12, 13.
Estimation of Serum Biochemistry
The assessment of biochemical parameters like SGOT, SGPT, ALP, and lipid profile was done by using the standard commercial kit method.
Antioxidant Analysis
For the determination of LPO (serum lipid peroxidation) TBARS (thiobarbituric acid reactive substances) was measured that is expressed in the content of MDA (malondialdehyde) by using Ohkawa method14. The activity of catalase was determined by using the method of Aebi15. The method of Marklund and Marklund16 was used for the estimation of SOD (superoxide dismutase) activity and the assay of FRAP (ferric reducing antioxidant power assay) was performed by using the method of Benzie and Strain17. The level of GSH (reduced glutathione) was estimated by using Beutler’s method18.
Histopathology
The tissues of liver and kidney were obtained from autopsied experimental broilers and were fixed in 10% formalin and then dehydrated gradually by using a series of ethanol and embedded in paraffin wax. Sections of embedded tissues of liver and kidney were cut at the 5-μm thickness and then using hematoxylin and eosin-stained slides were prepared which was observed under the microscope.
Statistical Analysis
The results obtained from the body and organ weight and biochemical analysis were mentioned in mean ± standard error of the mean (SEM) and for the evaluation of statistical variances one-way analysis of variance (ANOVA) and post hoc mean separation tests were used.
Results and Discussion
Non steroidal anti-inflammatory drugs (NSAIDs) have been used to reduce inflammation and pain for many years. In mid of 1990s most commonly used anti-inflammatory drug diclofenac, which is banned nowadays is responsible for drastic decline in the population of vultures because they consumed the carcass of diclofenac-treated animals19. Many other NSAIDs like meloxicam, flunixin, ibuprofen, carprofen, ketoprofen, aceclofenac, and phenylbutazone are currently used in veterinary practices20. Meloxicam chemically known as 4-hydroxy-2-methyl N-(5-methyl-2-thiazolyl)-2H-1,2-benzothiazine-3- carboxamide-1,1-dioxide is a member of NSAIDs that has an enolic acid group. The present study was carried out to elucidate the toxicity effect of meloxicam on Gallus domesticus.
Non-significant changes were observed in the body and organ weight of all treated groups except 30 and 60 days meloxicam treated groups which show a slight increase (P≤0.05) in the weight of liver of Gallus domesticus as compared to the control group (Table1). Similar results were also obtained earlier that showed no notable changes in the weight of body and organs in broiler chicks treated with meloxicam21.
Table 1: Body and organ weight of different duration dependent dose of Meloxicam treated Gallus domesticus.
| TREATMENT GROUPS |
BODY WEIGHT (kg) |
LIVER |
KIDNEY |
HEART |
|
Initial |
Final |
————-(gm/kg body weight) ———- |
|||
| Group I
(Intact Control) |
1.07±0.31 | 1.27±0.35 | 24.82±0.67 | 6.55±0.17 | 5.87±0.38 |
| Group II
(0.1 mg-15 days) |
1.35±0.14 | 1.39±0.24 | 23.33±0.89 d | 6.53±0.57 d | 5.78±0.35 d |
| Group-III
(0.1 mg-30 days) |
1.62±0.21 | 1.64±0.08 | 21.11±1.38 a | 6.32±0.21 d | 4.94±1.43 d |
| Group-IV
(0.1 mg-60 days) |
1.45±0.27 | 1.81±0.26 | 26.06±0.41 a | 7.89±0.33 b | 5.36±0.21 d |
Hematological parameters such as HB, TRBC, HCT, MCV, MCH, MCHC, RDW, TLC, PLT, MPV, and PDW mainly show a relationship with the status of health and play important role in clinical evaluation of health state so that the hematological parameters considered as important indicators of pathophysiological and nutritional status of the animals22. Administration of NSAID meloxicam to Japanese quail showed non-significant changes in hematological parameters23. Our results also confirmed that meloxicam did not show any alteration in the parameters of hematology when treated to Gallus domesticus.
The non-significant change was observed in the level of total cholesterol in 15 and 30 days meloxicam-treated groups while 60 days treatment group shows significant increase (P≤0.01) in the level of total cholesterol as compared to the control group. The level of HDL-cholesterol was non-significantly changed in all treatment groups. The concentration of LDL-C was significantly increased (P≤0.001) in all meloxicam treated groups as compared to vehicle treated control group. The level of serum triglycerides and VLDL-C were increased significantly (P≤0.001) in 60 days meloxicam treated group and very slight increase in the level triglyceride and VLDL-cholesterol was observed in 30 days treatment of meloxicam as compared to the control group (Table 2). In male albino rats after administration of meloxicam elevation in total cholesterol and LDL-C was observed24.
Table 2: Lipid profile of different duration dependent dose of Meloxicam treated Gallus domesticus.
| TREATMENT GROUPS | TOTAL- C
(mg/dL) |
HDL-C (mg/dL) | LDL-C
(mg/dL) |
VLDL-C (mg/dL) | TRIGLYCERIDE
(mg/dL) |
TC/HDL
|
LDL/HDL |
| Group I
(Intact Control) |
145.4
±7.66 |
36.8
±1.30 |
66.2
±1.66 |
21.05
±1.36 |
71.8
±1.31 |
3.94
±0.23 |
1.79
±0.40 |
| Group II
(0.1 mg-15 days) |
152.58
±5.60 d |
39.2
±1.58 d |
92.98
±1.93 c |
20.4
±1.09 d |
70.57
±1.15 d |
3.80
±0.45 d |
1.64
±0.44 d |
| Group-III
(0.1 mg-30 days) |
154.9
±2.44 d |
34.22
±1.54 d |
97.42
±1.13 c |
24.26
±1.06 a |
75.2
±1.46 a |
4.52
±0.43 d |
2.84
±0.43 a |
| Group-IV
(0.1 mg-60 days) |
177.17
±7.98 b |
39.23
±2.55 d |
102.08
±3.78 c |
35.86
±1.14 c |
78.52
±1.16 b |
4.51
±1.12 d |
3.49
±0.34 b |
Serum Glutamic Pyruvic Transaminase (SGPT) and Serum Glutamic-Oxaloacetic Transaminase (SGOT) are the major enzymes specifically present in the cells of the liver. The elevated level of SGPT and SGOT enzyme in the bloodstream is an indicator of liver cell damage. In the present study administration of meloxicam at the dose of 0.1mg/kg body weight for 15 and 30 days showed a slight increase (P≤0.01) in the level of serum SGPT and SGOT. Whereas a significant increase (P≤0.001) was observed in 60 days treatment of meloxicam in Gallus domesticus as compared to vehicle treated control group (Fig.1&2). Similarly, Zollinger et al also reported that intramuscular treatment of carprofen drug to Columba livia for 7 days showed a significant increase in the level of SGPT and SGOT25. Alkaline phosphatase (ALP) is isozymes, that is present in the liver thus any alteration in the concentration of ALP in the blood showed dysfunction of the liver. In the present investigation meloxicam treatment showed significant (P≤0.001) elevation in the concentration of serum ALP level in Gallus domesticus (Fig.3). Intramuscular administration of 0.1 mg/kg diclofenac and meloxicam treatment for 30 days showed significant increase in the concentration of serum alkaline phosphatase in broilers26. Yousef et al. also reported similar increase in the level of ALT and AST27.
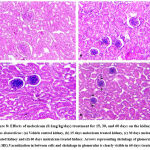
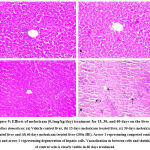
The histology of liver in control and 15 days treatment of meloxicam (0.1mg/kg body weight) groups revealed a normal structure of liver tissue with normal hepatic cords, hepatic sinusoids and central vein (Fig.9a & 9b). However, the histology of the liver in groups III and IV (meloxicam treatment 30 and 60 days) showed degeneration of hepatic cells, congested central vein and slight vacuolization observed in hepatic cells (Fig.9c & 9d). Our findings revealed that a short duration meloxicam not affected the structure of the liver but the use of meloxicam for a long duration causes some histoarchitectural changes in the liver. Administration of intramuscular carprofen for days in pigeon causes lesions in the hepatic cells25.
It was previously reported that NSAIDs inhibited the activities of COX-1 and COX-2 (cyclo-oxygenase-1 and 2). COX-1 and COX-2 are playing important roles in the regulation of kidney function. COX-2 shows a protective effect via producing prostaglandins and COX-1 maintain blood flow in the renal arteries by suppressing vasoconstriction of the afferent arteriole. Long term uses of NSAIDs cause long term inhibition of COX-1 that leads to glomerular infiltration and ischemic renal injury28, 29. In the present study the histological observations of the kidney of control, 15 and 30 days meloxicam treatment groups showed normal structures of renal epithelium, glomerulus and renal tubules that show meloxicam not affected the structure of kidney at short duration (Fig.8a, 8b & 8c). The structure of kidney of 60 days meloxicam treatment at the dose of 0.1mg/kg body weight showed shrinkage in glomeruli, vacuolization in renal epithelium lining, and renal necrosis was observed that showing the long term harmful effects of meloxicam on renal tissues, it may be due to long term uses of meloxicam inhibited COX-2 that leads to dysfunctioning of renal cells (Fig. 8d). Degeneration of glomeruli and dilation of renal tubules was observed in budgerigars treated with meloxicam30.
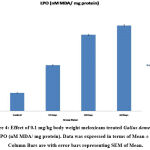
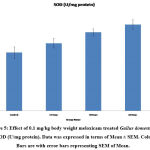
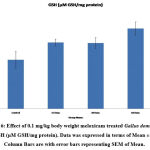
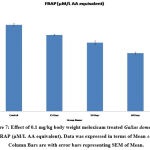
Antioxidant tests like SOD, LPO, CAT, GSH, and FRAP are associated with a prevention of cardiovascular, oxidative stress, and neurological illness like degenerative diseases31. Mainly antioxidants provide front-line defense against reactive oxygen species. In rabbits elevated SOD, Catalase, GSH and LPO were reported in day’s dependent manner after treatment with enrofloxacin, levofloxacin and meloxicam32. In the present investigation the content of LPO was significantly increased (P ≤ 0.001) and FRAP was significantly decreased (P ≤ 0.001) in 15, 30 and 60 days meloxicam treated Gallus domesticus (Fig. 4 & 7) and it was previously demonstrated that increased level of LPO and decrease the level of FRAP are an indicator of reduction in the status of antioxidant. Slight increase that is negligible was observed in SOD and GSH content in the meloxicam treated group (Fig. 5 & 6) denoted that meloxicam may increase oxidative stress in the body via increasing reactive oxygen species and reducing thereby meloxicam the status of antioxidant.
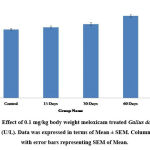 |
Figure 1: Effect of 0.1 mg/kg body weight meloxicam treated Gallus domesticus on SGOT (U/L). Data was expressed in terms of Mean ± SEM. Column Bars are with error bars representing SEM of Mean. |
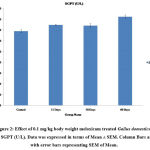 |
Figure 2: Effect of 0.1 mg/kg body weight meloxicam treated Gallus domesticus on SGPT (U/L). Data was expressed in terms of Mean ± SEM. Column Bars are with error bars representing SEM of Mean. |
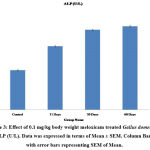 |
Figure 3: Effect of 0.1 mg/kg body weight meloxicam treated Gallus domesticus on ALP (U/L). Data was expressed in terms of Mean ± SEM. Column Bars are with error bars representing SEM of Mean. |
Conclusion
It can be concluded that the serum levels of SGOT, SGPT, and ALP as well as the histoarchitecture of the liver and kidneys were found to have changed negatively as a result of long-term use of meloxicam. The serum antioxidant assays LPO and SOD were significantly increased and FRAP readings were significantly decreased after 60 days of meloxicam treatment in current study. These changes, which are inconsistent with the biochemical parameters and histology of the treated groups in comparison to the control group, were seen in the treated groups but not in the control group. Degenerative alterations in the histology of the liver, kidney, and biochemical parameters are justified by higher levels of LPO and SOD, as is evident from increased LPO, SOD, and lower FRAP values. The capability of the antioxidant system is diminished when FRAP levels are low. Meloxicam belongs to the class of NSAIDs that inhibit COX-2, a regulator of kidney function. Chronic inhibition of COX-2 results in renal failure. Long-term meloxicam use also damages hepatic cells and raises oxidative stress by lowering antioxidant status. The aforementioned study’s findings suggest that meloxicam use over an extended period of time reduces the body’s antioxidant potential, which in turn causes the liver and kidney to malfunction.
Acknowledgment
We are thankful to the Head and Department of Zoology, Jai Narain Vyas University, Jodhpur, Rajasthan for providing facilities for the above research work. We are also thankful to UGC (UGC-Ref. No.: 940/(CSIR-UGC NET DEC.2017), CSIR-New Delhi (File No:09/098(0137)/2019-EMR-I) and SERB/CRG/002090/2019 for providing financial support. Special thanks to Professor A.K. Purohit and Dr. Heera Ram for their guidance and support.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Martel-Pelletier J, Lajeunesse D, Reboul P and Pelletier J. P. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann. Rheum. Dis., 2003; 62:501-509.
- Shaikh R. U, Pund M. M and Gacche R. N. Evaluation of anti-inflammatory activity of selected medicinal plants used in Indian traditional medication system in vitro as well as in vivo. Tradit.Complement. Med., 2016; 6(4): 355-361.
- Livshits A and Seidman D. S. Role of Non-Steroidal Anti-Inflammatory Drugs in Gynaecology. Pharmaceuticals, 2010; 3:2082-2089.
- Harris J. The conservation of Accipitridae vultures of Nepal: a review. J. Threat. Taxa., 2013; 5(2): 3603–3619.
- Hussain I, Khan M. Z, Khan A, Javed I and Saleemi M. K. Toxicological effects of diclofenac in four avian species. Avian Pathol., 2008; 37(3): 315-321.
- Green R. E, Newton I, Shultz S, Cunningham A. A, Gilbert M, Pain D. J and Prakash V. Diclofenac poisoning as a cause of vulture population declines across the Indian subcontinent. J. Appl. Ecol., 2004; 41: 793–800.
- Oaks J. L, Gilbert M, Virani M. Z, Watson R. T, Meteyer C. U, Rideout B, Shivaprasad H. L, Ahmed S, Chaudhry M. J. I, Arshad M, Mahmood S, Ali A and Khan A. A. Diclofenac residues as the cause of vulture population declines in Pakistan. Nature, 2004; 427: 630–633.
- Shultz S, Baral H. S, Charman S, Cunningham A. A, Das D, Ghalsasi G. R, Goudar M. S, Green R. E, Jones A, Nighot P, Pain D. J and Prakash V. Diclofenac poisoning is widespread in declining vulture populations across the Indian subcontinent. Proc. Royal Soc. Lond. Ser., 2004; B 271 (Suppl.): S458–S460.
- Swan G. E, Cuthbert R, Quevdeo M, Green R. E, Pain D. J, Bartels P, Cunningham A, Duncan N, Oaks J. L, Parry-Jones J, Taggart M, Verdoorn G and Wolter K. Toxicity of diclofenac to Gyps Biol. Lett., 2006a; 2: 279–282.
- Swan G, Naidoo V, Cuthbert R, Green R. E, Pain D. J, Swarup D, Prakash V, Taggart M, Bekker L, Das D, Diekmann J, Diekmann M, Killian E, Meharg A, Patra R. C, Saini M and Wolter K. Removing the Threat of Diclofenac to Critically Endangered Asian Vultures. PLoS Biol., 2006; 4(3): e66.
- Jones C. J and Budsberg S. C. Physiologic characteristics and clinical importance of the cyclooxygenase isoforms in dogs and cats. Am. Vet. Med. Assoc., 2000; 217(5): 721-729.
- Merchant M. A and Modi D. N. Acute and chronic effects of aspirin on haematological parameters and hepatic ferritin expression in mice. Indian J. Pharmacol., 2004; 36(4):226–30.
- Ramesh N, Jayakumar K, Honnegowda, and Narayana K. Effect of diclofenac and nimesulideon haematology in dogs. Indian J. Anim. Sci., 2001; 71(3):221–3.
- Ohkawa H, Ohishi N and Yagi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Biochem., 1979; 95(2):351-358.
- Aebi H. Catalase in Vitro. In Methods in Enzymology; Packer, L., Ed.; Academic Press Inc.: San Diego, CA, USA, 1984; 105: 121–126.
- Marklund S and Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem., 1974; 47(3):469–474.
- Benzie I and Strain J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of Antioxidant Power: The FRAP Assay. Ana. Biochem., 1996; 239:70-76.
- Beutler E, Duron O and Kelly B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med., 1963; 61:882–890.
- Senacha K. R, Taggart M. A, Rahmani A. R, Jhala Y. V, Cuthabert R, Pain D. J and Green R. E. Diclofenac level in livestock carcasses in India before the ban. J. Bombey Nat. Hist. Soc., 2008; 105: 148-161.
- Gilbert M, Virani M. Z, Watson R. T, Oaks J. L, Benson P. C and Khan A. A. Breeding and mortality of Oriental white-backed vulture Gyps bengalensis in Punjab Province, Pakistan. Bird Conserv. Intl., 2004; 12: 311-326.
- Ghodasara P. D, Pandey S, Khorajiya J. H, Prajapati K. S, Ghodasara D. J and Joshi B. P. Toxicopathological studies of meloxicam, ibuprofen and diclofenac sodium in broiler chicks. Indian J. Vet. Pathol., 2014; 38(4):250-255.
- Toghyani M, Tohidi M, Gheisari A. A and Tabeidian S. A. Performance, immunity, serum biochemical and hematological parameters in broiler chicks fed dietary thyme as alternative for an antibiotic growth promoter. J. Biotechnol., 2010; 9(40):6819-6825.
- Sinclair K. M, Church M. E, Farver T. B, Lowenstine L. J, Owens S. D and Paul-Murphy J. Effects of meloxicam on hematologic and plasma biochemical analysis variables and results of histologic examination of tissue specimens of Japanese quail (Coturnix japonica). Am. J. Vet. Res., 2012; 73(11): 1720–1727.
- Amin H. M, El-Feki M. A, Abdalla A. A and Youssef M. A. Hematological and Biochemical Effects of Meloxicam in Male Albino Rats. Curr. Sci. Int., 2017; 06 (1): 23-33.
- Zollinger J, HooverJ. P, Payton M. E and Schiller C. A. Clinicopathologic gross necropsy and histologic findings after intramuscular injection of carprofen in a pigeon (Columba livia) model. J. Avian Med. Surg., 2011; 25(3):173-84.
- Saran R. P, Purohit A and Ram H. A comparative patho-physiological study of diclofenac and meloxicam induced toxicity in Gallus domestics. J. Pharm. Health Res., 2016; 4(11): 1-15.
- Yousef M I, Abbassy M. S and Yacout M. H. Assessment of cypermethrin and dimethoate toxicity in Barki sheep: biochemical and histological changes and tissue residues. J. Anim. Prod., 1999; 36(1): 25-41.
- Turner P. V, Chen H. C and Taylor W. M. Pharmacokinetics of meloxicam in rabbits after single and repeat oral dosing. Med., 2006; 56(1):63-67.
- Pelligand L, Suemanotham N, King J. N, Seewald W, Syme H, Smith K, Lees P and Elliott J. Effect of cyclooxygenase (COX)-1 and COX-2 inhibition on furosemide-induced renal responses and isoform immunolocalization in the healthy cat kidney. BMC Vet. Res., 2015; 11: 296.
- Pereira M. E and Werther K. Evaluation of the renal effects of flunixin meglumine, ketoprofen and meloxicam in budgerigars (Melopsittacus undulatus). The Rec., 2007; 160(24): 844-6.
- Gohari A. R, Hajimehdipoor H, Saeidnia S, Ajani Y and Hadjiakhoondi A. Antioxidant activity of some medicinal species using FRAP assay. Med. Plant, 2010; 10(37):1-9.
- Khan A. M, Rampal S and Sood N. K. Effect of repeated oral administration of levofloxacin, enrofloxacin, and meloxicam on antioxidant parameters and lipid peroxidation in rabbits. Hum. Exp. Toxicol., 2017; 36(1): 42–50.