Manuscript accepted on :16-11-2022
Published online on: 30-11-2022
Plagiarism Check: Yes
Reviewed by: Dr. Niharika Kondepudi
Second Review by: Dr. Susmita
Final Approval by: Dr. Ian James Martin
Abdel-Nasser El-Shorbagi 1,2* , Sachin Chaudhary 1
, Sachin Chaudhary 1 , Anurag Chaudhary3
, Anurag Chaudhary3 , Garima Agarwal3, Prabhash Nath Tripathi3
, Garima Agarwal3, Prabhash Nath Tripathi3 , Shweta Dumoga3
, Shweta Dumoga3 .
.
1Department of Medicinal Chemistry, College of Pharmacy, University of Sharjah, Sharjah, United Arab Emirates.
2Faculty of Pharmacy, University of Assiut, Assiut, Egypt.
3Department of Pharmaceutical Technology, Meerut Institute of Engineering and Technology, NH-58, Baghpat Road Crossing, Bypass Road, Meerut, Uttar Pradesh, India.
Corresponding Author E-mail: aelshorbagi@sharjah.ac.ae
DOI : https://dx.doi.org/10.13005/bpj/2519
Abstract
β-lactam antibiotics are considered the safest bactericides, and upon wide clinical use of benzyl penicillin G in 1945, outbreaks of resistance came out. The frequent semi-synthetic strategies revealed β-lactam generations that are of broad-spectrum activity. The new agents as well as their concomitant use with known inhibitors of β-lactamases potentiate their effectiveness versus higher numbers of resistant pathogens. However, the extremely resistant pathogens are still representing a burden. Efforts had been continued to find more inhibitors of β-lactamases to combine with β-lactams to provide good management of infections by extremely resistant microbes. The purpose of this work is to overview the conventional and the recently introduced β-lactamases in clinical applications, as well as some reported effective inhibitors of β-lactamases. The review pinpoints the inhibitors that can be mixed and/or merged with the beta-lactam antibiotics to effectively treat the microbial infections producing resistant-β-lactamases. ClogP for these drugs and candidate inhibitors is introduced as suggestions to open a door for developers to admix derivatives with suitable pharmacokinetics.
Keywords
Antibacterial; β-lactamases inhibitors; Clinical investigations; Multi-resistant strains
Download this article as:| Copy the following to cite this article: El-Shorbagi A. N, Chaudhary S, Chaudhary A, Agarwal G, Tripathi P. N, Dumoga S. Beta-Lactamases Inhibitors: A Perspective on the Existing and the Potential Admixtures to Synergize Beta-lactams Versus Resistant Superbugs. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: El-Shorbagi A. N, Chaudhary S, Chaudhary A, Agarwal G, Tripathi P. N, Dumoga S. Beta-Lactamases Inhibitors: A Perspective on the Existing and the Potential Admixtures to Synergize Beta-lactams Versus Resistant Superbugs. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3AXCj3M |
Introduction
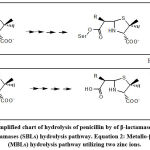
The strategies for the discovery of new β-lactam-antibiotics related to subclasses as penicillins, cephalosporins and monobactams in addition to carbapenems are not enough policies to kill superbugs. Historically, Abraham and Chain 1 noticed manifestation of resistance against penicillin from some bacterial cultures. The resistance involving deactivation by enzymes, mentioned later as β-lactamase.2,3 Most of the β-lactam-antibiotics are vulnerable to inactivation by β-lactamases. The persistent exposure of some bacterial strains to a multitude of β-lactam-antibiotics has led to overproduction and mutation of β-lactamases. β-Lactamases are produced by many gram-positive and gram-negative strains.2,4 Many β-lactamases have been reported and two systems were introduced to verify their types. The first one is Ambler system (1980) which utilizes amino acid sequence to define molecular phylogenies and grouping β-lactamases into four broad classes: A, B, C, and D.4 The second is Karen Bush in 1988. Ambler A, C, and D classes are serine β-lactamases (SBLs, Figure 1, Equation 1) while class B enzymes are Metallo-β-lactamases (MBLs, Figure 1, Equation 2). The Figure 1 simply configures the generalized fate of penicillin-β-lactamase-mediated hydrolysis to ring-opened penicilloic acid.1 In continuation of our interest in medicinal chemistry area we report this work since it complies with our efforts in skeletons of specified biological activities and targeting as well as naturally active agents5-25. This is an updated review describing most of β-lactamases, along with reports on the ongoing status and information concerning the recent inhibitors to date 2021. In addition, a demonstration, concerning the strategies applied during the process of drug discovery to identify new β-lactamase inhibitors is also introduced.
β-Lactamases Hydrolyzing Effects
As mentioned by WHO in 2019, superbug’s infections of the lower respiratory system represented the third top reason of worldwide deaths (https://www.who.int/data/gho/data/themes/ mortality-and-global-health-estimates) and if one adds superbug’s infections in other body organs, it will be shifted to the top reason of deaths. In addition, a warning alarm mentions that mortality resulting from infections by resistant microbes will be increased by 2050 and will kill more people than cancer do.26 There are attempts for production of wide-range-spectrum of β-lactamase inhibitors able to inhibit many β-lactamases, such as cephalosporinases along with serine-based carbapenemase, (Table 1) which severely limit therapeutic options by hydrolyzing β-lactam entity in β-lactam antibiotics. The (Figure 1), simply configures the generalized fate of penicillin-β-lactamase-mediated hydrolysis to ring-opened penicilloic acid. 1,25-28
Table 1: Classification of β-lactamases, substrates, sensitivity to common inhibitors and examples. Ambler and Bush et.al systems 58-61.
| Ambler | Bush et. al | Substrate | Inhibition | Enzymes | ||
| Mol class | Sub-group | Name | Clavulanic | Tazobactam | EDTA | |
| A | 2a | Penicillins | Yes | No | No | AmpC, ACT-1 |
| 2b | Penicillins & 1st, 2nd ceplalosporins | Yes | No | No | GC-1, CMY-37 | |
| 2be | Extended spectrum cephalosporins, Monobactams | Yes | No | No | PC-1 | |
| 2br | Penicillins | No | No | No | TEM-1, TEM-2, SHV | |
| 2c | Carbenicillin | Yes | No | No | TEM-3, SHV-2, CTX-M-15 | |
| 2ce | Carbenicillin, cefepime | Yes | No | No | TEM-30, SHV-10 | |
| 2e | Extended spectrum cephalosporins | Yes | No | No | CepA | |
| 2f | Carbapenems | Variable | Variable | No | KPC-2, SME-1 | |
| B | 3a | Carbapenems | No | Yes | Yes | IMP-1, NDM-1 |
| 3b | Carbapenems | No | Yes | Yes | CphA, Sfh-1 | |
| C | 1 | Cephalosporins | No | No | Ampc, ACT-1 | |
| 1e | Cephalosporins | No | Yes | GC-1, CMY-37 | ||
| D | 2d | Cloxacillin | Variable | No | No | OXA-1, OXA-10 |
| 2de | Extended spectrum cephalosporins | Variable | No | No | OXA-11, OXA-15 | |
| 2df | Carbapenems | Variable | No | No | OXA-23, OXA-48 | |
Clinically Useful β-Lactamases Inhibitors
To date the clinically used inhibitors of β-lactamases must be newly classified as follows:
Beta (β)-lactam β-lactamase inhibitors such as clavulanic acid, sulbactam and tazobactam.
Gamma (γ)-lactam β-lactamase inhibitors such as avibactam and relabactam.
Oxaborinane β-lactamase inhibitors such as vaborbactam.
The combination of β-lactams with β-lactamase inhibitors led to effective therapeutic properties. The known common combination are Amoxicillin combined with Clavulanic acid (Table 2, Compound 1) approved by FDA 1984 (https://www.medicinenet.com/amoxicillinclavulanic acid_tablet_875mg125mg/article.htm), Ticarcillin with Clavulanic acid, approved 1985, Piperacillin combined with Tazobactam (Table 2, Compound 2) approved by 1993, and Ampicillin combined with Sulbactam (Table 2, Compound 3) approved 1997. All of these combinations are introduced and widely used as major drugs for community-acquired contaminations as well as hospital-infection. 4
Recently, Relebactam (MK‐7655, Table 2, Compound 4) is a bicyclic none β-lactam β-lactamase inhibitor, gained FDA approval as part of the combination product RecarbrioⓇ in July 2019. 29-30 It is currently available in a combination product includes Imipenem and Cilastatin to treat complicated urinary tract infections (UTIs), pyelonephritis, and complicated intra-abdominal infections in adults. It is a last-line treatment option.
Avibactam (NLX104) (Table 2, Compound 5) 31-32 is another new none β-lactam β-lactamase inhibitor that is available in combination with Ceftazidime (AvycazⓇ).33-34 The FDA approved this combination in 2015 for the treatment of complicated intra-abdominal infections in combination with metronidazole, and the treatment of complicated urinary tract infections caused by resistant-and multi-drug resistant gram-negative bacterial pathogens. Avibactam is a potent and broad-spectrum inhibitor than the previously discussed inhibitors such as the widely prescribed clavulanic acid. It maintains the ability to covalently acylate β-lactamases. 32
Vaborbactam (Table 2, Compound 6) is a β-lactamase inhibitor; a cyclic boronic acid derivative approved by FDA 2017 as VabomereⓇ consists of Vaborbactam and Meropenem. Used mainly for complicated urinary tract infections (UTI) by intravenous administration. Vaborbactam is intended for serine beta-lactamases, Ambler class A and C enzymes.35-39
Β-Lactam Antibiotics and Superbugs Resistance
β-Lactam bearing drugs are among the most used antibiotics. 40-46 Their main mechanism is to target and interrupt biosynthesis of cell wall via irreversible inhibition of trans-peptidases, and what is recognized as penicillin-binding proteins (PBPs). PBPs represent a group of enzymes that are included in the final steps of peptidoglycan cross-linking of bacterial cell walls. 46-50 Superbugs represent a serious global health threat in this century. 51-52 Existing drugs become less effective against these resistant pathogens even in the presence of therapeutic-dose levels of the drugs because of their production of β-lactamases that irreversibly hydrolyze β-lactam ring. 54-55
The Resistant Superbugs and β-Lactamases Classes
The resistance to antibiotics by superbugs gained through chromosomal mutation side-by-side with horizontal transfer of resistance genes by bacterial plasmids. The bacterial families bearing resistance are related to: 1- Some Gram-positive bacteria such as Staphylococcaceae (Staphylococcus aureus). 2- Gram-negative bacteria such as Enterobacteriaceae (Klebsiella pneumonia, Citrobacter, Proteus vulgaris, Morganella, Salmonella, Shigella, Escherichia coli), Pasteurellaceae (Haemophilus influenza), Neisseriaceae (Neisseria gonorrhoeae), Pseudomonadaceae (Pseudomonas aeruginosa) and 3- Neither Gram-positive nor Gram-negative; acid-fast bacteria such as Mycobacteriaceae (Mycobacterium tuberculosis).
Because of the variety of β-lactamases discovered the Ambler system is identified as molecular based classes depending on the sequence of amino acid. Classes are declared by letters A, B, C, and D. The second classification system recognized as the Bush that focuses on different aspects of β-lactamases, such as enzyme inhibition profile, hydrolysis rate, and binding affinity categorized as 3 groups based on their substrate and inhibitory profiles. Ambler’s system appears to be more widely accepted. 56-64 Ambler and Bush classification systems and the main enzymes involved are outlined in Table 1. 43-66 Enzymes are characterized according to the sequencing of proteins. 62 Ambler’s classes A, C, and D utilizes serine-OH group as nucleophile (Figure 1, equation 1) while class B (metallo-β-lactamases) involves divalent zinc as metal ions (Figure 1, equation 2) for substrate hydrolysis. 43,66 Extended-Spectrum β-Lactamases (ESBLs) are rapidly growing group.65 Examples are class A TEM-type β-lactamases (plasmid-mediated) frequently encountered in E. coli and K. pneumoniae as well as in some strains of Gram-negative bacteria. 61,66 TEM 1 differs from TEM-2 by single amino acid and from TEM-3 by two amino acids. Other TEM-types differ by 3, 4 or more amino acids different from the parent TEM-1 and to-date over 140 TEM-enzymes were identified.67-69
Class A, SHV-1 (sulfhydryl SH) is also a chromosomally encoded-enzyme detected in K. pneumonia isolates, and isolated among samples of Enterobacteria.66-71 The gene encoding SHV-1 incorporated is later included within the plasma which facilitated its spread to Enterobacteria species.72 SHV-2 differs from SHV-1 by replacement of glycine by serine at particular position in the active site. 71 SHV-1 β-lactamase has structure resemblance with TEM-1.62 SHV-5 and SHV-12 are among the common types of ESBLs.61
Class A, CTX-M-type β-lactamases are resistant to cephalosporins and originated in Kluyvera species. These enzymes acquire gene transfer via plasmid and detected in multiple strains of Salmonella enteric, Typhimurium, E. coli, and other Enterobacteriaceae.69-73 To date, more than 172 CTX-M protein variants have been reported classed under 6 groups based on their amino acid sequencing. They include CTX-M-1, CTX-M-2, CTXM-8, CTX-M-9, CTX-M-25 and KLUC named after the first group which was detected. 60-62 It has been proven that the part associated with CTX-M β -lactamases is the serine residue at position 237.70-72
Class A carbapenemases enzymes were obtained from Enterobacteriaceace, in which they are involved with chromosomal encoding. 72-75 Carbapenemases represent the most diverse of the β-lactamase family that composed from two classes the Class A-serine-type and Class-B metallo-β-lactamases depending on the reactive site of the enzymes. They can be categorized under 3 different Ambler and 2 different Bush-Jacoby groups.76-78 The enzymes identified in this Class A-type include the chromosomally encoded (NMC-A, SME, and IMI-1) and others are the plasmid-encoded (KPC, IMI-2, some GES variants). 74-76
Class C-serine cephalosporinases 77-79 mentioned as AmpC are isolated from Enterobacteriaceae and identified as two types the chromosomal (inducible) Amp and plasmid mediated AmpC enzymes. Plasmid mediated type are becoming prevalent and generated through the transfer of chromosomal genes on to plasmids.79-81
Class D-serine such as oxacillinases are over 498 OXA β-lactamases have been detected and divided into OXA ESBLs and carpabenemases detected in P. aeruginosa widespread in Enterobacteriaceae, Acinetobacter and in K. pneumoniae. They vary in amino acid sequence and mentioned as OXA-11, OXA-14, OXA-16, OXA-19, OXA-31, etc. (60, 69), OXA-23, OXA-48, OXA-51, OXA-143 and OXA-48 which is detected in K. pneumoniae.61-83 Subgroups 2d, 2de, and 2df are related to molecular class D known as oxacillinases.84
Class B; metallo-β-lactamases (MBLs) also known as metallo-carbapenemase, that necessitate zinc ions in their active sites with mechanistic differences.72-85 Metallo-β-lactamases have been recognized in P. aeruginosa and their encoding genes can be either chromosomal structure or situated on plasmids that can be spread among species. MBLs are subdivided into three classes; subclass B1 (includes IMP, VIM and NDM variants) and subclass B3 require two zinc (II) ions in their active sites. In contrast, subclass B2 enzymes utilize only one zinc (II) ion.84-87
Brief Mechanisms of Inhibition of β-Lactamases by FDA Approved Drugs
β–lactamases from classes A, C, and D follow the same interaction with the β–lactam-antibiotic and similarly with inhibitors. The (Figure 2), represents the initial location at the binding sites of enzymes and the FDA-approved drugs such as clavulanic acid (representing the 4-membered β–Lactamases group such as clavulanic acid, sulbactam and tazobactam), avibactam (representing 5-membered lactams such as avibactam and relebactam) and vaborbactam as borane derivative. The main amino acids catalyzing the interaction are mention.88 The mechanism of interaction is reported elsewhere in which the amino acids lining the binding site interact and stretch the inhibitor molecule (similar to the antibiotics penicillins or cephalosporins) and facilitating the nucleophilic attack of ser-70 to form covalent bond with the vulnerable group of the inhibitor.57-84
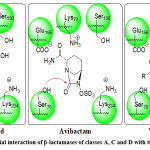 |
Figure 2: Simplified initial interaction of β-lactamases of classes A, C and D with the β-lactamase inhibitors. |
The interaction of these β-lactamases with the inhibitors of different types (Figure 3) lead to hanging-up these enzymes through the formation of variable types of bonding.
In case of the first group of inhibitors namely clavulanic acid, sulbactam and tazobactam an irreversible covalent bond (ester) between the ser-70 hydroxyl group with the carbonyl of the inhibitor 89. Further strong covalent bond may form between amino group of lys-73 or ser-130 with carbon-6 followed by inhibitor degradation and inactivation of the enzyme (Figure 3: A, B, C). 90
In case of the second group of inhibitors namely avibactam and relebactam a carbamate ester can be formed between ser-70 hydroxyl group with the carbonyl of theses inhibitors. Carbamate esters are less susceptible for hydrolysis (Figure 3: D).
In case of vaborbactam which represents a different type of inhibitors, a strong co-ordinate covalent bond is formed between ser-70 hydroxyl group with boron of the inhibitor (Figure 3: E).
In contrast to serine β-lactamases; MBLs are not associated with a covalent bond between the β-lactam antibiotic and an amino acid lining the active site. The metal-particles in MBLs are two zinc(II) ions the first zinc(II) ion coordinates to His-residues while the second zinc(II) ion coordinated with Asp120, His, and Cys-residues. The first zinc (II) ion acts as a Lewis acid and decreases the pKa value of the bridging water molecule which results in the formation of hydroxide ion that initiates the nucleophilic attack on the β-lactam antibiotic or the inhibitor vulnerable groups.52-91
Another important point is that the amino acids lining the MBLs active site and interacting with the β-lactam antibiotic differ than those of classes A, C and D. Payne et. al. descried the interactions between the enzyme and a natural tricyclic structure SB236050 (Table 2, Compound 7) mentioned as MBLs inhibitor and provided antibacterial synergy with meropenem on some MBLs.92 This compound has inhibitory activity versus IMP-1, and CfiA. Zn (II) coordinated with water and Asn193, His99, His101, His162, Zn (II) also coordinated with the same water molecule Asp103, Cys181, His223, and the inhibitor.
Structures of FDA-Approved Drugs, Patents and Developments of β-lactamase Inhibitors to Date
There is a serious need to search for new β-lactamase inhibitors in light of emerging of resistant superbug. To conquer this risk, scientists could introduce inhibitors to synergize the β-lactams effects. Developing inhibitors is a difficult aspect because of the different classes of β-lactamases. As an example, MBLs and OXA β-lactamases create a challenge and represent a highly significant problem due to their ability to inactivate nearly all β-lactam antibiotics.93-95
Regardless of the worldwide spread of MBLs, their inhibitors have not yet emerged in clinical use.94 MBLs inhibitors have been reported, but the mechanism of inhibition is unknown for many of these inhibitors yet becoming more prevalent and problematic. On the other hand, the OXA species are extremely diverse with other 500 variants; among these species they differ in their hydrolytic activity with the other serine-based mechanism, thus a single β-lactam-β-lactamase inhibitor combination strategy that targets all clinically significant β-lactamases seems improbable.86-97
Table 2 outlines the structures of FDA-approved drugs, codes of registered compounds or by numbers for the compounds that reported of high activity. The structures, and the references that introduce and/or discuss the mechanisms of these compounds are also mentioned in this table. The cLog P from chemdraw (https://perkinelmerinformatics.com/products/research/chemdraw/) for each structure are computed and added in (Table 2) is to understand structure’s hydrophilicity and hydrophobicity.
Clavulanic acid (Table 2, Compound 1) was the first β-lactamase inhibitor introduced into clinical. It is mixed with amoxicillin (Augmentin ®), and this allows the effective dose of amoxicillin to be decreased. Mixed with ticarcillin (Timentin) to be used (i.v.) to inhibit the enzyme β-lactamase and providing prompt effect. The success of clavulanic acid stimulated the development of semisynthetic penicillinic acid sulfones. It is classed as sentry drug because it’s able to make two covalent bonds and the molecule will be cleaved (Figure 3: B & C).96-99
Penicillinic acid Sulfones as FDA- approved drugs are synthetized penicillanic acid derivatives. The sulfur atom is made as sulfonyl group to increase its electron withdrawal effect on C-5 to be similar in its electrophilicity and reactivity with nucleophies at the binding site to that of clavulanic acid for its inhibition mechanism (Figure 3: B& C).
Sulbactam (Table 2, Compound 2) is a penicillanic acid sulfone of broader spectrum but slightly less potent than clavulanic acid. It is mixed with ampicillin as capsules, pills or (i.v.) injectables (https://go.drugbank.com/drugs/DB09324 ).
Tazobactam (Table 2, Compound 3) is another penicillanic acid sulfone β-lactamase inhibitor of equal potency with clavulanic acid but much broader. The combination piperacillin-tazobactam acquires activity against TEM-type ESBL’s, in which the enzymes are more susceptible to piperacillin-tazobactam than SHV type ESBL’s. Moreover, such combination provides a 10-fold high activity against CTX-M when compared with calvulanic acid 98-100.
Relebactam (Table 2, Compound 4) is a bicyclic system bearing a five-membered lactam ring bearing an electrophilic carbonyl-carbon that forms ester when attacked by the nucleophile of the active site. The ester in this case is a stable urethane (carbamate ester).
Avibactam (Table 2, Compound 5) has the same bicyclic system of relebactam bearing a five-membered lactam ring with no piperidine moiety on the amide side chain. The acylation mechanisms are reported elsewhere and enzyme binding with either avibactam or relebactan is represented in Figure 3: D.31-33
Vaborbactam (Table 2, Compound 6) is a β-lactamase inhibitor; a cyclic boronic acid derivative approved by FDA 2017 as VabomereⓇ consists of Vaborbactam and Meropenem. Used mainly for complicated urinary tract infections (UTI) by intravenous administration. Vaborbactam is intended for serine beta-lactamases, Ambler class A and C enzymes in Figure 3: E.35-37
Candidates and Active Derivatives as β-Lactamase Inhibitors
The following monographs collect the effective derivatives under common structural titles and verification names:
Chaeto-Chromones
These were isolated from crude extract of fungus Chaetomium indicum (CBS. 860.68).90-101 Group of potent inhibitors versus metallo-beta lactamases are mentioned from those polyketides SB236050 (Compound 7, Table 2) had been investigated at the active site of a metallo-beta lactamase enzyme in crystal structure.90-92
Four-Membered-Ring Bearing Scaffolds as Inhibitors
SYN 1012 and BRL 42715 (Compounds 8 and 9, Table 2) are methylidene-penem derivatives possessing strong inhibition of serine β-lactamases of class A, C and D enzymes.100-102 These agents having increased cell permeability and acting by a different mechanism than clavulanic acid.101-105 Penems (Compounds 10 and 11, Table 2) are other methylidene penems related to compound 9 and aimed mainly to improve stability in solution and increase lipophilicity. The compounds have proven to be potent inhibitors of class A, C, and D β-lactamases.104-108 The two compounds are effective inhibitors of class D OXA-1 β-lactamases. 107-110
AM-112 (Compound 12, Table 2)
It is a derivative of clavulanic acid. This compound was unstable however it gives a lead zwitterion compound which has a potent inhibition property against class C and D β-lactamases.109-113
LK-157 (Compound 13, Table 2)
It is a fused tricyclic bearing carbapenem structure. It significantly inhibits AmpC-lactamase with 2,000-fold more potency than clavulanic acid and about 28-fold more active than tazobactam.114 The introduction of a methoxy group at C-4 position shows affinity towards both Class A and Class C.113 Ethylidene entity at C-6 was intended to maintain stability of the β-lactamase inhibitor/β-lactamase complex, in addition it was speculated that the hydrophobic rings at position C-3 and C-4 intended to block water molecule from penetrating the acyl-enzyme complex and thus preventing enzyme recovery.114-116
β-Methylcarbapenems (Compound 14-17, Table 2)
These are carbapenem derivatives that contain a methyl group at C-1 and substituents at C-2 that are important in this scaffold for the inhibition of class B metallo-β–lactamases. Among these derivatives; J-110,441 (Compound 14, Table 2) was the most potent inhibitor of class B metallo- β-lactamases (IC50 of 0.1 mM).115-117
SA2-13 (Compound 18, Table 2)
It is a tazobactam-related derivative β-lactamase inhibitor. It is developed to increase the lifetime of the trans-enamine intermediate compared to tazobactam when interacting with the enzyme. SA2-13: SHV-1 intermediate has a 10-fold lower de-acylation rate than tazobactam: SHV-1 intermediate.86-88
BAL29880 (Compound 19, Table 2)
It has a chelating property to be combined with MBL-resistant monobactam antibiotic that is affected by cephalosporinases class C type AmpC and ESBLs. BAL29880 inhibits AmpC furthermore, clavulanic acid blocks the activity of other class A β-lactamases including ESBLs.116-118
MK-8712 (Compound 20 and 21, Table 2)
The first has better AmpC inhibitory activity and shows synergistic effects with imipenem than the latter.119-121
Ro 48-1220 (Compound 22, Table 2)
(Z)-2 – acrylonitrile penam sulfone is a potent inhibitor. Comparing its inhibition with tazobactam, Ro 48-1220 was 15 times more effective against class C -lactamases. In another study, the inhibitor enhanced the activity of ceftriaxone and ceftazidime against producers of TEM-1 type class A -lactamases.122
6-Substituted penam sulfones (Compound 23-27, Table 2)
These are nM to low M inhibitors of serine –lactamases.88 Changing the stereochemistry and functional groups on the C-6 position of penam sulfones can affect the selectivity of inhibition to the different classes of -lactamases.121-124
The compounds 23-25 (Table 2) providing IC50 = 1.6, 0.7 and 0.6 M versus TEM-1, respectively and were found to be potent inhibitors of class A -lactamases. The other compounds having C-6-hydroxy-akyl and mercapto-alkyl penam sulfones and the compounds 26 and 27, (Table 2) were designed to act as chelators for metals in MBLs. Modification of the hydroxymethyl group by a mercaptomethyl group results in broader spectrum of inhibition, targeting both serine and metallo- -lactamases.122-126
Non -Lactam -Lactamase Inhibitors
Boronic Acid Analogs
Boron atom has three electrons in its valence shell. Covalent bonding favor only six surrounding electrons and thus it represents a favored electrophilic center (as monobasic Lewis acid of boron). To reach octet it must receive electrons from attacking nucleophiles. Boronic acid β-lactamases inhibitors are one of the most promising classes of β-lactamase inhibitors in development.125-129 They have been explored as serine β-lactamases inhibitors of mainly class A and C β-lactamases.
Boronic acids act as competitive inhibitors, forming a tetrahedral intermediate by binding to the catalytic serine residues of the enzymes through a coordinate covalent bond. The enzyme: inhibitor complex resembles the tetrahedral structure of the high-energy intermediate formed during the mechanism of β-lactam hydrolysis (Figure 3: E).130
ZBTH2BB (Compound 28, Table 2)
It is the benzothiophene-2-boronic acid, a potent nanomolar non-β-lactam inhibitor for class C β-lactamases with a Ki of 27 nM. Another compound, 1-amido-2-(meta-carboxyphenyl) ethane boronic acid (Compound 29, Table 2) possess features to make it closer to β-lactams antibiotics during hydrolysis.131
Phosphonate Derivatives
Among non- β-lactam inhibitors, phosphonates showed good inhibition against serine β-lactamases. They inhibit the enzyme by acylation and phosphylration of the active site serine-70 residue and formation of a stable tetrahedral phosphonyl-enzyme complex.132
Phosphonate monoesters bearing phenyl rings (Compound 30 and 31, Table 2) have shown inhibition against class A and C β-lactamases. In addition, compound 30 has a weak antibacterial activity as demonstrated by its inhibition of the D-ala-D-ala trans peptidases in Streptomyces R61. Compound 32 (Table 2) was found to inhibit class C enzymes.130-134 The acyl phosphonate Compound 33 (Table 2) showed irreversible inhibition of β-lactamases. Adding proper hydrophobic substituents on diacyl phosphonates Compound 34 (Table 2) can increase the potency of inhibition, achieving an inhibition constant in the piconanomolar range.132-136 Other analogs like, cyclic phosphonates (Compound 35 & 36, Table 2), The acyl phosphonates showed irreversible inhibition of β-lactamases. Adding proper hydrophobic substituents on diacyl phosphonates can increase the potency of inhibition, achieving an inhibition constant in the piconanomolar range. 130-135
Thiol Derivatives (Captopril)
Captopril is a well-known antihypertensive agent that inhibits the zinc-containing angiotensin-converting enzyme in humans to treat hypertension.134-135 It has also shown effective inhibition against all classes of metallo- -lactamases, which include classes of B1, B2 and B3 enzymes. Both diastereoisomers of captopril are capable of inhibiting these enzymes. However, the D-diastereoisomers (Compound 37, Table 2) is more potent in inhibiting some MBLs than the L- diastereoisomers (Compound 38, Table 2). The thiol group is important for binding. Lengthening of carboxylate group decreases the activity against the enzymes via complex formation (MBL-Zn(II)-inhibitor). The sulfhydryl group bridges the two active site zinc ions, displacing the nucleophilic water molecule that act as the nucleophile to attack the carbonyl carbon on -lactam antibiotics.134-138
Thiomendalic acid (Compound 39)
It is a simple thiol-containing compound able to inhibit different subclasses of MBLs, demonstrating that potent inhibitors are possible to design. Against the dizinc BcII enzyme thiomandelic acid provided Ki value of 0.09 µM for the R-isomer and 1.28 µM for the S-isomer, respectively. Varying the functional groups at the para-position of benzene bearing analogues didn’t provide more promising derivatives. The inhibitory activity of thiomandelic acid was found to be highly dependent on the thiol group, which chelates the two Zn (II) atoms in the active site of MBLs.134-141
Biphenyl tetrazoles
The inhibitory activity of biphenyl tetrazoles through screening and molecular modeling studies of metallo-β-lactamase structure from imipenem-resistant B. fragilis had been realized. Biphenyl moiety form hydrogen bonds with the NH of Asn176 and His145, along with the NH2 of Lys187, enhancing the binding affinity to the enzyme. However, changing the position of the tetrazole ring from the ortho– to the meta– or para-position, relative to the biphenyl rings, has resulted in a drastic loss of the inhibitory activity against the enzyme. L-809, 022 (Compound 40, Table 2) have shown to be a weak inhibitor of the MBL enzyme of B. fragilis, however, the added methyl group in L-158, 507 (Compound 41) improved the potency of the compound by 5-folds. 140-145
Conjugation of the biphenyl system with substituted heterocyclic aromatic rings increased the potency.140-146 For example, replacement of the methyl group in the parent compound with a Z-methyl-benzimidazole moiety provided L-809, 559 (Compound 42, Table 2) of 40-fold increase in potency. The addition of the substituted imidazo[4,5-b] pyridinyl entity gave L-158,678 (Compound 43, Table 2) of 50-fold increase in inhibition over the parent compound. L-159, 061 (Compound 44, Table 2) with R= Z-butyl-6-hydroxylquinazolinone, showed the highest potency with a 100-fold greater activity than the parent compound. 121,140-148
Proposed selections of proper inhibitors for admixing with β-lactams
The (Table 2), includes CLogP parameter obtained from chemdraw ultra. The number of candidates proved potent inhibitory activity to metallo-β-lactamases are 16 candidates, each representing the top of a series of compounds. Six of those are β–lactam-bearing derivatives. The CLogP of these 6 derivatives drop in hydrophobic area ranging from 0 to 3.4. The generally approved β-lactamase inhibitors that are in clinical use are of balanced hydrophilicity and hydrophobicity having CLogP range -1.75 to 0.53.147-151 All of those admixtures are active versus classes A, C and D β-lactamase. On this basis, we can consider the mentioned physicochemical figure for the selection of β-lactamase inhibitors. The presence of polar groups is important to chelate one metal ion, at least, in the active site of metallo-β-lactamase (Figure 1, equation 2). However, the hydrophobicity also is of high importance in case of metallo-β-lactamase.152
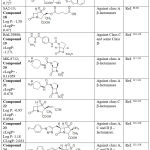 |
Table 2: The list of active derivatives as β-lactamase inhibitors. |
In addition, it might be effective also if we mix broad-spectrum β-lactams with suitable amounts of the earlier generations such as phenoxymethyl-penicillin (Pen V). Pen V is a good substrate for β-lactamases and thus acts as suicidal inhibitor allowing the broad-spectrum β-lactams to be effective versus super bugs.153-155
Conclusion
The impact of antimicrobials has brought upon benefits to medicine. However, harms include the β-lactamase enzymes that are resistant to either one or multiple antibiotics; as a result, limiting their use as single agents. In this review, we revisited the classification systems used to categorize these enzymes; in addition, we collected the various scaffolds of β-lactamase inhibitory activities. Most of the candidates mentioned mainly focused on targeting broader classes of β-lactamases against all types.
Acknowledgements
The authors would like to acknowledge University of Sharjah, Sharjah, United Arab Emirates.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise with this work.
References
- Abraham EP, Chain E. An enzyme from bacteria able to destroy penicillin. 1940. Rev. infect. dis., 1988; 10(4): 677-688.
- Abraham E. Selective reminiscences of beta-lactam antibiotics: early research on penicillin and cephalosporins. Bioessays., 1990; 12(12): 601-606.
- Hamilton-Miller JM, Newton GG, Abraham EP. Products of aminolysis and enzymic hydrolysis of the cephalosporins. Biochem. J., 1970; 116(3): 371-384.
- Bush K. The ABCD’s of beta-lactamase nomenclature. J. Infect. Chemother., 2013; 19(4): 549-559.
- Chaudhary S, El-Shorbagi A-N, Chaudhary A, Agarwal G, Tripathi PN, Dumoga S. The Recent Updates on Neoteric Variants of Covid-19 Virus and Therapeutic Effectiveness of Vaccines against the Variants. Biomed. Pharmacol. J., 2022; 15(3): 1201-1211.
- Chaudhary S, El-Shorbagi A-N, Gupta RK, Kumar A. The recent updates on approaches and clinical trials status of Covid-19 vaccines developed globally. Biomed. Pharmacol. J., 2021; 14(3): 1109-1124.
- Abdel-Moty SG, Sakai S, Aimi N, Takayama H, Kitajima M, El-Shorbagi A, Ahmed A, Omar N. Synthesis of cytotoxic 1-polyhydroxyalkyl-β-carboline derivatives. Eur. J. Med. Chem., 1998; 32(12): 1009-1017.
- Abd-Elrahman MI, Ahmed MO, Ahmed SM, Aboul-Fadl T, El-Shorbagi A. Kinetics of solid state stability of glycine derivatives as a model for peptides using differential scanning calorimetry. Biophy. Chem., 2002; 97(2-3): 113-120.
- Aboul-Fadl T, El-Shorbagi AN, Hozien ZA, Sarhan AWAO. Investigation of alkylating, antineoplastic and anti-HIV potentials of the chalcones: 2-(3-arylpropenoyl)benzimidazole and their corresponding N1-methyl derivatives. Boll. Chim. Farm., 2000; 139(5): 228-234.
- Amin EN, Abdel-Alim AAM, Abdel-Moty SG, El-Shorbagi ANA, Abdel-Rahman MS. Synthesis of new 4,5-3(2H)pyridazinone derivatives and their cardiotonic, hypotensive, and platelet aggregation inhibition activities. Arch. Pharm. Res., 2010; 33(1): 25-46.
- Chaudhary S, Gupta RK, Gupta MK, Verma HC, Kumar H, Kumar A, Swain SR, El-Shorbagi AN. Hepatoprotective response of Cordia sebestena fruit against simvastatin induced hepatotoxicity. J. Pharm. Pharmacogn. Res., 2020; 8(4): 327-335.
- El-Gendy MA, Omar N, Farag HH, El-Shorbagi AN, Sakai SI. Imidazo[2,1-b]benzothiazoles: II: Synthesis and Antiinflammatory Activity of Some Imidazo[2,1-b]benzothiazoles. Chem. Pharm. Bull., 1989; 37(11): 2971-2975.
- El-Shorbagi AN. New tetrahydro-2H-1,3,5-thiadiazine-2-thione derivatives as potential antimicrobial agents. Arch. Pharm., 2000; 333(9): 281-286.
- El-Shorbagi AN, El-Naggar M, Tarazi H, Chaudhary S, Abdu-Allah H, Hersi F, Omar H. Bis-(5-substituted-2-thiono-1,3,5-thiadiazinan-3-yl) butane as a scaffold of anti-proliferative activity, blended by a multicomponent process. Med. Chem. Res., 2018; 27(4): 1103-1110.
- El-Shorbagi AN, Sakai SI, El-Gendy MA, Omar N, Farag HH. Imidazo[2,1-b]benzothiazoles. I. Chem. Pharm. Bull., 1988; 36(12): 4760-4768.
- El-Shorbagi ANA, Husein MA. Synthesis and investigation of antihypertensive activity using anaesthetizednormotensive nonhuman primates of some 2-aryl-4-(substituted) pyrimido [1,2-a] benzimidazoles. Der Pharma Chem., 2015; 7(4): 190-200.
- El-Shorbagi ANA, Husein MA. An approach to hypertension crisis: Evaluation of new fused banzazoles; 2-arylethenyl and 2,4-bis(arylethenyl) derivatives derived from 2,4-dimethylpyrimido [1,2-a] benzimidazole. Der Pharma Chem., 2015; 7(5): 319-328.
- Emara S, El-Gindy A, El-Shorbagi AN, Hadad G. Utility of copper(II) oxide as a packed reactor in flow injection assembly for rapid analysis of some angiotensin converting enzyme inhibitors. Anal. Chim. Acta., 2003; 489(1): 115-123.
- Emara S, Razee S, El-Shorbagi AN, Masujima T. Flow injection method for the determination of methotrexate with a column-packed oxidizing agent. Analyst., 1996; 121(2): 183-188.
- Aboul-Fadl T, El-Shorbagi AN. New carriers for representative peptides and peptide drugs. Arch. Pharm., 1997; 330(11): 327-332.
- El-Shorbagi AN. Model for delivery of amines through incorporation into a tetrahydro-2H-1,3,5-thiadiazine-2-thione structure. Eur. J. Med. Chem., 1994; 29(1): 11-15.
- Chaudhary S, Verma HC, Gupta MK, Kumar H, Swain SR, Gupta RK, et al. Antidiabetic aptitude of cordia sebestena and its outcome on biochemical parameters, serum electrolytes, and hematological markers. Pharmacogn. J., 2019; 11(2): 418-423.
- El-Shorbagi AN, Chaudhary S. Monobactams: A unique natural scaffold of four-membered ring skeleton, recent development to clinically overcome infections by multidrugresistant microbes. Lett. Drug Des. Discov., 2019; 16(12): 1305-1320.
- Mohamed MH, El-Shorbagi ANA. (±)-termisine, a novel lupine alkaloid from the seeds of Lupinus termis. J. Nat. Prod., 1993; 56(11): 1999-2002.
- Soliman S, Hamoda AM, El-Shorbagi ANA, El-Keblawy AA. Novel betulin derivative is responsible for the anticancer folk use of Ziziphus spina-christi from the hot environmental habitat of UAE. J. Ethnopharmacol., 2019; 231: 403-408.
- San Millan A. Evolution of Plasmid-Mediated Antibiotic Resistance in the Clinical Context. Trends Microbiol., 2018; 26(12): 978-985.
- Barnaud G, Labia R, Raskine L, Sanson-Le Pors MJ, Philippon A, Arlet G. Extension of resistance to cefepime and cefpirome associated to a six amino acid deletion in the H-10 helix of the cephalosporinase of an Enterobacter cloacae clinical isolate. FEMS Microbiol. lett., 2001; 195(2): 185-190.
- Odegaard K, Solberg O. Isolation of a penicillinase producing strain of Neisseria gonorrhoeae. Acta Pathol. Microbiol. Scand. B., 1976; 84b(6): 458-460.
- Papp-Wallace KM, Barnes MD, Alsop J, Taracila MA, Bethel CR, Becka SA, Duin DV, Kreiswirth BN, Kaye KS, Bonomo RA. Relebactam is a potent inhibitor of the KPC-2 β-lactamase and restores imipenem susceptibility in KPC-producing Enterobacteriaceae. Antimicrob. Agents. Chemother., 2018; 62(6): e00174-18.
- Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, Alberti S, Bush K, Tenover FC. Novel carbapenemhydrolyzing-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother., 2001; 45:1151-1161.
- Livermore DM, Mushtaq S, Barker K, Hope R, Warner M, Woodford N. Characterization of β-lactamase and porin mutants of Enterobacteriaceae selected with ceftaroline + avibactam (NXL104). J. Antimicrob. Chemother., 2012; 67(6): 1354-1358.
- Zhanel GG, Lawson CD, Adam H, Schweizer F, Zelenitsky S, Lagacé-Wiens PRS, Denisuik A, Rubinsein E, Gin AS, Hoban DJ, Lynch JP, Karlowsky JA. Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination. Drugs., 2013; 73(2): 159-177.
- Wang DY, Abboud MI, Markoulides MS, Brem J, Schofield CJ. The road to avibactam: the first clinically useful non-β-lactam working somewhat like a β-lactam. Future Med. Chem., 2016; 8(10): 1063-1084.
- Abboud MI, Damblon C, Brem J, Smargiasso N, Mercuri P, Gilbert B, Rydzik AM, Claridge TDW, Schofield CJ, Frere JM. Interaction of avibactam with class B metallo-β-lactamases. Antimicrob. Agents Chemothe., 2016; 60(10): 5655-5662.
- Lomovskaya O, Sun D, Rubio-Aparicio D, Nelson K, Tsivkovski R, Griffith DC, Dudley MN. Vaborbactam: spectrum of beta-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob. Agents. Chemother., 2017; 61(11): e01143-17.
- Sun D, Rubio-Aparicio D, Nelson K, Dudley MN, Lomovskaya O. Meropenem-vaborbactam resistance selection, resistance prevention, and molecular mechanisms in mutants of KPC-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother., 2017; 61(12): e01694-17.
- Pfaller MA, Huband MD, Mendes RE, Flamm RK, Castanheira M. In vitro activity of meropenem/vaborbactam and characterisation of carbapenem resistance mechanisms among carbapenem-resistant Enterobacteriaceae from the 2015 meropenem/vaborbactam surveillance programme. Int. J. Antimicrob. Agents., 2018; 52(2): 144-150.
- Bush K. A resurgence of β‐lactamase inhibitor combinations effective against multi-drug resistant gram-negative pathogens. Int. J. Microb. Agents., 2015; 46(5): 483- 493.
- Werner JP, Mitchell JM, Taracila MA, Bonomo RA, Powers RA. Exploring the potential of boronic acids as inhibitors of OXA‐24/40 β‐ Protein. Sci., 2017; 26(3): 515-526.
- Kapoor G, Saigal S, Elongavan A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol., 2017; 33(3): 300-305.
- Dincer I, Ergin A, Kocagoz T. The vitro efficacy of beta-lactam and beta-lactamase inhibitors against multidrug resistant clinical strains of Mycobacterium tuberculosis. Int. J. Antimicrob. Agents., 2004; 23(4): 408-411.
- Guo Z, Ma S. Recent advances in the discovery of metallo- β-lactamase inhibitors for β-lactam antibiotic-resistant reversing agents. Curr. Drug Targets., 2014; 15(7): 689-702.
- Bush K, Bradford PA. beta-Lactams and beta-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med., 2016; 6(8): a025247.
- King DT, Sobhanifar S, Strynadka NC. One ring to rule them all: Current trends in combating bacterial resistance to the beta-lactams. Protein. Sci., 2016; 25(4): 787-803.
- Gonzalez-Bello C. Antibiotic adjuvants- A strategy to unlock bacterial resistance to antibiotics. Bioorg. Med. Chem. Lett., 2017; 27(18): 4221-4228.
- Ouyang X, Chang YN, Yang KW, Wang WM, Bai JJ, Wang JW, Zhang YJ, Wang SY, Xie BB, Wang LL. A DNA nanoribbon as a potent inhibitor of metallo-beta-lactamases. Chem. Comm., 2017; 53(63): 8878-8881.
- Pontes DS, de Araujo RSA, Dantas N, Scotti L, Scotti MT, de Moura RO, et al. Genetic Mechanisms of Antibiotic Resistance and the Role of Antibiotic Adjuvants. Current topics in medicinal chemistry. 2018;18(1):42-74.
- Choi H, Paton RS, Park H, Schofield CJ. Investigations on recyclisation and hydrolysis in avibactam mediated serine beta-lactamase inhibition. Org. Biomol. Chem., 2016; 14(17): 4116-4128.
- Fisher JF, Meroueh SO, Mobashery S. Bacterial resistance to beta-lactam antibiotics: compelling opportunism, compelling opportunity. Chem. Rev., 2005; 105(2): 395-424.
- Xiang Y, Chang YN, Ge Y, Kang JS, Zhang YL, Liu XL, Oelschlaeger P, Yang KW. Azolylthioacetamides as a potent scaffold for the development of metallo-beta-lactamase inhibitors. Bioorg. Med. Chem. Lett., 2017; 27(23): 5225-5229.
- Shortridge D, Pfaller MA, Castanheira M, Flamm RK. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa collected from patients with bloodstream infections isolated in United States hospitals (2013-2015) as part of the Program to Assess Ceftolozane-Tazobactam Susceptibility (PACTS) surveillance program. Diagn. Microbiol. Infect. Dis., 2018; 92(2): 158-163.
- Watkins RR, Papp-Wallace KM, Drawz SM, Bonomo RA. Novel beta-lactamase inhibitors: a therapeutic hope against the scourge of multidrug resistance. Frot. Microbiol., 2013; 4: 392.
- Zaman SB, Hussain MA, Nye R, Mehta V, Mamun KT, Hossain N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus., 2017; 9(6): e1403.
- Krishnan NP, Nguyen NQ, Papp-Wallace KM, Bonomo RA, van den Akker F. Inhibition of Klebsiella beta-Lactamases (SHV-1 and KPC-2) by Avibactam: A Structural Study. PLoS One., 2015; 10(9): e0136813.
- Lee SY, Brem J, Pettinati I, Claridge TD, Gileadi O, Schofield CJ, McHugh PJ. Cephalosporins inhibit human metallo beta-lactamase fold DNA repair nucleases SNM1A and SNM1B/apollo. Chem. Comm., 2016; 52(40): 6727-6730.
- Munita JM, Arias CA. Mechanisms of Antibiotic Resistance. Microbiol. Spectr., 2016; 4(2):1128/microbiolspec.
- Gama JA, Zilhao R, Dionisio F. Impact of plasmid interactions with the chromosome and other plasmids on the spread of antibiotic resistance. Plasmid., 2018; 99: 82-88.
- Willyard C. The drug-resistant bacteria that pose the greatest health threats. Nat. News., 2017; 543(7643): 15.
- Bush K. Proliferation and significance of clinically relevant beta-lactamases. Ann. N. Y. Sci., 2013; 1277: 84-90.
- Viana Marques DA, Machado SEF, Ebinuma VCS, Duarte CAL, Converti A, Porto ALF. Production of beta-Lactamase Inhibitors by Streptomyces Antibiotics., 2018;7(3): 61.
- Livermore DM. Defining an extended-spectrum beta-lactamase. Clin. Microbiol. Infect., 2008; 14(1): 3-10.
- Ur Rahman S, Ali T, Ali I, Khan NA, Han B, Gao J. The Growing Genetic and Functional Diversity of Extended Spectrum Beta-Lactamases. BioMed. Res. Int., 2018; 2018: 9519718.
- Shaikh S, Fatima J, Shakil S, Rizvi SMD, Kamal MA. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi. J. Biol. Sci., 2015; 22(1): 90-101.
- Jacoby GA. AmpC beta-lactamases. Clin. Microbiol. Rev., 2009; 22(1):161-82.
- Shirley M. Ceftazidime-Avibactam: A Review in the treatment of serious gram-negative bacterial infections. Drugs., 2018; 78(6): 675-692.
- Bush K. Bench-to-bedside review: The role of beta-lactamases in antibiotic-resistant gram-negative infections. Crit. Care., 2010; 14(3): 224.
- Rasool U, S P, Parveen A, Sah SK, S H. Efficacy of andrographis paniculata against extended spectrum beta-lactamase (ESBL) producing E. coli. BMC Complement Altern Med., 2018; 18(1): 244.
- Nolte O. Antimicrobial resistance in the 21st century: a multifaceted challenge. Protein Pept. lett., 2014; 21(4): 330-335.
- Zhao WH, Hu ZQ. Epidemiology and genetics of CTX-M extended-spectrum beta-lactamases in gram-negative bacteria. Crit. Rev. Microbiol., 2013; 39(1): 79-101.
- Smet A, Martel A, Persoons D, Dewulf J, Heyndrickx M, Herman L, Haesebrouck F, Butaye P. Broad-spectrum beta-lactamases among Enterobacteriaceae of animal origin: molecular aspects, mobility and impact on public health. FEMS Microbiol Rev., 2010; 34(3): 295-316.
- Ghafourian S, Sadeghifard N, Soheili S, Sekawi Z. Extended Spectrum Beta-lactamases: Definition, Classification and Epidemiology. Curr. Issues Mol. Biol., 2015; 17: 11-21.
- Shaikh S, Fatima J, Shakil S, Rizvi SM, Kamal MA. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J. Biol. Sci., 2015; 22(1): 90-101.
- D’Andrea MM, Arena F, Pallecchi L, Rossolini GM. CTX-M-type beta-lactamases: a successful story of antibiotic resistance. Int. J. Med. Microbiol., 2013; 303(6-7): 305-317.
- Zander E, Fernandez-Gonzalez A, Schleicher X, Dammhayn C, Kamolvit W, Seifert H, Higgins PG. Worldwide dissemination of acquired carbapenem-hydrolysing class D beta-lactamases in acinetobacter spp. other than acinetobacter baumannii. Int. J. Antimicrob Agents. 2014; 43(4): 375-377.
- Hrabak J, Chudackova E, Papagiannitsis CC. Detection of carbapenemases in Enterobacteriaceae: a challenge for diagnostic microbiological laboratories. Clin. Microbiol. Infect., 2014; 20(9): 839-853.
- Jeon JH, Lee JH, Lee JJ, Park KS, Karim AM, Lee CR, Jeong BC, Lee SH. Structural basis for carbapenem-hydrolyzing mechanisms of carbapenemases conferring antibiotic resistance. Int. J. Mol. Sci., 2015; 16(5): 9654-9692.
- Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: the phantom menace. J. Antimicrob chemother., 2012; 67(7): 1597-1606.
- Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing enterobacteriaceae: An emerging public-health concern. Lancet Infect. Dis., 2008; 8(3): 159-166.
- Singhal N, Pandey D, Somendro Singh N, Kumar M, Virdi JS. Molecular characteristics of “BlaB-Like” chromosomal inducible cephalosporinase of yersinia enterocolitica biotype 1A strains. Microb. Drug Resist., 2019; 25(6): 824-829.
- Jones RN. Important and emerging beta-lactamase-mediated resistances in hospital-based pathogens: the Amp C enzymes. Diagn. Microbiol. Infect. Dis., 1998; 31(3): 461-466.
- Lahiri SD, Johnstone MR, Ross PL, McLaughlin RE, Olivier NB, Alm RA. Avibactam and class C beta-lactamases: mechanism of inhibition, conservation of the binding pocket, and implications for resistance. Antimicrob. Agents Chemother., 2014; 58(10): 5704-5713.
- Bush K, Jacoby GA. Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother., 2010; 54(3): 969-976.
- Antunes NT, Lamoureaux TL, Toth M, Stewart NK, Frase H, Vakulenko SB. Class D beta-lactamases: are they all carbapenemases? Antimicrob. Agents Chemother., 2014; 58(4): 2119-2225.
- Meini MR, Llarrull LI, Vila AJ. Overcoming differences: The catalytic mechanism of metallo-beta-lactamases. FEBS Lett,. 2015; 589(22): 3419-3432.
- Salahuddin P, Kumar A, Khan AU. Structure, Function of serine and metallo-beta-lactamases and their inhibitors. Curr. Protein Pept. Sci. 2018; 19(2):130-144.
- Diene SM, Pinault L, Keshri V, Armstrong N, Khelaifia S, Chabriere E, Caetano-Anolles G, Colson P, La Scola B, Rolain JM, Pontarotti P, Raoult D. Human metallo-beta-lactamase enzymes degrade penicillin. Sci. Rep., 2019; 9(1): 12173.
- Khan AU, Maryam L, Zarrilli R. Structure, Genetics and Worldwide Spread of New Delhi Metallo-beta-lactamase (NDM): a threat to public health. BMC Microbiol., 2017; 17(1): 101.
- Drawz SM, Bonomo RA. Three decades of beta-lactamase inhibitors. Clin. Microbiol. Rev., 2010; 23(1): 160-201.
- Padayatti PS, Helfand MS, Totir MA, Carey MP, Carey PR, Bonomo RA, van den Akker F. High resolution crystal structures of the trans-enamine intermediates formed by sulbactam and clavulanic acid and E166A SHV-1 β-lactamase. J. Biol. Chem., 2005; 280(41): 34900-34907.
- Tooke CL, Hinchliffe P, Bragginton EC, Colenso CK, Hirvonen VH, Takebayashi Y, Spencer J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol., 2019; 431(18): 3472-3500.
- Meini MR, Llarrull LI, Vila AJ. Evolution of Metallo-beta-lactamases: Trends Revealed by Natural Diversity and in vitro Antibiotics., 2014; 3(3): 285-316.
- Payne DJ, Hueso-Rodríguez JA, Boyd H, Concha NO, Janson CA, Gilpin M, Bateson JH, Cheever C, Niconovich NL, Pearson S, Rittenhouse S, Tew D, Diez E, Perez P, de la Fuente J, Rees M, Sagredo AR. Identification of a series of tricyclic natural products as potent broad-spectrum inhibitors of metallo-β-lactamases. Antimicrob. Agents Chemother., 2002; 46(6): 1880-1886.
- Nichols DA, Renslo AR, Chen Y. Fragment-based inhibitor discovery against beta-lactamase. Future Med. Chem., 2014; 6(4): 413-427.
- Eiamphungporn W, Schaduangrat N, Malik AA, Nantasenamat C. Tackling the antibiotic resistance caused by class A beta-lactamases through the use of beta-lactamase inhibitory protein. Int. J. Mol. Sci., 2018; 19(8): 2222.
- Papp-Wallace KM, Bonomo RA. New beta-Lactamase Inhibitors in the Clinic. Infect. Dis. Clin. North Am., 2016; 30(2): 441-464.
- Ju LC, Cheng Z, Fast W, Bonomo RA, Crowder MW. The continuing challenge of metallo-beta-lactamase inhibition: mechanism matters. Trends Pharmacol. Sci., 2018; 39(7): 635-647.
- Chen YL, Chang C-W, Hedberg K. Synthesis of a potent β-lactamase inhibitor-1,1-dioxo-6-(2-pyridyl)methylenepenicillanic acid and its reaction with sodium methoxide. Tetrahedron Lett. 1986; 27(30): 3449-3452.
- Maveyraud L, Golemi-Kotra D, Ishiwata A, Meroueh O, Mobashery S, Samama J-P. High-Resolution X-ray Structure of an Acyl-Enzyme Species for the Class D OXA-10 β-Lactamase. J. Am. Chem. Soc., 2002; 124(11): 2461-2465.
- Reading C, Cole M. Structure-activity relationships amongst beta-lactamase inhibitors. J. Enzyme Inhib., 1986;1(2):83-104.
- Hayashi Y, Roberts JA, Paterson DL, Lipman J. Pharmacokinetic evaluation of piperacillin-tazobactam. Expert Opin. Drug Metabol. Toxicol., 2010; 6(8): 1017-1031.
- Lu K, Zhang Y, Li L, Wang X, Ding G. Chaetochromones A and B, two new polyketides from the fungus Chaetomium indicum (CBS. 860.68). Molecules., 2013; 18(9): 10944-10952.
- Ramirez MS, Tolmasky ME. Aminoglycoside modifying enzymes. Drug Resist. Updat., 2010; 13(6): 151-171.
- Matagne A, Ledent P, Monnaie D, Felici A, Jamin M, Raquet X, Galleni M, Klein D, Francois I, Frere JM. Kinetic study of interaction between BRL 42715, beta-lactamases, and D-alanyl-D-alanine peptidases. Antimicrob. Agents Chemother., 1995; 39(1): 227-231.
- Michaux C, Charlier P, Frère J-M, Wouters J. Crystal structure of BRL 42715, C6-(N 1-methyl-1, 2, 3-triazolylmethylene) penem, in complex with enterobacter c loacae 908R β-lactamase: evidence for a stereoselective mechanism from docking studies. J. Am. Chem. Soc., 2005; 127(10): 3262-3263.
- Venkatesan AM, Agarwal A, Abe T, Ushirogochi H, Yamamura I, Kumagai T, Petersen PJ, Weiss WJ, Lenoy E, Yang Y, Shlaes DM, Ryan JL, Mansour TS. Novel imidazole substituted 6-methylidene-penems as broad-spectrum β-lactamase inhibitors. Bioorg. Med. Chem., 2004; 12(22): 5807-5817.
- Venkatesan AM, Gu Y, Santos OD, Abe T, Agarwal A, Yang Y, Petersen PJ, Weiss WJ, Mansour TS, Nukaga M, Hujer AM, Bonomo RA, Knox JR. Structure-activity relationship of 6-methylidene penems bearing tricyclic heterocycles as broad-spectrum β-lactamase inhibitors: crystallographic structures show unexpected binding of 1, 4-thiazepine intermediates. J. Med. Chem., 2004; 47(26): 6556-6568.
- Higgins PG, Pérez-Llarena FJ, Zander E, Fernández A, Bou G, Seifert H. OXA-235, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother., 2013; 57(5): 2121-2126.
- Weiss WJ, Petersen PJ, Murphy TM, Tardio L, Yang Y, Bradford PA, Venkatesan AM, Abe T, Isoda T, Mihira A, Ushirogochi H, Takasake T, Projan S, Connell JO, Mansour TS. In vitro and in vivo activities of novel 6-methylidene penems as β-lactamase inhibitors. Antimicrob. Agents Chemother. 2004; 48(12): 4589-4596.
- Thomson JM, Distler AM, Prati F, Bonomo RA. Probing active site chemistry in SHV β-lactamase variants at ambler position 244 understanding unique properties of inhibitor resistance. J. Biol. Chem., 2006; 281(36): 26734-26744.
- Venkatesan AM, Agarwal A, Abe T, Ushirogochi H, Ado M, Tsuyoshi T, Santos OD, Li Z, Francisco G, Lin YI, Petersen PJ, Yang Y, Weiss WJ, Shlaes DM, Mansour TS. 5, 5, 6-Fused tricycles bearing imidazole and pyrazole 6-methylidene penems as broad-spectrum inhibitors of β-lactamases. Bioorg. Med. Chem., 2008; 16(4): 1890-1902.
- Pfaendler HR, Weisner F, Metzger K. Synthesis and antibacterial activity of (1′ R, 5R, 6R)-2-tert-butyl-6-(1′-hydroxyethyl) oxapenem-3-carboxylic acid. Bioorg. Med. Chem. Lett., 1993; 3(11): 2211-2218.
- Jamieson CE, Lambert PA, Simpson IN. In vitro and in vivo activities of AM-112, a novel oxapenem. Antimicrob. Agents Chemother., 2003; 47(5): 1652-1657.
- Jamieson CE, Lambert PA, Simpson IN. In vitro activities of novel oxapenems, alone and in combination with ceftazidime, against gram-positive and gram-negative organisms. Antimicrob. Agents Chemother., 2003; 47(8): 2615-2618.
- Paukner S, Hesse L, Preželj A, Šolmajer T, Urleb U. In vitro activity of LK-157, a novel tricyclic carbapenem as broad-spectrum β-lactamase inhibitor. Antimicrob. Agents Chemother., 2009; 53(2): 505-511.
- Vilar M, Galleni M, Solmajer T, Turk B, Frère J-M, Matagne A. Kinetic study of two novel enantiomeric tricyclic β-lactams which efficiently inactivate class C β-lactamases. Antimicrob. Agents Chemother., 2001; 45(8): 2215-2223.
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob. Agents Chemother., 2011; 55(11): 4943-4960.
- Nagano R, Adachi Y, Imamura H, Yamada K, Hashizume T, Morishima H. Carbapenem derivatives as potential inhibitors of various β-lactamases, including class B metallo-β-lactamases. Antimicrob. Agents Chemother., 1999; 43(10): 2497-2503.
- Karpiuk I, Tyski S. Looking for the new preparations for antibacterial therapy. II. Clinical trials; new beta-lactam antibiotics and beta-lactamase inhibitors. Przegl. Epidemiol., 2013; 67(1): 51-6, 135-40.
- Buynak JD. β-Lactamase inhibitors: a review of the patent literature (2010–2013). Expert Opin. Ther. Pat. 2013; 23(11): 1469-81.
- Blizzard TA, Chen H, Kim S, Wu J, Young K, Park Y-W, Ogawa AM, Raghoobar S, Painter RE, Wisniewski D, Hairston N, Fitzgerald P, Sharma N, Scapin G, Lu J, Hermes J, Hammond ML. Side chain SAR of bicyclic β-lactamase inhibitors (BLIs). 2. N-alkylated and open chain analogs of MK-8712. Bioorg. Med. Chem. Lett., 2011; 21(14): 4267-4270.
- Wetson GS, Blazquez J, Baquero F, Shoichet BK. Structure-based enhancement of boronic acid-based inhibitors of AmpC beta-lactamase. J. Med. Chem., 1998; 41(23): 5477-4586.
- Mascaretti OA, Danelon GO, Setti EL, Laborde M, Mata EG. Recent advances in the chemistry of beta-lactam compounds as selected active-site serine beta-lactamase inhibitors. Current Pharm. Des., 1999; 5(11): 939-954.
- Mezes PS, Clarke AJ, Dmitrienko GI, Viswanatha T. 6-β-(Trifluoromethane sulfonyl)-amido-penicillanic acid sulfone: A potent inhibitor for β-lactamases. FEBS Lett. 1982; 143(2): 265-267.
- Payne DJ, Du W, Bateson JH. Beta-lactamase epidemiology and the utility of established and novel beta-lactamase inhibitors. Expert Opin. Investig. Drugs., 2000; 9(2): 247-261.
- Ahmad I, Shagufta S. An important class of organic compounds with diverse biological activities. Int. J. Pharm. Sci., 2015; 7: 19-27.
- Dmitrienko GI, Copeland CR, Arnold L, Savard ME, Clarke AJ, Viswanatha T. Inhibition of β-lactamase-I by 6-β-sulfonamidopenicillanic acid sulfones: evidence for conformational change accompanying the inhibition process. Bioorg. Chem., 1985; 13(1): 34-46.
- Caselli E, Romagnoli C, Vahabi R, Taracila MA, Bonomo RA, Prati F. Click chemistry in lead optimization of boronic acids as β-lactamase inhibitors. J. Med. Chem., 2015; 58(14): 5445-5458.
- Ghannoum MA, Rice LB. Antifingal agents: Mode of action, mechanism of resistance, correlation of these mechanisms with bacerial resistance. Clin. Microbiol. Rev., 1999; 12(4): 501-517.
- Georgopapadakou NH, Walsh TJ. Antifungal agents: Chemotherapeutic targets and immunologic strategies. Antimicrob. Agents Chemother., 1996; 40: 279-291.
- Holz RW. The effect of the polyene antibiotics nystatin and amphotericin B on thin lipid membranes. Ann. N Y Acad. Sci., 1974; 235: 469-479.
- Tondi D, Venturelli A, Bonnet R, Pozzi C, Shoichet BK, Costi MP. Targeting class A and C serine β-lactamases with a broad-spectrum boronic acid derivative. J. Med. Chem., 2014; 57(12): 5449-5458.
- Zervosen A, Sauvage E, Frère J-M, Charlier P, Luxen A. Development of new drugs for an old target-the penicillin binding proteins. Molecules., 2012; 17(11): 12478-12505.
- Bebrone C, Lassaux P, Vercheval L, Sohier J-S, Jehaes A, Sauvage E, Galleni M. Current challenges in antimicrobial chemotherapy. Drugs., 2010; 70(6): 651-679.
- Kaur K, Pratt R. Mechanism of reaction of acyl phosph (on) ates with the β-lactamase of Enterobacter cloacae P99. Biochem., 2001; 40(15): 4610-4621.
- Drawz SM, Bonomo RA. Three decades of β-lactamase inhibitors. Clin. Microbiol. Rev., 2010; 23(1): 160-201.
- Bonnet R. Growing group of extended-spectrum β-lactamases: the CTX-M enzyme. Antimicrob. Agents Chemother., 2004; 48: 1-14.
- McGeary RP, Tan DT, Schenk G. Progress toward inhibitors of metallo-β-lactamases. Future Med. Chem., 2017; 9(7): 673-691.
- Mollard C, Moali C, Papamicael C, Damblon C, Vessilier S, Amicosante G, Schofoeld CJ, Galleni M, Frere JM, Roberts GCK. Thiomandelic acid, a broad spectrum inhibitor of zinc β-lactamases kinetic and spectroscopic studies. J. Biol. Chem., 2001; 276(48): 45015-45023.
- Damblon C, Jensen M, Ababou A, Barsukov I, Papamicael C, Schofield CJ, Olsen L, Bauer R, Robers GCK. The inhibitor thiomandelic acid binds to both metal ions in metallo-β-lactamase and induces positive cooperativity in metal binding. J. Biol. Chem., 2003; 278(31): 29240-29251.
- Antony J, Piquemal JP, Gresh N. Complexes of thiomandelate and captopril mercaptocarboxylate inhibitors to metallo‐β‐lactamase by polarizable molecular mechanics. Validation on model binding sites by quantum chemistry. J. Comput. Chem., 2005; 26(11): 1131-1147.
- Karsisiotis AI, Damblon CF, Roberts GC. Solution structures of the Bacillus cereus metallo-β-lactamase BcII and its complex with the broad spectrum inhibitor R-thiomandelic acid. Biochem. J., 2013; 456(3): 397-407.
- Toney JH, Fitzgerald PM, Grover-Sharma N, Olson SH, May WJ, Sundelof JG, Vanderwall DE, Cleary KA, Grant SK, Wu JK, Kozarich JW, Pompliano DL, Hammond GG. Antibiotic sensitization using biphenyl tetrazoles as potent inhibitors of bacteroides fragilis metallo-beta-lactamase. Chem. Biol., 1998; 5(4): 185-196.
- Faridoon, Ul Islam N. An update on the status of potent inhibitors of metallo-beta-lactamases. Sci. Pharm., 2013; 81(2): 309-327.
- Toney JH, Cleary KA, Hammond GG, Yuan X, May WJ, Hutchins SM, Ashton WT, Vanderwall DE. Structure-activity relationships of biphenyl tetrazoles as metallo-beta-lactamase inhibitors. Bioorg. Med. Chem. Lett., 1999; 9(18): 2741-2746.
- Dudley J, Feinn L, deFrancesco H, Lindsay E, Coca A, Roberts EL. Antibacterial assessment of heteroaryl, vinyl, benzyl, and alkyl tetrazole compounds. Med. Chem., 2018; 14(6): 550-555.
- Yang Y, Rasmussen BA, Shlaes DM. Class A beta-lactamases–enzyme-inhibitor interactions and resistance. Pharmacol. Ther., 1999; 83(2): 141-151.
- Beharry Z, Chen H, Gadhachanda VR, Buynak JD, Palzkill T. Evaluation of penicillin-based inhibitors of the class A and B β-lactamases from Bacillus anthracis. Biochem. Biophy. Res. Comm., 2004; 313(3): 541-545.
- Shapiro AB. Kinetics of sulbactam hydrolysis by β-lactamases, and kinetics of β-lactamase inhibition by sulbactam. Antimicrob. Agents. Chemother., 2017; 61(12): e01612-17.
- Bou G, Oliver A, Martínez-Beltrán J. OXA-24, a novel class D β-lactamase with carbapenemase activity in an Acinetobacter baumannii clinical strain. Antimicrob. Agents. Chemother., 2000; 44(6): 1556-1561.
- Poirel L, Marqué S, Héritier C, Segonds C, Chabanon G, Nordmann P. OXA-58, a novel class D β-lactamase involved in resistance to carbapenems in acinetobacter baumannii. Antimicrob. Agents. Chemother., 2005; 49(1): 202-208.
- Gutmann L, Kitzis M, Billot-Klein D, Goldstein F, Van Nhieu GT, Lu T, Carlet J, Collatz E, Williamson R. Plasmid-mediated β-lactamase (TEM-7) involved in resistance to ceftazidime and aztreonam. Rev. Infect. Dis., 1988; 10(4): 860-866.
- Griffith DC, Loutit JS, Morgan EE, Durso S, Dudley MN. Phase 1 study of the safety, tolerability, and pharmacokinetics of the β-lactamase inhibitor vaborbactam (RPX7009) in healthy adult subjects. Antimicrob. Agents. Chemother., 2016; 60(10): 6326-6332.
- Mendelsohn LD. ChemDraw 8 ultra, windows and macintosh versions. J. Chem. Inf. Comput. Sci., 2004; 44(6): 2225-2226.
- Mall TR, Tumber A, John T, Brewitz L, Strain-Damerell C, Owen CD, Lukacik P, Chan HTH, Maheswaran P, Salah E, Duarte F, Yang H, Rao Z, Walsh MA, Schofield CJ. Mass spectrometry reveals potential of β-lactams as SARS-Cov-2 Mpro inhibitors. Chem. Commun., 2021; 57: 1430-1433.
- Mojica MF, Rossi MA, Vila AJ, Bonomo RA. The urgent need for metallo-β-lactamase inhibitors: an unattended global threat. Lancet Infect. Dis., 2022; 22(1): e28-e34.