Manuscript accepted on :28-04-2020
Published online on: 27-05-2020
Plagiarism Check: Yes
Reviewed by: M Mohan Varma
Second Review by: Suraj Sundaragiri
Final Approval by: Dr. Josphert Ngui Kimatu
Ezz El Din Mostafa Abd El Wahed Shalaby1 ,Nagla Ahmed ElNabarawy2,Dalia Abd Elwahab Hassan3
,Nagla Ahmed ElNabarawy2,Dalia Abd Elwahab Hassan3 ,Mohamed Sherif Negm4 and Sarah A. Khater5
,Mohamed Sherif Negm4 and Sarah A. Khater5
1Department of Forensic Medicine And Clinical Toxicology, Faculty of Medicine, Cairo University, Egypt.
2National Egyptian Center of Environmental And Toxicological Research, Faculty of Medicine, Cairo University, Egypt.
3Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Beni Suef University, Egypt
4Department of Pathology, Faculty of Medicine, Cairo University, Egypt.
5Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Misr University for Science and Technology, Egypt
Corresponding Author E-mail : ezz.shalaby@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1940
Abstract
Aluminum phosphide (ALP) is a cheap highly toxic pesticide. It inhibits cytochrome oxidase, oxidative phosphorylation, and producing an energy crisis in the cells. It is, widely available worldwide, with a lot of reported intoxicated cases and no specific antidotes are available. This study aims to investigate the effect of oral iron, copper and coconut oil as possible oral antidote in treatment of ALP toxicity. 48 Rats were divided into 6 groups with 8 rats each (4 males and 4 females) Group A served as control. (Untreated rats) Group B received ALP. Group C: received ALP + oral copper after half hour of ingestion of ALP. Group D: received ALP +oral iron ferric hydroxide poly maltose after half hour of ingestion of ALP. Group E: received ALP +oral virgin coconut oil after half hour of ingestion of ALP. Group F: received ALP +oral copper + oral Iron (ferric hydroxide poly maltose) + virgin coconut oil daily, after half hour of ingestion of ALP. Investigations were done to all rats including: cardiac enzymes: CK, CK-MB, AST and LDH, liver function tests: Bilirubin total and direct, AST and ALT, kidney function tests: urea and creatinine. Also, pathological assessment of lung, liver, and kidney were done.Copper has amelioration effect on ALP toxicity (important for function of cytochrome oxidase). However, iron showed an ineffective role in treatment of ALP toxicity. In addition, coconut oil can be added to the treatment of ALP toxicity. Pathological assessment also confirmed the results of lung, liver, and kidney in rats. We recommend to use copperas oral antidote in treatment of Aluminum phosphide poison. And oral coconut oil to be added in treatment plan.
Keywords
Aluminum Phosphide; Copper; Iron; Coconut Oil; Cytochrome Oxidase.
Download this article as:| Copy the following to cite this article: Shalaby E. E. D. M. A. E. W, ElNabarawy N A, Hassan D. A. E, Negm M. S, Khater S. A. Evaluation of Role of Iron, Copper and Coconut Oil in Treatment of Acute Aluminum Phosphide Poison in Experimental Rats. Biomed Pharmacol J 2020;13(2). |
| Copy the following to cite this URL: Shalaby E. E. D. M. A. E. W, ElNabarawy N A, Hassan D. A. E, Negm M. S, Khater S. A. Evaluation of Role of Iron, Copper and Coconut Oil in Treatment of Acute Aluminum Phosphide Poison in Experimental Rats. Biomed Pharmacol J 2020;13(2). Available from: https://bit.ly/2TG40Iw |
Introduction
Aluminum phosphide (ALP) is a cheap, widely available worldwide, especially in developing countries, highly toxic pesticide, with a lot of reported cases especially suicidal in many countries including Egypt.
ALP is readily available as a fumigant for stored cereal grains is highly toxic, especially when consumed from a freshly opened container. [1] The causes of death are from profound shocks, myocarditis and multi-organ failures. [2]
Human toxicity occur due to the ingestion of ALP or the injury from phosphine inhalation (uncommon) or even after absorption through skin (rare). [3]
Many reports have demonstrated deaths occurred in a dose of 150-500 mg, in spite of the lethal dose which is 1-1.5 gm. [4] ALP, when ingested, liberates a lot of phosphine gas which lead to inhibition of the cytochrome oxidase of mitochondria, resulting in blocking the electron transfer chain, oxidative phosphorylation, and producing an energy crisis in the cells. [5, 6] The severe toxicity of ALP manifests as profound and refractory hypotension, congestive heart failure, ECG abnormalities and myocarditis, pericarditis and sub endocardiac infarction. The hypotension varied from 76% to 100%, which is a cardinal feature in ALP toxicity. [7] Metabolic acidosis is common due to accumulation of lactic acid caused by blockage of oxidative phosphorylation and poor tissue perfusion. [8, 9] This deleterious effect is considered a prognostic indicator in ALP toxicity. [10]
Cytochrome oxidase, also known as complex IV, is the terminal, or final, enzyme of the electron transport system. It is a transmembrane molecule found in the mitochondria of eukaryotes and in the cellular space of aerobic prokaryotes. This molecule is a proton pump that plays a vital role in producing energy, in the form of ATP. [11] This energy produced from the reaction is used to pump H+ ions from the matrix to the inter-membrane space of the mitochondria. [12] Cytochrome oxidase is made up of two identical proteins. The two proteins in cytochrome oxidase mirror one another. The system uses multiple metals to complete its function including the two irons in the two hemes, three coppers, one magnesium, and one zinc, the irons in the two hemes and the three copper molecules are vital to the success of the enzyme in aerobic respiration. [12]
Our aim in the current study is to investigate the effect of oral iron, copper and coconut oil as possible oral antidote in treatment of ALP toxicity.
Materials and Methods
In our study all efforts were made to minimize animal suffering and reduce the number of animals used. All experimental procedures were conducted according to the principles of care and use of laboratory animals in the research. They were kept in separate cages at room temperature for one week before starting the study. [13]
Forty eight Wister rats with average weight 200-250 gm were used in this study. Rats were divided into 6 groups with 8 rats each (4 males and 4 females)
Group A served as control received 1 ml corn oil. (Untreated rats)
Group B received ALP at dose of 15 mg/ kg dissolved in 1 ml corn oil. [14]
GROUP C: received ALP at dose of 15 mg/kg dissolved in 1 ml corn oil + oral copper at dose of 200 mg /kg after half hour of ingestion of ALP. [15]
Group D: received ALP at dose of 15 mg/ kg dissolved in 1 ml corn oil + oral iron ferric hydroxide poly maltose at dose of 10 mg/kg after half hour of ingestion of ALP. [16]
Group E: received ALP at dose of 15 mg/kg dissolved in 1 ml corn oil + oral virgin coconut oil at dose of 1.42 ml/kg daily after half hour of ingestion of ALP. [17]
Group F: received ALP at dose of 15 mg/kg dissolved in 1 ml corn oil + oral copper at dose of 200mg/kg + oral Iron(ferric hydroxide poly maltose) at dose of 10 mg/kg daily + virgin coconut oil at dose of 1.42 ml/kg daily, after half hour of ingestion of ALP.
All these groups are kept under observation for 6 hours starting from 8 am till 3 pm with observation of vital signs and manifestation of toxicity. After 2 hours we noticed that all the rats in Group B that received ALP have developed progressive severe hypotension and became drowsy. Blood samples were collected then the rats were sacrificed within 15 minutes to collect liver, kidneys, and lungs for pathological examinations.
After 3 hours we noticed that all rats except one male from Group D received ALP and oral iron. Moreover, all the rats from Group E that received ALP and coconut oil have developed severe hypotension and became drowsy. Blood samples were collected then the rats were sacrificed within 20 minutes to collect liver, kidneys, and lungs for pathological examinations which were kept in formalin 10%.
Only one male rat from Group D tolerated for 3 hours and 30 minutes then developed progressive severe hypotension and became drowsy. Blood samples were collected then the rats were sacrificed within 15 minutes to collect liver, kidneys, and lungs for pathological examinations which were kept in formalin 10%.
Group C rats received ALP and Copper and Group F received ALP + Copper + Iron + coconut oil were all alive for 6 hours without significant hypotension except one female rat from group C died after 5 hours and developed mild hypotension. Blood samples were collected then the rats were sacrificed within 15 minutes to collect liver, kidneys, and lungs for pathological examinations which were kept in formalin 10%.
Blood samples were collected from the retro-orbital plexus of all groups in capillary glass tubes and were incubated at 37°C until blood clotted and then were centrifuged to separate serum.
Serum samples were analyzed for measurement of bilirubin, ALT, AST, urea, Creatinine, ALK, LDH, CK, CK MMB according to the manufacture.
All rats were investigated by the following laboratory assessment (was done in kaser Al Ani central laboratory) and histopathological examinations
Cardiac enzyme: CK, CK-MB, AST and LDH.
Liver function tests: Bilirubin total and direct, AST and ALT.
Kidney function tests: urea and creatinine.
Pathological assessment of:
Lung- liver- kidney and heart.
Statistical Analysis
Recorded data were analyzed using the statistical package for social sciences, version 20.0 (SPSS Inc., Chicago, Illinois, USA). Quantitative data were expressed as mean± standard deviation (SD). Qualitative data were expressed as frequency and percentage. A one-way analysis of variance (ANOVA) followed by Post Hoc test: Least Significant Difference (LSD) for multiple comparisons between different variables and Chi-square (x2) test of significance to compare proportions between qualitative parameters.
The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant as the following:
Probability (P-value)
P-value <0.05 was considered significant.
P-value <0.001 was considered as highly significant.
P-value >0.05 was considered insignificant.
Results
The results of the present study are demonstrated in the following tables and figures
In Our Study
According to the sex, there is no statistical significant difference between different groups as shown in table 1. Regarding biochemical results by comparison between group regarding bilirubin there is high significant statistical difference between groups with result high normal in Group B receive Aluminum phosphide poison Alone, Group D receive Aluminum phosphide poison+ Iron orally and Group E receive Aluminum phosphide poison+ pure coconut oil by comparison with control group, group C receive Aluminum phosphide poison+ copper orally and Group F receive Aluminum phosphide poison+ copper+ Iron+ coconuts shown in table 2 ,as shown in figures 1,2.
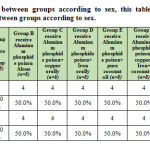 |
Table 1: Comparison between groups according to sex |
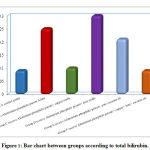 |
Figure 1: Bar chart between groups according to total bilirubin. |
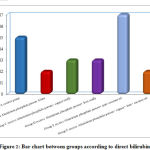 |
Figure 2: Bar chart between groups according to direct bilirubin. |
Regarding liver enzyme ALT,AST is high in all group by comparison with control group but highly significant statistical difference between groups with result high in Group B receive Aluminum phosphide poison Alone and Group D receive Aluminum phosphide poison+ Iron orally by comparison with control group, group C receive Aluminum phosphide poison+ copper orally ,Group F receive Aluminum phosphide poison+ copper+ Iron+ coconut and Group E receive Aluminum phosphide poison+ pure coconut oil as shown in table 2 ,as shown in figures 3,4.
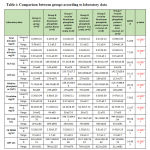 |
Table 2: Comparison between groups according to laboratory data |
Using: One Way Analysis of Variance
P-value>0.05 NS; *p-value <0.05 S; **p-value <0.001 HS
This table shows highly statistically significant difference between groups according to laboratory data.
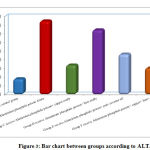 |
Figure 3: Bar chart between groups according to ALT. |
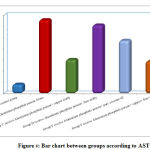 |
Figure 4: Bar chart between groups according to AST. |
Regarding Alkaline Phosphatase there is high significant statistical difference between groups with result high in Group B receive Aluminum phosphide poison Alone, Group D receive Aluminum phosphide poison+ Iron orally and Group E receive Aluminum phosphide poison+ pure coconut oil by comparison with control group, group C receive Aluminum phosphide poison+ copper orally and Group F receive Aluminum phosphide poison+ copper+ Iron+ coconut as shown in table 2, as shown in figure 5.
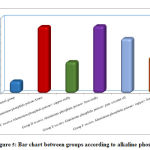 |
Figure 5: Bar chart between groups according to alkaline phosphatase. |
Regarding creatinine by comparison between groups, there is high significant statistical difference between groups with result high normal in Group B receive Aluminum phosphide poison Alone, Group D receive Aluminum phosphide poison+ Iron orally and Group E receive Aluminum phosphide poison+ pure coconut oil by comparison with control group, group C receive Aluminum phosphide poison+ copper orally and Group F receive Aluminum phosphide poison+ copper+ Iron+ coconut, but all groups creatinine is normal as mostly creatinine need hours to raise after renal impairment.as shown in table 2 ,as shown in figure 6.
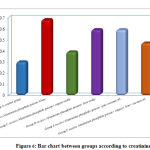 |
Figure 6: Bar chart between groups according to creatinine. |
Regarding urea, CK,CK MB and LDH, result by comparison between groups, there is high statistical significant difference between groups with result high in Group B receive Aluminum phosphide poison Alone, Group D receive Aluminum phosphide poison+ Iron orally and Group E receive Aluminum phosphide poison+ pure coconut oil by comparison with control group, group C receive Aluminum phosphide poison+ copper orally and Group F receive Aluminum phosphide poison+ copper+ Iron+ coconuts as shown in table 2 ,as shown in figure 7,8,9,10.
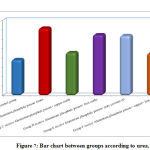 |
Figure 7: Bar chart between groups according to urea. |
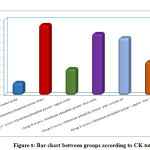 |
Figure 8: Bar chart between groups according to CK total. |
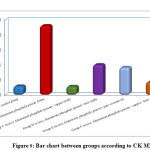 |
Figure 9: Bar chart between groups according to CK MMB. |
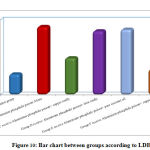 |
Figure 10: Bar chart between groups according to LDH. |
Histopathological Assessment
Lung
Regarding Bronchitis there was highly significant statistical difference between groups as group B showed 4 rats with marked bronchitis, 2 rat with marked bronchitis with abscess and 2 rat with moderate bronchitis. Group C and F showed 4 rats with mild bronchitis and Groups D and E showed 2 rat with mild bronchitis and 6 rats with moderate bronchitis as shown in table 3.
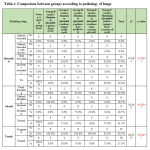 |
Table 3: Comparison between groups according to pathology of lungs |
X2-Chi-square test;
*p-value <0.05 S; **p-value <0.001 HS
This table shows statistically highly significant difference between groups according to pathology of lungs.
In alveoli inflammation and sloughing there was high significant statistical difference the between groups: group B had 8 rats with marked inflammation and sloughing. Groups C and F had 2 rat with focal sloughing and Group D had 2 rat with focal inflammation and sloughing and 6 rats with moderate inflammation and sloughing. Finally, Group E had 6 rats with mild inflammation and sloughing and 2 rat with moderate inflammation and sloughing as shown in table 3, as shown in figures 17,18,19,20.
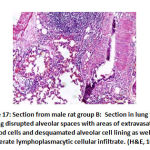 |
Figure 17: Section from male rat group B |
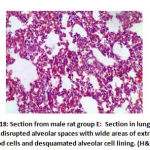 |
Figure 18: Section from male rat group E |
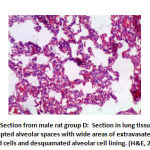 |
Figure 19: Section from male rat group D: |
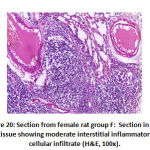 |
Figure 20: Section from female rat group F |
Furthermore, the vascular congestion had high statistical significance between the groups: Group B showed 8 rats with vascular congestion, Group C showed 4 rats with vascular congestion, Group D and E showed 8 rats with vascular congestion. Also, Group F showed 2 rat with vascular congestion as shown in table 3.
Liver
There was high significant difference in portal inflammation between the groups: Group B showed 4 rats with mild inflammation and 4 rats with moderate portal inflammation, group C and F showed 2 rat with mild portal inflammation, group D showed 8 mild portal inflammation and group E showed 4 rats with mild portal inflammation as shown in table 4, as shown in figure 23, 25.
In addition, in vessels congestion there was high significant difference between groups as: group B showed 2 rat with marked congestion and 6 rats with moderate vessels congestion, group C showed 2 rat with mild vessels congestion, group D showed 8 with mild vessels congestion and group E showed 4 rats with mild vessels congestion, as shown in table 4, as shown in figures 21, 22.
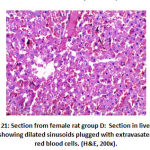 |
Figure 21: Section from female rat group D |
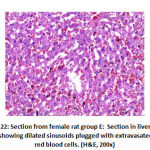 |
Figure 22: Section from female rat group E |
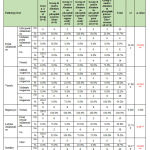 |
Table 4: Comparison between groups according to pathology of livers. |
X2-Chi-square test;
P-value >0.05 NS; *P-value <0.05 S; **P-value <0.001 HS
This table shows highly statistically significant difference between groups according to pathology of livers at portal inflammation, vessels and interface.
This table shows statistically significant difference between groups according to lobular inflammation.
Regarding interface there was significant difference between groups as: Group B 6 rats with interface and group D 2 rat with interface.as shown in table 4, as shown in figures 23, 25.
However, hepatocytes pathology showed no statistical significance between groups and lobular inflammation show statistically significant difference between groups as group B with 6 rats with focal lobular inflammation, group D and group E 2 rats with focal lobular inflammation as shown in table 4, as shown in figures 23, 25.
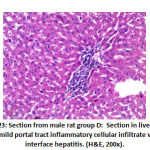 |
Figure 23: Section from male rat group D |
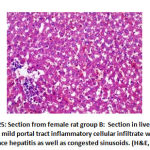 |
Figure 25: Section from female rat group B |
Kidney
Regarding mesangial proliferation in glomeruli: there was significant difference between groups as group B showed 8 rats with mesangial proliferation, groups C and F showed 2 rats with mesangial proliferation, group D with 6 rats with mesangial proliferation and group E 4 rats with mesangial proliferation.as shown in table 5,as shown in figures11,12,13,14.
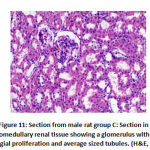 |
Figure 11: Section from male rat group C |
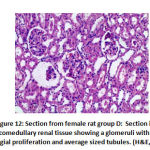 |
Figure 12: Section from female rat group D |
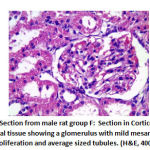 |
Figure 13: Section from male rat group F |
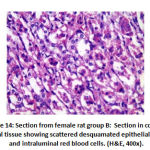 |
Figure 14: Section from female rat group B |
Regarding renal tubules there was statistical significance difference between groups with slough with or without hydropic changes in all rats of B group as shown in table 5.
 |
Table 5: Comparison between groups according to pathology of kidneys. |
X2-Chi-square test;
P-value >0.05 NS; *p-value <0.05 S
This table shows statistically significant difference between groups according to pathology of kidneys at glomeruli, tubules, vessels and interstitium.
Also, in renal vessels congestion: there was significant difference between groups as group B 4 rats with moderate congestion and 4 rats with sever congestion, groups C and F showed 2 rat with mild congestion, group D with 2 rat with mild congestion, 4 rats with moderate congestion and 2 rats with sever congestion group E showed 4 rats with mild congestion. As shown in table 5 as shown in figure 16.
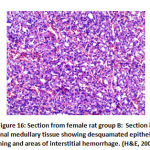 |
Figure 16: Section from female rat group B |
Finally, interstitial inflammation: there was a significant difference between groups as group B depicted 6 rats with mild interstitial inflammation and 2 rat with moderate interstitial inflammation groups C and F showed no interstitial inflammation, group D depicted 6 rats with mild interstitial inflammation and group E showed 4 rats with mild interstitial inflammation as shown in table 5 as shown in figure 14, 16.
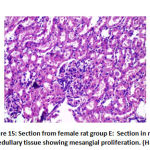 |
Figure 15: Section from female rat group E |
 |
Figure 24: Section from male rat group C |
 |
Figure 26: Section from male rat group F |
Discussion
ALP was the major cause of death in developing countries especially Egypt, because it is cheap, available, and highly toxic. [18] It is better absorbed by the gastrointestinal tract after oral intake. Mechanism of action of Phosphine gas and cyanide are similar. Both inhibit cytochrome oxidase enzyme in the mitochondria resulting in cellular oxygen utilization, inhibit oxidative respiration by 70% and ultimately results in a marked decrease in mitochondrial membrane potential. [19-20]
There is no specific therapy for ALP poisoning and no oral antidote. The management of ALP intoxication is mainly supportive and dependent on the administration of H 2 receptor antagonists or proton pump inhibitors to reduce the gastric acidity and release phosphine gas. Magnesium plays a key role in the formation of several antioxidants therefore, administration of intravenous magnesium has been recommended in several cases of ALP poisoning. Hence, Hypomagnesaemia is a deleterious result of phosphine gas. [21]
In our study coconut oil has a mild role in treatment of aluminum phosphide toxicity in both laboratory and pathological assessment Shadnia et al,.(2005) concluded that coconut oil has a positive clinical significance and can be added to the treatment protocol of acute ALP poisoning in humans. [22]
The results collaborated with Bajwa et al; (2010) who stated that coconut oil reduces the catalytic reaction of phosphide with HCL and inhibits the release of phosphine. [23]
Moreover, in our study copper has a significant role in treatment of aluminum phosphide toxicity in both laboratory and pathological examinations which in agreement with Velid; (2018) who reported that copper exhibits biological impacts, including anti-oxidative stress, anti-aging, anti-toxicity and anticancer. Accumulating evidences also found that the phototherapeutic agents play a crucial role in the removal of mercury from the tissue and in reducing oxidative stress. [24]
The nutrient antioxidants, vitamins A, C and E, and the minerals copper, zinc and selenium are found in certain foods and may prevent some of the damage caused by free radicals by neutralizing them. [25]
Copper is a component of several enzymes necessary for normal metabolic functions in humans especially cytochrome oxidase. Food sources rich in copper include shellfish, organ meats, nuts, beans and cocoa. [26]
Also, Pena et al ;( 1999) approved that aerobic life depends on cellular copper homeostasis and distribution. Copper ions serve as important catalytic cofactors in redox chemistry for proteins that carry out fundamental biological functions. A copper-containing metalloenzyme, mitochondrial cytochrome c oxidase (COX), is the final electron acceptor in the mitochondrial electron transport chain and is required for aerobic ATP production. [27]
Moreover, Varotsis et al,.(1993) showed the importance of copper and iron in structure and activation of cytochrome oxidase as Two electrons are passed from two cytochrome c’s, through the Cu A and cytochrome a sites to the cytochrome a3– Cu b binuclear center, reducing the metals to the Fe2+ form and Cu+. The hydroxide ligand is protonated and lost as water, creating a void between the metals that is filled by O2. [28] In contrast to our results in Iron, JuanDu et al ;( 2015) who hypothesized that increasing intracellular iron would enhance ascorbate-induced cytotoxicity .Treatment of cells with the iron-chelators decreased the flux of H2O2 generated by pharmacological ascorbate and reversed ascorbate-induced toxicity. [29]
Conclusion
ALP is highly toxic, cheap and available poison till now no antidote or treatment are available. Therefore, we tried copper, iron and coconut oil as a possible oral antidote for ALP toxicity. Copper showed amelioration effect on ALP toxicity (important for function of cytochrome oxidase) however, iron showed an ineffective role in treatment of ALP toxicity. In addition, coconut oil can be added to the treatment of ALP toxicity. Pathological assessment also confirmed our results of lung, liver, and kidney tissues in rats.
Compliance with Ethical Standards
The study was ethically approved by the Institutional Animal Care and use committee (IACUC) Cairo University with number (CU/81 /12 / 2019/).
Acknowledgment
Thanks for Animal house team in Cairo University, Faculty of medicine for their hard working in care of animals and research
Competing Interests
The authors declare that they have no competing interests.
Funding
Not applicable as no fund was obtained for the study.
References
- Chung, SN, Dushyant, Ram, S, Arora, B and Malhotra, KC. Incidence and outcome of aluminum phosphide poisoning in a hospital study .The Indian Journal of medical research.(1991); 94:232-5.
- Mathai, A and Bhanu, M. Acute aluminum phosphide poison: Can we predict mortality? Indian Journal of Anesthesia. (2010); 54 (4):302-7.
- Mohan G, Arvind K B, Afzal A and Kalpana S. Managing aluminum phosphide poisonings .Journal of emergencies, Trauma and shock (2011); 3(4):378-384.
- Siwach SB, Yadav DR, Arora B, Dalal S and Jagdish M. Acute aluminum phosphide poisoning – An epidemiological, clinical and histo-pathological study. J Assoc Physicians India. (1988); 36:594–6.
- Mehrpour O, Jafarzadeh M and Abdollahi M. A systematic review of aluminum phosphide poisoning. Arh Hig Rada Toksikol. (2012); 63:61–73
- Ranga GS, Dwivedi S, Agarwal M and Kumar D. Aluminum phosphide poisoning in a young adult: A suicidal cardiotoxin simulating myocardial ischemia. J Indian Acad Clin Med (2004); 5:369.
- Siwach SB, Jagdish K, Katyal VK, Dhall A and Bhardwaj G. Prognostic indices in aluminum phosphide poisoning: Observations on acidosis and central venous pressure. J Assoc Physicians India. (1997); 45:693–5.
- Nejad FT, Mohammadi AB, Behnoush B, Kazemifar A, Nahandi MZ, Dabiran S, et al. Predictors of poor prognosis in aluminum phosphide intoxication. Iran J Toxicol. (2012); 6:610–4.
- Jaiswal S, Verma RK, Tewari N. Aluminum phosphide poisoning: Effect of correction of severe metabolic acidosis on patient outcome. Indian J Crit Care Med. (2009); 13:21–4.
- Shadnia S, Rahimi M, Pajoumand A, Rasouli MH and Abdollahi M. Successful 10-treatment of acute aluminum phosphide poisoning: possible benefit of coconut oil. Hum Exp Toxicol J. (2005); 24(4):215-8.
- Goodsell, D. Cytochrome c Oxidase. Protein Database (PDB) (2000). (Accessed Jan. 23, 2018).
- Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science (2002).
- David K and Traci P. Panax Ginseng, Am Fam Physician J. (2005); 15; 68(8):1539-1542.
- Ahmadi J ,Joukar S , Anani H ,et ,al. Dihydroxyacetone as a definitive treatment for aluminum phosphide poisoning in rats. Arh Hig Rada Tokdikol. (2018); 1; 69(2):169-177.
- Kumar V. Kalita J, Misra UK and Bora HK. A study of dose response and organ susceptibility of copper toxicity in a rat model. J Trace Elem Med Biol. (2015); 29:269-74.
- Elisabet L, Jonathan S and Magda R. Recovery from dietary iron deficiency anemia in rats by the intake of microencapsulated ferric saccharate. J food Sci Technol. (2017); 54(9):2913-2918.
- Kamisah Y, Periyah V, Lee KT, et al. cardio protective effect of virgin coconut oil in heated palm oil diet –induced hypertensive rats. Pharm Biol. (2015), 53(9):1243-9.
- Chugh, SN; Dushyant; Ram, S; Arora, B and Malhotra, KC. “Incidence & outcome of aluminum phosphide poisoning in a hospital study”. The Indian Journal of Medical Research. (1991), 94: 232–5.
- Mathai, A and Bhanu , M. “Acute aluminum phosphide poisoning: Can we predict mortality?” .Indian Journal of Anesthesia. (2010), 54 (4): 302–7.
- Valmas N, Zuryn S and Ebert PR. Mitochondrial uncouples act synergistically with the fumigant phosphine to disrupt mitochondrial membrane potential and cause cell death. Toxicology. (2008), 252:33-39.
- Chugh SN, Kumar P, Aggarwal HK, Sharma A, Mahajan SK and Malhotra KC. Efficacy of magnesium sulphate in aluminum phosphide poisoning – comparison of two different dose schedules. J Assoc Physicians India (1994), 42:373-375.
- Shadnia S, Rahimi M, Pajoumand A, Rasouli MH and Abdollahi M . Successful treatment of acute aluminium phosphide poisoning: possible benefit of coconut oil.Hum Exp Toxicol. (2005), 24(4):215-8.
- Bajwa SJ, Bajwa SK, Kaur J, Singh K and Panda A. Management of celphos poisoning with a novel intervention: A ray of hope in the darkest of clouds. Anesth Essays Res. (2010), 4:20–4.
- Velid U. Natural Phytotherapeutic Antioxidants in the Treatment of Mercury Intoxication-A Review Adv Pharm Bull. (2018), 8(3): 365–376.
- Lobo.V, Patil. A, Phatak. A, and Chandra. N. Free radicals, antioxidants and functional foods: Impact on human health, Pharmacogn Rev. (2010), 4(8): 118–126.
- https://www.who.int › nutrition › publications › guide_food_fortification_mi…
- Pena MM, Lee J and Thiele DJ. A. delicate balance: homeostatic control of copper uptake and distribution. J. Nutr. (1999), 129:1251–60.
- Varotsis C, Zhang Y, Appelman E H and Babcock G T .Resolution of the reaction sequence during the reduction of O2 by cytochrome oxidase. PNAS. (1993), 90 (1) 237-241.
- JuanDu, Brett A. Wagner , Garry R. Buettner and Joseph J.Cullen. Role of labile iron in the toxicity of pharmacological ascorbate Free Radical Biology and Medicine. (2015), Volume 84, Pages 289-295







