Chemical Composition and Nutritive Value of Astragalus podolobus Seeds Growing Wild in South of Iran
Atieh Amirpour Kumleh1, Jinous Asgarpanah2 and Parisa Ziarati*3
1Pharmaceutical sciences Branch and Pharmaceutical Sciences Research Center, Islamic Azad University, Tehran-Iran (IAUPS).
2 Department of Pharmacognosy, Faculty of Pharmacy, Pharmaceutical Sciences Branch, Islamic Azad University, Tehran – Iran (IAUPS).
3 Department of Medicinal Chemistry, Faculty of Pharmacy, Pharmaceutical Sciences Branch, Islamic Azad University, Tehran-Iran (IAUPS).
*Corresponding Author E-mail: ziarati.p@iaups.ac.ir
DOI : https://dx.doi.org/10.13005/bpj/1058
Abstract
This study on the utilization of plants by local community of the district shows the rich diversity of plants and also the rich local knowledge on plant usage for food, medical, decoration, fuel and other various purposes and also on the application purposes and methods. The interest in chemical constituents of various species of the genus Astragalus has been increasing during the recent years. Literature survey revealed that there was no biological investigation on Astragalus podolobus seeds worldwide. The mature seeds of A. podolobus was collected in August 2015 from Geno, Bandar Abbas, Hormozgan Province, Iran to evaluate their nutritional value and explore a new source for nutritional purposes. Specimen was identified by R. Asadpour and voucher was deposited in the Herbarium of Faculty of Pharmacy, Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS). The samples were analyzed by wet digestion method and analysis of mineral element contents analyzed by Atomic Absorption Spectrophotometer. The result indicates that the protein content of the seeds are 13.23% carbohydrate: 71.74%, fat /Lipids (EE) 3.15%, crude fiber 6.02%, Total Ash 10.42 % of the seeds. Mineral metallic analysis revealed the order Zn> K> Fe> Mn >Se> Ca>Na>Mg>Cu >Mo, Li in the seeds of A. podolobus samples. Phytochemical analysis revealed high levels of Nutritive value of seeds of A. podolobus was high on a dry matter (DM basis: 33.84% ) and this medicinal plant has good nutritive value, which supports its use as food, fodder and good source of various important nutrients for livestock. The crude protein, fat and fiber on DM basis shows comparatively high protein, fiber content also carbohydrate and fat in sufficient amount of suitable mineral element and showing high Nutritive value. Seem to be good for younger people, anemic people and common local food and diet for people in the region of south of Iran.
Keywords
Astragalus podolobus; seed; Iran; Nutritive value; Selenium; Geno Mountain
Download this article as:| Copy the following to cite this article: Kumleh A. A, Asgarpanah J, Ziarati P. Chemical Composition and Nutritive Value of Astragalus podolobus Seeds Growing Wild in South of Iran. Biomed Pharmacol J 2016;9(3). |
| Copy the following to cite this URL: Kumleh A. A, Asgarpanah J, Ziarati P. Chemical Composition and Nutritive Value of Astragalus podolobus Seeds Growing Wild in South of Iran. Biomed Pharmacol J 2016;9(3). Available from: http://biomedpharmajournal.org/?p=9656 |
Introduction
Plants are non-separable parts of human being’s life, and it is important to conserve them and also the knowledge attached to them. This study on the utilization of plants by local community of the district shows the rich diversity of plants and also the rich local knowledge on plant usage for food, medical, decoration, fuel and other various purposes and also on the application purposes and methods. Astragalus L. (Fabaceae) is generally considered as the largest genus of vascular plants with an estimated 2500 to 3000 species. Astragalus species are widely distributed in temperate regions of the Northern Hemisphere. The greatest numbers of species are found in the arid, continental regions of western North America (400 species) and central Asia (2000 to 2500 species). Iran by the vast diversity of climate and abundant plant genetic resources is known as one of the richest in terms of national resources and natural talents [1]. Iran also has the world’s most important source of astragal growth and over 804 species exist in Iran flora [2, 3]. Many Astragalus species are useful as forage plants, to control erosion, as ornamentals or as medicinal plants [4, 5]. Due to the diversity in species, astragals are valuable from various aspects of medicine, industry, sand dune fixation and forage production, so that some of its barb species like Astragalus adscendens has industrial aspects and manna is obtained from it [2, 3]. A. adscendens (Boiss. and Haussk) counts as an important plant in Iran, which is used for a special manna (called Gaz-angabin) production [6-7].
The historical use of culturally significant plants is an interest to many tribal people and to the general public [8]. People from each region use a variety of useful plants in their surroundings [9]. Local people in Hormozgan province are using plant species in different ways for food, medicinal, decoration, fuel and other purposes. Also the results clarify why similar natural areas in Iran are mostly considered as forage resources and other ecosystem functions are usually ignored, since 92 % of the region’s plants are utilized for feeding domestic animals. The Hormozgan province is located in the southern part of Iran with around 1.000 km of coastline along the warm waters of the Oman Sea and the Persian Gulf, in correspondence with the Strait of Hormuz, at the entrance of the Persian Gulf. The inner part is mainly mountainous, including the southern tip of the Zagros Range. The Zagros Mountains contain several ecosystems. Prominent among them are the forest and forest steppe areas with a semi-arid climate and is home to a rich and complex flora.
Additionally, there are four internationally important wetlands characterized as intertidal areas with extensive mudflats, lagoons and creeks, some with extensive mangrove vegetation. Many of these unique ecoregions from North to South have not yet been studied intensively and, as a consequence, it is not yet possible to draw complete and correct biogeographic conclusions about the Chrysididae fauna of Hormozgan as well as the one present in Iran.
Plants which are used for decoration purposes due to their esthetic characteristics are some tulips or thorny Astragalus with beautiful flowers. An overall review of other studies shows that this application type is mostly ignored in other areas, whereas 16 % of plant species in the area are used for decorating purposes, which facilitates their accessibility to the symbols of nature.
The interest in chemical constituents of various species of the genus Astragalus has been increasing during the recent years. Astragalus species are well suited with respect to seed oil chemical components. The storage lipids of legume seeds are a major of dietary fat [10]. Literature survey revealed that there was no biological investigation on A. podolobus seeds worldwide. Hence the current study includes the characterization of A. podolobus seeds to evaluate their nutritional value and explore a new source for nutritional purposes.
Material and Methods
Study Area
The experiment was conducted in August 2015 from Geno, Bandar Abbas, Hormozgan Province, Iran: altitude 2200 m : (27°23’34” N 56°23’59” E, 100m). Specimen was identified by R. Asadpour and voucher was deposited in the Herbarium of Faculty of Pharmacy, Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS) Tehran by Vocher No : 422-PMP/A. Hormozgan province district is situated in the southeast of Iran. More than 70% of the province is covered by mountains and hills thus it is a mountainous region [11-13]. Site characterized by deciduous forest trees, Palm and pasture plants. Geno Mountain is a protected area, the Malaise trap was placed on the southern slop between deciduous forest trees and pasture plants. This province is located between northern latitude 25⁰ 24′ to 28⁰ 57′ and eastern longitude 53⁰ 41′ to 59⁰ 15′. It occupies an area of 70697 km2 [11-14].
The study area (Bandar Abbas region in Hormozgan province) is located in northern costal zones of the Persian Gulf in south of Iran (Fig. 1). The extent of the study area was about 18,000 ha and was situated in mountain and hill area having elevation ranging from 860 m to 3,081 m. Annual rainfall is 214 mm, mean temperature is 24.33°C, average maximum temperature is 31.25°C, and average minimum is 17.35°C. The soils of the study area are mostly shallow and therefore are only suitable as rangeland for herd grazing. According to the American system of soil taxonomy [15], the soils of the region are classified in as Entisols.
 |
Figure 1: Location of A. podolobus samples collection |
Plain part of the region includes much of the southern, eastern and northern part of the strip consisted of alkaline and saline soils contain large amounts of soluble salts such as chloride, sulfate and carbonate of Ca, Mg, sodium, and potassium[ 16,17].
Sampling Method
The present study was carried out determining the nutritional value and study on some mineral and essential elements of A. podolobus seeds. A. podolobus mature seed samples drying at (80 C ° ) for 2 h to a constant weight, the samples were separated and weighed individually. The dried samples were homogenized and grounded using a mortar.
Crude Fiber
Five grams of the grounded A. adscendens mature dry seeds samples were digested in 50 ml of 1.25% H2SO4. The solutions were boiled for 45 minutes and then were filtered and washed with hot distilled water. The filtrates were digested in 50 ml of 1.25% Sodium Hydroxide solutions. For 50 minutes these solutions were heated, filtered and washed with hot deionized water and over dried. The final oven-dried residues were ignited in a furnace at 550oC. The weights of the left after ignition were measured as the fiber contents and were expressed in term of the weights of the samples before ignition [15, 18].
Crude Protein
The protein nitrogen in one gram of the dried samples were converted to ammonium sulphate by digestion with concentrated H2SO4 (Merck 96.5%) and in the presence of CuSO4 and K2SO4 (20-21). The solutions were heated and the ammonia evolved were steam distilled into Boric acid 2%. The nitrogen from ammonia were deduced from the titrations of the trapped ammonia with 0.1M HCl with Tashirus indicator (methyl red: methylene blue 2:1) until a purplish pink color were obtained. Crude proteins were calculated by multiplying the valve of the deduced nitrogen by the factor 6.25 mg [15-18, 22].
Ash Content
One gram of the oven-dried samples in powder from was placed in acid washed crucible by known weight. They were ignited in a muffle furnace for 5 hours at 550 oC. After cooling crucibles they were weighed and the ash contents were expressed in terms of the oven-dried weight of the sample [18, 22]
Zinc, Manganese, Copper and Potassium Determination
All stock solutions and working standards were stored at 4°C and brought to room temperature (25 0C) before use. For Zinc, Manganese, Copper and Selenium concentration in A. podolobus, powered seed samples were dried in oven for 48 hours at a temperature of 85°C. The samples were then ground and sieved through 0.5 mm sieve. The powdered samples then subjected to the acid digestion using concentrated nitric acid (65% Merck), Sulfuric acid (96.5% Merck) and per chloric acid (70% sigma). Analar grade hydrogen peroxide (about 30%) also was used for the digestion. Application of concentrated HNO3 along with thirty percent hydrogen peroxide H2O2 (Merck) for mineralization of samples to the complete digestion of samples [15-23] following Environmental Protection Agency (EPA) Method 3052 was done [21-27].
Two gram of air-dried of each homogeneously A. podolobus samples accurately weighed and 30.0 mL of the digestion mixture (2 parts by weight of nitric acid: 1 parts of Sulfuric acid & 4 parts by weight perchloric acid) and heated slowly by an oven and then rise the temperature. The remaining dry inorganic residues were dissolved in 30.0 mL of concentrated nitric acid and the solution used for the determination of trace and essential mineral elements. Blanks and samples were also processed and analyzed simultaneously. All the chemicals used were of analytical grade (AR). Standardized international protocols were followed for the preparation of material and analysis of heavy metals contents [22-27]. The samples were analyzed by Flame Emission Spectrophotometer Model AA-6200 (Shimadzu, Japan) using an air-acetylene, flame temperature: 2800°C, acetylene pressure: 0.9–1.0 bar, air pressure: 4.5–5 bar, reading time: 1–10 sec (max 60 sec), flow time: 3-4 sec (max 10 sec), using at least five standard solutions for each metal and determination of potassium content was followed by FDA Elemental analysis [28]. In order to verify of reliability of the measuring apparatus, periodic testing of standard solutions was performed. The accuracy was checked using quality control test for fungi and their substrate samples to show the degree of agreement between the standard values and measured values; the difference was less than 5%.
Iron Determination
The aliquot was passed through the atomic absorption spectrophotometer to read the iron concentration. Standards were prepared with a standard stock of 10 mg/L using ferrous ammonium sulphate where 3 – 60 ml of iron standard solution (10 mg /L) were placed in stepwise volumes in 100 ml volumetric flasks. 2 ml of hydrochloric acid were added and then brought to the volume with distilled water. The concentration of iron in the aliquot was measured using the atomic absorption spectrophotometer in mg/L. The whole procedure was replicated three times [14-17].
Calcium, Sodium and Magnesium Determination
5 ml of the aliquot were placed in a titration flask using a pipette and diluted to 100 ml with distilled water and subsequently 15 ml of buffer solution, ten drops of Eriochrome black T indicator and 2 ml of triethanolamine were added. The mixture was titrated with Ethylene-Diamine-Tetra-Acetate (EDTA) solution from red to clear blue [15-18].
Selenium Determination
Stock standard solutions for selenium were 1000 g /mL solution. All reagents and standards were of analytical grade ( Merck, Germany) .The palladium matrix modifier solution was prepared by the dilution (10 g/ L) Pd(NO3)2 and iridium AA standard solution, 1000 g/ mL in 20% HCl , 0.1 % V/V nitric acid prepared by dilution trace pure 65 % nitric acid and 0.1 % Triton X-100 were used. Doubly distilled water was used in all operations. The samples were analyzed by Flame Emission Spectrophotometer Model AA-6200 (Shimadzu, Japan). The analyze performed according by Analytical Method ATSRD[15-18, 29-30].
Crude Protein – Nitrogen Crude Protein (CP)
Nitrogen content was measured by modified Kjeldahl method (involving sulphuric acid digestion). It was assumed all N present in the feed was derived from protein and that all protein contains 16%N. Thus CP = N x 100/16 = N x 6.25 [31].
Ether soluble Extract
An ether soluble extract was prepared by mixing the feed using petroleum ether for a defined period. The mixture was then filtered. The filtrate was allowed to evaporate and the remaining residue was weighed to determine the mass of the ether soluble extract (EE)[15,31].
Crude Fiber
Crude Fiber (CF) was measured by taking the residual food following ether extraction, and then boiling it with acid and then alkali for defined periods. The residue was then dried, weighed, ashed and weighed again. CF was weight of residue less weight of ash. CF equates to the indigestible part of ration (for non-ruminants) and consists of cellulose, hemicellulose, lignin, i.e. plant cell walls [31].
Nitrogen free extractive
This is an indirect determination of the non-fiber carbohydrate content of the feed. Nitrogen free extractive (N-FE) as a percentage is 100% minus the rest. This will include sugars, starches, and water soluble vitamins (will also measure some cellulose, hemicellulose, resins, pigments, tannins, pectin) [31].
Results and Discussion
The mean content of trace and essential mineral elements (mg/kg DW) in the mature dry seed of A. podolobus samples is shown in table 1. The samples were analyzed by wet digestion method and standardized international protocols were followed for the preparation of material and analysis of mineral contents and analyzed by Atomic Absorption Spectrophotometer in Research Laboratory in Pharmaceutical Sciences Branch, Islamic Azad University.
Table 1: The Mean content (mg/kg DW± SD) composition of the mature dry seeds of A. podolobus samples from Hormozgan Province, Iran
|
Minerals |
Mean content ± SD*
(mg/Kg DW) |
Minerals | Mean content ± SD*
(mg/Kg DW) |
| Sodium | 3 6.21± 1.46 | Boron | 0.016 ± 0.013 |
| Potassium | 78.67 ± 1.036 | Phosphor | 3.743 ± 0.011 |
| Calcium | 10.13± 0.012 | Iodine | 1.076 ± 0.032 |
| Magnesium | 22.07 ± 0.023 | Manganese | 3.048 ± 0.005 |
| Iron | 65.76 ± 1.44 | Sulphur | 1.002 ± 0.002 |
| Copper | 0.102 ± 0.001 | Fluorine | 0.005± 0.001 |
| Selenium | 13.765 ± 0.822 | Lithium | 0.001 ± 0.0002 |
| Zinc | 102.26 ± 1.302 | Molybdenum | 0.001± 0.0001 |
*SD = Standard Deviation
Mineral metallic analysis revealed the order Zn> K> Fe>Mn>Se>Ca>Na>Mg > Cu >Mo, Li in the seeds of of A. podolobus samples from Hormozgan Province, Iran.
Copper has the role of assisting in the formation of haemoglobin, helping to prevent anemia as well as being involved in several enzymes. Iron is the central metal in the haemoglobin molecule for oxygen transport in the blood and is portion of myoglobin located in muscles. Manganese is one of the co-factors in a number of enzymes as is molybdenum. Selenium has several roles such as regulating the thyroid hormone as well as being part of an enzyme that protects against oxidation [32], Selenium has also been reported as assisting in deactivating heavy metals. Calcium is responsible for strong bones and teeth and accounts for ninety percent of the calcium in the body whereas the other one percent is circulating in fluids in order to ionize calcium. The metal’s function is related to transmitting nerve impulses; contractions of muscles; blood clotting; activation of some enzyme reactions and secretion of hormones Magnesium has many roles including supporting the functioning of the immune system; assists in preventing dental decay by retaining the calcium in tooth enamel; it has an important role in the synthesis of proteins, fat, nucleic acids; glucose metabolism as well as membrane transport system of cells. Magnesium also plays a role in muscle contraction and cell integrity. Potassium and sodium work together in muscle contraction nerve transmission. Sodium is important in muscle contraction and nerve transmission Sodium ions are the main regulators of extra cellular fluid and volume [13, 32] .Zinc is an essential trace element and plays an important role in various cell processes including normal growth, brain development, behavioral response, bone formation and wound healing. Zinc deficient diabetics fail to improve their power of sensitivity and it cause loss of sense of touch and smell [13, 33-34]. Proximate composition and physicochemical characteristics of the samples has shown in figure 2 , based on the fresh weight. The seeds of A. podolobus are tiny but have great nutritive value.
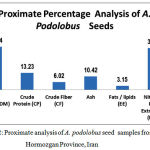 |
Figure 2: Proximate analysis of A. podolobus seed samples from Hormozgan Province, Iran. |
The seeds of A. podolobus are tiny but have great nutritive value. Potassium was higher in the studied mature seeds but they contained less sodium; Na and K. take part in ionic balance of the human body and maintain tissue excitability, carry normal muscle contraction, help in formation of gastric juice in stomach [35], K help in release of chemicals which acts as nerve impulses, regulate heart rhythms, deficiency causes nervous irritability mental disorientation, low blood sugar, insomnia and coma [36]. Iron sufficient in A. podolobus, it make body tendons and ligaments, certain chemicals of brain are controlled by presence or absence of Iron, it is essential for formation of hemoglobin, carry oxygen around the body [37] Iron deficiency causes anemia, weakness, depression, poor resistance to infection [38]. Calcium is high in A. podolobus seeds . Calcium play important role in building and maintaining strong bones and teeth also large part of human blood and extra cellular fluids. It is also necessary for normal functioning of cardiac muscles, blood coagulation, milk clotting and regulation of cell permeability [39]. Calcium deficiency causes rickets, back pain, osteoporosis, indigestion, irritability, premenstrual tension and cramping of the uterus [40]. The content of Zinc by 102.26 ± 1.302 (mg/kg DW± SD) is relatively high though the percentage of copper is very low in all samples. Cu was an important component of many enzyme systems such as cytochrome oxidase, lysyl oxidase and ceruloplasmin, an iron oxidizing enzyme in blood [41]. Cu deficiency has been associated with cardiac abnormalities in human and animal, cause’s anemia and neutropenia [41]. Zinc maintain various reactions of the body which help to construct and maintain DNA, required for growth and repair of body tissues, important element of ligaments and tendons [42]. Zinc deficiency causes clinical consequences, including growth dela, diarrhea, pneumonia, distributed neuropsychological performance and abnormalities of fetal development [43]. Phosphorous is medium by 3.743 ± 0.011 (mg/kg DW± SD). Phosphorous maintain blood sugar level, normal heart contraction dependent on phosphorous [44] also important for normal cell growth and repair, needed for bone growth, kidney function and cell growth. It plays important role in maintaining body’s acid-alkaline balance [45]. The wild plant serves as an indispensable constituent of the human diet supplying the body with minerals, vitamins and certain hormone precursors, in addition to protein and energy and the results of current study revealed that the protein content of the seeds are 13.23% carbohydrate: 71.74%, fat /Lipids (EE) 3.15%, crude fiber 6.02%, Total Ash 10.42 % of the seeds. The Crude fiber of these plant ranges from (6.02%) which is low. Crude fiber in food or plant is an indication of the level of non-digestible carbohydrate and lignin. Crude fiber enhances digestibility, lowering blood cholesterol and blood sugar. It is known to reduce the risk of disease such as obesity, diabetes, breast cancer and gastro intestinal disorder [46]. The plants contain protein which was 13.23%. The recommended dietary allowance (RDA) for protein is 56g for individual weighing 70kg and 46g for adult weighing 50kg; children may consume 2kg/day [47]. Abortifacient protein with ribosome- inhibiting properties has been isolated from several cucurbits [48]. The ash percentage of this seed is 10.42%. Ash is the inorganic residue remaining after water and organic matter have been removed by heating which provides a measure of total amount of minerals within the food. Minerals are not destroyed by heating and they have a low volatility as compared to other food components [49-50]. The results obtained in current study show that the seeds of A. podolobus contains appreciable amount of nutrients which can contribute to the nutrient and energy requirement of man.
Conclusion
Nutritive value of seeds of A. podolobus was high on a dry matter (DM basis) this medicinal plant has good nutritive value, which supports its use as food, fodder and good source of various important nutrients for livestock. The crude protein, fat and fiber on DM basis shows comparatively high protein, fiber content also carbohydrate and fat in sufficient amount of suitable mineral element and showing high Nutritive value. Seem to be good for younger people, anemic people and common local food and diet for people in the region of Hormozgan province in the south of Iran.
Acknowledgement
Supports from Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS) is gratefully acknowledged.
Conflicts of Interest
None of the authors have any conflicts of interest associated with this study.
References
- Tavili, A. Effect of some Treatments on Germination Improvement of Two Astragalus Species. International Conference on Biological, Environment and Food Engineering (BEFE-2014) August 4-5, 2014 Bali (Indonesia).Available online: http://iicbe.org/upload/2307C814066.pdf .
- Masumi, A.A., Ghahraman, A. Iran Astragals, Research Institute of Forests and Rangelands, 2006, pp 786 .
- Ghaderi, A., Kamkar, F., Soltani, A. Seed science and technology (translation), Mashhad University Jihad Press, 2008; pp 512 , Mashhad, Iran.
- Hiroani, M. , Liu, H. Z. , Furuya, T. , 1994; 36: 665 .
- Baratta, F. A., Ruberto, G. Planta Med., 1997; 63: 280 .
- Gerami, B. Gas of Khansar: The Manna of Persia. Bot., 1998;52(2): 183-91.
- Esmaeili, Gh., Azizi M. , Aroei, H., Samiei, L. Micropropagation of Astragalus adscendens: A Source of Gaz-angabin Manna in Iran (Persian Manna) , Agr. Sci. Tech., 2016; 18: 741-50.
- Casey, P. A. , Wynia, R. L. Culturally significant plants. USDA-Natural Resources Conservation Service, Kansas Plant Materials Center: Manhattan, KS. 2010.
- Khajoei, N. F., Khosravi, A. R. Ethno-botanical study of medicinal plants of Sirjanin Kerman Province, Iran.Journal of Ethnopharmacology, 2014; 154: 190–7.
- Bagci, E. Nat. Comps., 2006; 42:645.
- Available in Site: http://www.doe.ir/Portal/File/ShowFile.aspx?ID=7c52e9d6-451d-45a4-a424-666cc4850e31 .
- Kermanshah, A., Ziarati, P., Asgarpanah, J., Qomi, M. International Journal of Plant, Animal and Environmental Sciences. 2014; 4: 380-8.
- Abbasian , K., Asgarpanah , J., Ziarati, P. Chemical Composition profile of Acacia Nilotica Seed Growing Wild in South of Iran. Oriental Journal of Chemistry. 2015; 31(2): 1027-33.
- Sabzian, M. Iran tourism, a complete source book. Tehran: Kamel Publications; 2008. pp. 560–81.
- Sefidanzadeh, S., Ziarati, P., Mohammadi Motammed, S. Chemical Composition of Suaeda vermiculata Seeds Grown in Hormozgan in the South of Iran. Bioscience and Biotechnology Research Asia, 2015; 12(3): 1923-9.
- Zarei ,M., Asgarpanah , J., Ziarati, P. Chemical Composition profile of Wild Acacia oerfota (Forssk) Schweinf Seed Growing in the South of Iran. Oriental Journal of Chemistry. 2015; 31 (4): 2311-8.
- Soil taxonomy: Keys to soil taxonomy, Sixth Edition, 1994, pp 177.
- Aryapak, S., Ziarati, P. Nutritive Value of Persian Walnut (Juglans regia L.) Orchards. American Eurasian J. Agric. & Environ. Sci. 2014; 14: 1228-35.
- Ziarati, P. Determination of some heavy metals in popular medicinal plants in Tehran’s market Journal of Pharmaceutical and Health Sciences. 2012; 1(3): 31-6.
- Ziarati, P. Determination of Contaminants in Some Iranian Popular Herbal Medicines .Journal of Environmental & Analytical Toxicology . 2012 ; 2(1). Available from: http://dx.doi.org.
- Ziarati, P., Khoshhal, Z., Asgarpanah, J., Qomi, M. Contaminations of Heavy Metals in Tea Leaves, Finished Tea Products and Liqour in Gilan Province, Iran. Intl J Farm & Alli Sci. 2013; 2 : 383- 7.
- Amini Noori,F., ziarati, P., Jafarpour, A. Nutritive Value of Persian Hazelnut (Corylus avellana L.) Orchards. Journal of Pharmaceutical and Health Sciences. 2016; 4(1): 71-7.
- Praveen, S. Application note Atomic Absorption, 2011. Available in site : perkinelmer.com.
- Ziarati, P., Tosifi, S. Comparing some physical and chemical properties of green olive (olea europea l.) in iran association with ecological conditions. International Journal of Plant, Animal and Environmental Sciences. 2014; 4(2): 519-28.
- Ziarati, P., Rabizadeh, H. The Effect of Thermal and Non Thermal of Food Processes and Cooking Method in Some Essential Mineral Contents in Mushroom (Agaricus bisporus) in Iran. Nov. Appl. Sci. 2013 ; 2 : 954-9.
- AOAC (Association of Official Analytical Chemists). Wet digestion for non –volatile metals in: AOAC official methods of analysis, 1998, 16th edition, 4th revision, vol.1, chapter 9. p32.
- Ziarati, P., Behbahani, P., Karbalaie Mohammad, N. Role of Unprofessional Storage methods on the heavy metal content of Rosa Damascena ( Gole Mohammadi) . Journal of Pharmaceutical and Health Sciences .2012; 1(4): 73-8.
- ORA Laboratory Manual FDA, 2004, 6. Document no.: iv-02, version no.: 1.5, effective date: 10-01-03 REVISED: 02-14-13. Available in Site: http://www.fda.gov/downloads.
- Ziarati , P., Azizi, N. Consequences of cooking method in essential and heavy metal contents in brown and polished alikazemi rice .International Journal of Plant, Animal and Environmental Sciences. 2014; 4: 280-7.
- Masamba, K.G. , Kazombo-Mwale, R. Determination and comparison of nutrient and mineral contents between cultivated and indigenous edible mushrooms in Central Malawi .African Journal of Food Science .2010; 4: 176- 179.
- Association of Official Analytical chemists, Official methods of analysis. USA: Washington, DC. 1990.
- Whitney, E.N., Rofles, S. R. Understanding Nutrition. 2002, 9 ed: Thomas Learning, Inc.
- Guide to Naturopathy published by Geddes& Grosset, Printed in UK, ISBN: 1- 85534-936-1, 1999, pp 51-3 .
- Jabeen, S., Shah, M.T.;Khan, S., Hayat, M.Q. Pak. Med. Plants Res. 2010; 4(7): 559- 66.
- Brody, Nutritional Biochemistry San Diego Academic press, 1998, pp 11 – 2.
- Gaeta,A., Robert Hider, C. The crucial role of metal ions in neuro degeneration; the basis for promising therapeutic strategy. Brit J. of Pharmalo. 2005; 146: 1041 – 59.
- Weight, L.M., Jalobes, P., Noakes, T.D. Dietary Iron deficiency and sports anemia Brit, J. of Nutri. 1992; 68: 253 – 60.
- Hasling, C. Sondergard, K ., Pand Moselkiloe,C . Calcium metabolism in postmenopausal osteoporotic woman is determined by dietary calcium and coffee intake . ins. of nutria. 1991; 23: 119 – 26.
- Smith, J.C. Copper nutritive and cardiovascular integrity in: Hemphill DD. Ed proceedings of 21st annual conference on trace substances in Environmental health, Columbia, 1987, pp 499 – 513.
- Diaz-Gomez, N.M., Domenech ,E. ,Barroso, F., Castells, S., Cortabarria, C., Jimenz, A. The effect of zinc supplementation on linear growth body composition and growth factors in preterm infants, pediatrics, 2003; 111 (5): 1002 – 9.
- Hambiadge, M. Human zinc deficiency Tour of Nutrition Denver, J. Nutr., 2000; 130: 1344 – 9.
- Linder , C., Manria, C. Nutritional Biochemistry and metabolism with clinical applications. Appleton and Lange, Norwalk, 1991;2: 191 – 212.
- Johns, T., Duquette, M. Deficiency of phosphorus in man. J. of clin. Nutr. 1991; 53: 448 – 56.
- Cooper, J. Structure and Biological activity of nitrogen and oxygen; coordinated nicotinic acid complexces of chromium, Inorganica chemica, ACTA, 1984; 91: 1 – 9.
- Shivraj, H. ,Nile , C.N., Khobragade, N. Determination of Nutritive Value and Mineral Elements of some Important Medicinal Plants from Western Part of India. Journal of Medicinal Plants. 2009; 8(5) .
- Saldanha, L. G. Fiber in the diet of United States children results of National Surveys. 1995;96: 994-6.
- Jones, M. M., Johnson, D.O.,Netlerville, J.T., Wood, J. I. and Joesten, M. D. Chemistry and Society 5th ed., Saunders College Publisher U.S. A., 1985; 521-77.
- Ng, T. B., Feng, Z., Li, W.W. and Yeung, H.W. Improved Isolation and further characterization of beta-trichosathin, a ribosome-inactivating and abortifacient protein from tubers of Trichosanthes cucumerina (Cucurbitaceae). J. Biochem., 1991;23:561- 7.
- Mishra, S. R., Mahunty, M.K., Das, S.P. Pattanaik, A. K. Production of Biodisel (methyl ether) from simarouba glauca oil. J. Chem.Sci. 2012; 2(5):66-71.
- Osuagwu, A. N. ,Edeoga, H. O. Nutritional Properties of the Leaf, Seed and Pericarp of the fruit of four cucurbitaceae Species from South- East Nigeria. IOSR Journal of Agriculture and Veterinary Science (IOSR-JAVS). 2014; 7(9): 41-4.








