Manuscript accepted on :November 24, 2008
Published online on: 09-11-2015
Plagiarism Check: Yes
C. H. Madhusudan Reddy, G. Mubeen* and Mamta Pal
Al-Ameen College of Pharmacy, Bangalore - 560 027 (India).
Abstract
HPTLC method for estimation of metformin HCl in single and in combination dosage form was developed and validated by using mobile phase consisting of methanol: chloroform: ammonium acetate (6:3:1 v/v/v). Densitometric analysis of metformin hydrochloride was carried out in the absorbance mode at 236 nm.The linearity was found to be in the concentration range of 100- 300 ng/spot. The % recovery of metformin hydrochloride was found to be 100.24-101.58%, indicating no interference from the excipients in the method.
Keywords
HPTLC; Metformin HCl; validation
Download this article as:| Copy the following to cite this article: Reddy C. H. M, Mubeen G, Pal M.HPTLC Method for Estimation of Metformin Hydrochloride.Biomed. Pharmacol. J.2008;1(2) |
| Copy the following to cite this URL: Reddy C. H. M, Mubeen G, Pal M.HPTLC Method for Estimation of Metformin Hydrochloride. Biomed. Pharmacol. J.2008;1(2). Available from: http://biomedpharmajournal.org/?p=505 |
Introduction
Metformin, an hypoglycemic drug, official in IP,Chemically 1,1-dimethyl biguanide hydrochloride, widely used to enhanced peripheral glucose uptake and utilization, inhibition of hepatic gluconeogenesis, increased muscle glyconeogenesis and reduction of net glucose absorption by the small intestine, indicated for patients with non-insulin dependent diabetes mellitus.IP reports UV method for estimation of metformin HCl. Literature survey reveals many HPLC method2-7 for the estimation of metformin HCl in biological fluids and formulations. So far no HPTLC method has been reported for estimation of metformin HCl in biological fluids. In our present study, a HPTLC method for estimation of Metformin HCl in single and combination dosage form was developed and validated.
Materials and Methods
Instruments
HPTLC was performed with a Camag Linomat V (Switzerland),twin-trough TLC chamber (10×10),a Camag TLC Scanner-3, a Wincats –version 1.3.3 software, Hamilton HPTLC syringe (100mcL), Acculab ALC 210.3 weighing balance and ultrasonicator were used during study.
Chemicals and Reagents
Metformin standard was procured as a gift sample from Franco-Indian Remedies Pvt. Ltd. Methanol, chloroform, ammonium acetate, glacial acetic acid and ammonia solution all are Analytical Reagent (AR) grade. Tablets containing metformin, single dosage form Glyciphase (500mg/tablets) and Obimet (500mg/tablets) and combination dosage form Dionorm, Diabetrol and Dibizide were purchased from local market.
Chromatographic Conditions
TLC was performed in the form of bands of width 6 mm with a Camag microlitre syringe on precoated silica gel aluminium plate 60 F-254, (10×10 cm) with 250 μm thickness; E. Merck, (Germany) using a Camag Linomat V (Switzerland). A constant application rate of 15 μl/s was
employed. The mobile phase consisted of methanol: chloroform: ammonium acetate (6:3:1 v/v/v). Linear ascending development of chromatogram was carried out in a Camag twin trough glass chamber saturated with the mobile phase. The chamber saturation time for mobile phase was optimized at 30 min. The length of chromatogram run was 7.5 cm. Subsequent to the
development; the TLC plates were dried with the help of a Camag TLC plate dryer. Densitometric scanning was performed on a Camag TLC scanner III in the absorbance mode at 236 nm. The plate was analysed on a TLC scanner 3® densitometer driven by the Wincats –version 1.3.3 software, dried by using TLC plate heater and scanned at 236nm with D2 lamp
Preparation of standard Metformin Hydrochloride Solution
50 mg of standard metformin HCl was accurately weighed and dissolved in methanol and the volume made up to 100 ml from this 0.5ml was withdrawn and diluted upto 10 ml to give concentration 25 μg/ml.
Estimation of Metformin HCl in single dosage form
The tablets were powdered and powder weight equivalent to 50 mg of drug was extracted in methanol. To ensure complete extraction of the drug it was shaken for 15min and the volume was made up to 100 ml. The resulting solution was filtered. 0.5 ml of the filtrate was taken and diluted up to 10 ml with methanol to get 25 ng/μl solution. Different volumes of this solution ranging from 5, 6 and 7 μl were spotted onto the plate, the chromatogram was developed and scanned at 236nm.(Glyciphase and Obimet label claim 500 mg/tablets respectively).
 |
Figure 1:
|
Estimation of Metformin HCl in combination dosage forms
The tablets were powdered and powder weight equivalent to 50 mg Metformin HCl of was extracted in methanol. To ensure complete extraction of the drug it was shaken for 15min and the volume was made up to 100 ml. The resulting solution was filtered. 0.5 ml of the filtrate was taken and diluted up to 10 ml with methanol to get 25 ng/μl solution.10 μl of these three solutions were spotted on a the plate. The chromatogram was developed and scanned as 236 nm.
( Dibizide (glipizide + metformin HCl) coded as glpm, Dianorm (gliclazide + metformin HCl) coded as gclm and Diabetrol (glibenclamide +metformin HCl) coded as mgl.
Method Validation
The HPTLC method was developed and validated by using various parameters like limit of detection, limit of quantification, linearity, range, specificity, intra day precision, inter day precision, robustness, ruggedness and recovery studies.
Results and discussion
The linearity range was found to be in the concentration of 100 – 300ng/spot. The LOQ and LODwas found to be 100 ng/spot and 25 ng/spot respectively. The RSD for intra and inter day was found to be 0.828-0.3318% and 1.093-0.5114% respectively. The percentage recovery in solution was found to be 100.24-101.58%. The percentage purity of metformin HCl in single dosage was found to be 99.2-100.6% w/w and in combination dosage form 98.9-103.2% w/
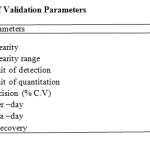 |
Table 1: Summary of Validation Parameters.
|
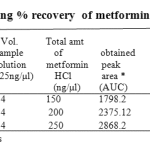 |
Table 1a: Data showing % recovery of metformin HCl (In solution).
|
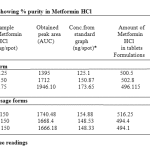 |
Table 2: Data showing % purity in Metformin HCl.
|
Average of three readings
Conclusion
The developed HPTLC method is found to be precise, specific accurate and reproducible for estimation for the metformin HCl in single dosage form and in combination dosage forms.
References
- Indian Pharmacopoeia. Ministry of Health and Family Welfare, New Delhi. 1996; I: P.469.
- Charles BG, Jacobsen NW, Ravenscroft PJ. Rapid liquid-chromatographic determination of metformin in plasma and urine. J Clinical Chem. 1981;27;434-6.
- Benzi L, Marchetti P, Cecchetti P. Navalesi R. Determination of metformin and phenformin in human plasma and urine by reversed-phase high-performance liquid chromatography. J Chromatogr Biomed Appl. 1986;48(1):184.
- Keal J, Somogyi A. Rapid and sensitive high-performance liquid-chromatographic assay for metformin in plasma and urine using ion-pair extraction techniques. J Chromatogr Biomed Appl. 1986;51(2):503-8.
- Tanabe S, Kobayashi T, Kawanabe K. Determination of oral hypoglycaemic biguanides
- by high-performance liquid chromatography with fluorescence detection. J Anal Sci. 1987;3(1):69.
- Huupponan R, Ojala KP, Rouru J, Koulu M, J. Determination of metformin in plasma by high-performance liquid chromatography. J Chromatogr Biomed Appl. 1992;121:270-3.
- Ohta M, Iwasaki M, Kai M, Ohkura Y. Determination of a biguanide, metformin, by
- high-performance liquid chromatography with pre-column fluorescence derivatization.
- J Anal Sci. 1993;9(2):217.
- ICH Guidelines (Q2A): validation of analytical procedure:terminology and definition.Geneva.Oct 1994 (CPMP/ICH/381/95).







