Manuscript accepted on :May 17, 2016
Published online on: 11-08-2016
Plagiarism Check: Yes
Ulduz Zamani Ahari1, Parisa Falsafi2, Firouz pouralibaba2 , Hosein Eslami2, Saleh Maleki1, Farzaneh Pakdel2*, and Mahdi Rahbar3
1Post Graduate Student of Oral Medicine, Dental Faculty of Tabriz University, Tabriz, Iran.
2Department of Oral Medicine, School of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran.
3Operative and Esthetic Dentistry Department, Dental Faculty of Tabriz University, Tabriz, Iran.
*Corresponding Author E-mail: Farzaneh_Pakdel@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/964
Abstract
Gestational diabetes is first diagnosed during pregnancy. There are Evidences of increased oxidative stress in pregnant women and on the other hand, the role of oxidative stress and impaired antioxidant system in complications during pregnancy such as premature delivery and miscarriage have been proved. Given that peroxidase as an enzymatic antioxidant and alpha-amylase as salivary enzyme, are important variables associated with diabetes, and regarding contradictory articles about their increase or decrease, assessing both factors in terms of reducing the complications of diabetes. In this cross-sectional study, unstimulated saliva of 31 patients with gestational diabetes and 59 non-diabetic pregnant patients were collected and amounts of salivary peroxidase alpha-amylase were assessed separately in these individuals. Data obtained from the study was statistically analyzed using descriptive statistics methods and Mann-Whitney test. P <0.05 was considered as significant in all tests. The mean value of salivary peroxidase level was -54.55 ± 48.7 in women with gestational diabetes and 24.71 ± 8.77 in non-diabetic pregnant women. The mean value of amylase level in the saliva was 609387.1± 132534.38 in women with gestational diabetes and 478559 ±.8581000.41 in non-diabetic pregnant women. According to the data obtained from this study, it could be stated that in women with gestational diabetes the level of alpha-amylase is increased but the level of salivary peroxidase is reduced, compared with non-diabetic pregnant women, but none of these changes are significant.
Keywords
alpha-amylase; salivary peroxidase; gestational diabetes mellitus
Download this article as:| Copy the following to cite this article: Ahari U. Z, Falsafi P, Pouralibaba F, Eslami H, Maleki S, Pakdel F, Rahbar M. Comparison of Salivary Alpha Amylase and Peroxidase Levels in Women with GDM and Non-Diabetic Pregnant Women. Biomed Pharmacol J 2016;9(2). |
| Copy the following to cite this URL: Ahari U. Z, Falsafi P, Pouralibaba F, Eslami H, Maleki S, Pakdel F, Rahbar M. Comparison of Salivary Alpha Amylase and Peroxidase Levels in Women with GDM and Non-Diabetic Pregnant Women. Biomed Pharmacol J 2016;9(2). Available from: http://biomedpharmajournal.org/?p=7507 |
Introduction
Unlike the previous categories which were based on the age of onset or type of treatment, currently diabetes is classified as the following based on the pathogenesis that can lead to hyperglycemia:
I- Type 1 diabetes (cell destruction, usually leading to absolute insulin deficiency): A. immune-mediated and Idiopathic
II- Type 2 diabetes (from dominant form of insulin resistance with partial insulin deficiency to dominant form of insulin secretion defect with variable insulin resistance)
III- Other specific types of diabetes
IV- Gestational diabetes mellitus (1-4)
Gestational diabetes is caused by a disorder in carbohydrate tolerance that is first diagnosed during pregnancy (5-8).
Women who are at average risk, are screened with 50 g oral glucose (GCT) at 28-24 weeks of gestation, but in high risk women including obese individuals, family history of diabetes, previous history of gestational diabetes, impaired glucose metabolism or Glucosuria, history of macrosomia or malformations in previous newborns and smoking during pregnancy, as soon as possible, they are evaluated in accordance with the above-mentioned guideline. If the result is negative, the test is repeated again in 28-24 weeks of pregnancy or when a person has symptoms and if the oral glucose tolerance test happens to be higher than recommended for two or more times, the person is considered to have gestational diabetes (3, 5, and 9).
Antioxidants are compounds that can neutralize free radicals and reactive oxygen species and prevent oxidation of lipids, nucleic acids and proteins. Antioxidants are able to act at four levels:
(A) Preventing formation of free radicals
(B) Preventing or interrupting the activity of free radicals after their formation
(C) To repair damages resulting from radical activity
(D) Increasing the excretion or absorption of damaged molecules (10)
Oxidative stress occurs due to an imbalance between the formations of reactive oxygen species (ROS) and antioxidant defenses. In normal conditions antioxidants prevent overproduction of ROS that lead to tissue damage (11-13).
The role of oxidative stress and impaired antioxidant system in various complications during pregnancy, such as preterm delivery, fetal growth restriction, preeclampsia, and abortion has been demonstrated (14). Moreover, there are evidences for increased oxidative stress in diabetic pregnant women especially with type II diabetes (12).
There are two types of antioxidants in the body (1) enzymatic, (2) non-enzymatic. The most important enzymatic antioxidants include superoxide dismutase (SOD), catalase and glutathione peroxidase (GPO) (15).
Saliva is a complex liquid consisting of water and organic and inorganic components and contains buffers, enzymes, growth factors, cytokines, immunoglobulins, mucins and other glycoproteins (1). Saliva has various defense systems including the antioxidant defense system that serves as the first line of defense against oxidative stress (2).
Saliva plays major roles including defensive functions that includes Lubrication, antimicrobial activity, growth factors, maintaining integrity of mucosa, washing and clearing the surface of mucosa and teeth, buffered properties and mineralization. Other functions of saliva include assisting in the preparation of ingested food, chewing and digestion, taste and speech. These multiple compounds in saliva, play roles not only in protecting oral tissues, they may be an indicator of some systemic diseases as well. So that salivary biomarkers are used as indicator tools for evaluating public health and early diagnosis of diseases (1, 2). Saliva, as a clinical tool, has many advantages over serum, because the collection and storage of saliva is easy and could be collected in sufficient amount for analysis. Besides, collecting saliva, as a non-invasive sampling techniques, causes less anxiety and discomfort in patients and provides a simple preparation of samples for long-term evaluation of one particular condition (13).
Since diabetes can lead to a Variety of other diseases including cardiovascular diseases and regarding the fact that peroxidase as an enzymatic antioxidant and Alfa amylase as a salivary enzyme, are two important variables associated with diabetes, and also considering the contradictory articles about their increase or decrease, it is very important to investigate both factors with regard to reducing the complications of diabetes (16).
Amylase is derived from the Greek word “amilone” which means starch. The main sources of Amylase in human is pancreas and salivary gland although it could be found in other tissues in small amounts. Alpha amylase enzyme belongs to Glycosyl hydrolase family of enzymes and is locally produced in the oral cavity by the salivary glands. Its biological function is to digest macromolecules such as carbohydrates and starch. Salivary alpha amylase is considered one of the most important salivary enzymes and is produced primarily in the parotid glands (16, 17).
For several years, due to low levels of serum amylase, disseminated pancreatic degradation was considered secondary to the advanced pancreatic diseases such as chronic pancreatitis. Recently, several clinical studies have shown that low levels of serum amylase is associated with the metabolic syndrome and diabetes. Impairment in serum amylase is associated with insulin-deficiency in patients with type 1 diabetes and with lower incidence with type 2 diabetes and pathogenesis of insulin resistance in obese animal models. Since serum amylase is from two types, salivary type and pancreatic type, it is necessary to differentiate between types of amylase for accurate clinical diagnosis. However, measurement of serum amylase is useful to determine the pathogenesis of many diseases (16, 17).
Diabetes not only causes defects in the metabolism of carbohydrates, but also associates with abnormal metabolism of fats and proteins as well. Thus, three key enzymes including lipase, protease and amylase are important in the treatment of diabetes because they help to digest all of these three groups of food (protein, fats and carbohydrates) (16, 17).
It is also much safer for Experts to collect saliva rather than blood test that pose health workers with the risk of contact with hepatitis viruses or HIV. Therefore, the diagnosis of disease based on the collection of saliva will be much more accessible and cheaper than other methods and entails minimal risk to the patient (1, 18). Diabetes increases the production of free radicals and is associated with disorders in antioxidant defenses and since few studies have studied the role of oxidative stress in diabetic pregnancy. Given the limited and contradictory studies in this area and in particular with regard to the importance of antioxidants in the prevention and treatment of many diseases and cancers, the aim of this study was to investigate amylase and peroxidase levels in the saliva of women with gestational diabetes in comparison with non-diabetic pregnant women.
Materials and Methods
In this cross-sectional study, the study population was selected After completing the consent form from patients referred to private clinics and Oral Medicine Department of Tabriz Dental medicine School and Al-Zahra Hospital in Tabriz in 1394 (2015). Being Diabetic and non-diabetic and having any systemic disease and drug consumption in pregnant women was confirmed by obstetricians using approved check list before sampling. According to the recommendation of American Diabetes Association (ADA) on routine screening for GDM during pregnancy, serum screening for GDM takes place between 26 to 30 weeks of pregnancy (19). Diagnosis of diabetes is made when FPG is 126 mg / 100 ml or more or when blood sugar, two hours after taking 75 -100 grams of glucose, is 200 mg / 100 ml or more in two repeated tests(3). All subjects were matched for age, Body Mass Index (BMI) and pregnancy with the control group.
Inclusion and exclusion criteria: 20-40 years old pregnant women having gestational diabetes and under diabetes treatment, lack of any systemic diseases in the last three months, no history of diabetes before, not using any other drug no-related with diabetes, no history of smoking and not using drugs that impairs the balance of antioxidant defense system. This information was obtained by studying medical records and clinical examination.
Saliva Collection method: Saliva sample collection was performed by Spitting method in which, the patient was allowed to collect saliva in the mouth and then transfer it into a special sterile plastic tubes (falcon). This is usually done every 60 seconds for 15-5 minutes. To get unstimulated samples, the person was asked to avoid eating or drinking or any other oral stimulation for 90 minutes before collecting the saliva. Using this method, approximately 5 mL of saliva was collected. Saliva was collected for all subjects between 8 to 9 am (fasting) in order to prevent day and night changes (20, 21). Collected Saliva was immediately put on ice and transported to the laboratory and centrifuged at 800g for 10 min at 4 ° C to separate squamous cells and debris. The samples were frozen at -80 ° C until preparation of all samples were (21, 22). When all Samples were collected, the necessary tests were carried out using purchased kits.
Measuring salivary peroxidase enzyme activity: Peroxidase was measured using the glycol method for which Bioris kit was used. In this method, glutathione is oxidized by hydro-peroxide, and then is converted to glutathione reduced form in the presence of glutathione reductase (GR).
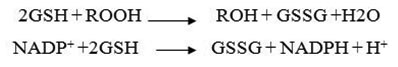
First of all, 50 ml saliva incubated with ml 1 dilution buffer for 5 minutes and then 1 ml Drubkin solution was added (dilution factor 1 to 41). Then, in a quartz cuvette (test cuvette) 10 μlit of diluted sample + 500 μlit reagent + 20 μlit Common hydrogen peroxide was added. In another cuvette other (blank cuvette) 10 μlit distilled water +500 μlit reagent + 20μlit Common hydro-peroxide is poured and both cuvettes were mixed separately and placed in spectrophotometer. After one minute, absorption of test and blank cuvettes was read. In 1 and 2 minutes later the absorption of both cuvettes was read again. To calculate the glutathione peroxidase activity, kit instructions was followed using the following formula:
U/L= 8412 × ΔA/min
The activities of blank and test cuvettes were calculated separately and then the activity of blank was subtracted from the activity of test. Peroxidase unit of measurement is unit / lit. After measuring the peroxidase activity in both groups (women with gestational diabetes and healthy women), the values were compared to each other.
Measuring alpha- amylase: After sending saliva samples to the laboratory, for producing a pure solution and separation of impurities, it was centrifuged at 3000 rpm for 3-5 minutes that resulted in the formation of two phases in Falcon tube. The lower contains impurities and the upper phase is pure saliva. In the next step, was sample dilution by 1:120 For this purpose, 800 μlit distilled water and 20 ml of pure saliva were shed in a test tube and 1:40 dilution was obtained. Then 1:30 dilution was obtained by shedding 200 ml of distilled water in a 1.5 micro tubes and 100 μlit of 1:40 dilution was added to it and then put the mixture in a shaker for 2-3 minutes. The sample finally was diluted 1:120. First, 1 μlit of Reagent 1 which was alpha amylase (Mg / l) was poured in falcon tube and placed in 37 ° C water bath for 5 minutes Along with the blank tube containing 1 μlit of Reagent 1 . Then to all tubes except the blank tube, 20 microliter sample with dilution of 1:120 was added and placed again in 37 ° C water bath for 2-3 minutes (16).
Finally, the data obtained from the study were analyzed by descriptive statistics (mean, standard deviation and frequency percentage) using SPSS.16 statistical analysis software. In This study p-value< 0.05 was considered statistically significant. For comparing values of the parameters, due to non-normal distribution of data in the case and control groups, Mann-Whitney test was used and always p <0.05 was considered significant. In addition to graphics, Kolmogorov-Smirnov test was also used to show normal distribution of parameters. It should be noted that in all patients before entering the study, written informed consent was obtained and patients were free to leave the study in case of unwillingness.
Results
From 90 cases, 31 cases were women with gestational diabetes and 59 others were non-diabetic pregnant women. Average peroxidase levels in the saliva of women with gestational diabetes was -54.55 ± 48.7 and in non-diabetic pregnant women was 8.77 ± 24.71. According to the data average, level of salivary peroxidase has decreased in women with gestational diabetes. However, comparison of salivary peroxidase levels in women with gestational diabetes and non-diabetic pregnant women with Mann-Whitney nonparametric test (with respect to non-normal data) showed that this difference was not significant (Figure A) (P-value = 0.69).
According to this study, the mean level of amylase in the saliva of women with gestational diabetes and non-diabetic pregnant women was 609387.1±132534.38 and 478559.85± 81000.41 respectively. According to the data average, the level of salivary amylase has increased in women with gestational diabetes. However, comparing these two levels with Mann-Whitney nonparametric test (with respect to non-normal data) showed that this difference was not significant (Figure Two) (P-value = 0.61).
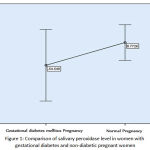 |
Figure 1: Comparison of salivary peroxidase level in women with gestational diabetes and non-diabetic pregnant women
|
 |
Figure 2: Comparison of alpha amylase in the saliva of women with gestational diabetes and non-diabetic pregnant women
|
Discussion
Gestational diabetes mellitus (GDM) is common during pregnancy and is associated with some complications for mother and fetus. Studies have shown that in societies that diabetes type II is more common, gestational diabetes is more common as well, but the risk and onset of the disease is quite variable (23).
This disease is one of the most common complications of pregnancy and the prevalence of almost 7 percent (more than two hundred thousand people per year) of all pregnancies have been reported in America (24). This metabolic disorder with increased severity of side effects, is an important risk factor for the mother and child in short term and long term. Children born to mothers with gestational diabetes are more vulnerable to obesity, glucose intolerance and diabetes in childhood and adolescence. Gestational diabetes increases the risk for the mother and fetus during pregnancy and in later stages of life (25, 26).
During normal pregnancy, oxidants, with their physiological functions, are very in the promotion and control cell fate and play a vital role in the natural development through cell signaling. Absence of increased antioxidant activity along with an increase in oxidants leads to oxidative stress. The level Oxidative stress is able to change the duration and severity of symptoms. Overproduction of ROS can damage the cell mass by acting on proteins, fats and DNA. In the case of systemic oxidative stress such as gestational diabetes, it can even cause biochemical disturbances in the fetus (27-30).
In the present study, 31 pregnant women with gestational diabetes and 59 non-diabetic pregnant women were studied in terms of the amount of hydrogen peroxidase antioxidant and salivary amylase. The mean level of peroxides in the saliva of women with gestational diabetes was -54.55 ± 48.7 and in non-diabetic pregnant women was 8.77±24.71. Additionally, the mean level of amylase in the saliva of women with gestational diabetes was 609387.1± 132534.38 and in non-diabetic pregnant women was 478559.85±81000.41.
Various studies have examined the salivary biomarkers which will be discussed and compared next. In a study that was conducted by Kornhauser and colleagues (31) about several biomarkers, including glutathione peroxidase, it was Concluded that the amount of this antioxidant is significantly lower in patients with type 2 diabetes mellitus so that The amount of this biomarker was reduced in the study group compared to the control group, although this reduction was not statistically significant. The findings of Chaudhari (32) and Kinalski (33) also showed that levels of peroxidase is significantly lower.
Unlike the above mentioned studies, Sharma and colleagues (9) examining the antioxidant levels in pregnant women suffering diabetes, reported that peroxidase was significantly higher in patients with gestational diabetes than normal pregnant women. In another study, Tosecu and colleagues also came to a similar result and stated that the amount of hydrogen peroxides in all women with diabetes, especially in people with type 2 diabetes is significantly higher (34).
These conflicting findings may be under the influence of various factors, including medication, duration of diabetes, gestational age, age, gender and other factors that could be assessed in the future research in similar samples.
However, it can be stated that oxidative stress management, coupled with rigorous control of blood sugar might be beneficial during both pre-pregnancy and pregnancy , But it is unclear if taking antioxidant supplements or eating a diet rich in antioxidants can improve the consequences of oxidative stress in children or not. Obviously more studies are required to fully understand the short-term and long-term benefits of reduced oxidative stress during gestational diabetes (30).
Indira et al (35) in 2015, studied the saliva of patients with type 2 diabetes and found that diabetes alters the composition of saliva. In this study, level of amylase was significantly lower in the case group rather than the control group. Similar results were also obtained by Yavuzyilmaz and colleagues in 1996 (36), Panchbhai and colleagues in 2010 (37), Nakajima et al in 2011 (38) and Mufeed and colleagues in 2014 (16), which attributed it to hormonal and metabolic changes in diabetic patients.
In another study in which Dawood compared the levels of alpha-amylase among diabetic patients(39), it was observed that there is an inverse relationship between type 1diabetes alpha-amylase level, this means that patients with type 1 diabetes have significantly lower amylase level compared to the control group. It was also indicated that with increasing disease duration, the more enzymes decreased. But unlike previous studies and in line with the results of the present study, evaluation of patients with type II diabetes (39) did not show any significant relationship with levels of alpha-amylase This attributable to a dire shortage of insulin in Type I diabetes which also affects Exocrine glands and its secretions like alpha-amylase as well. A study in India by Reddy and Shankarah (2011) (39) demonstrated a significant increase in levels of alpha-amylase in type II diabetes patients which is very similar to the present study in terms of higher levels of alpha-amylase in cases with the difference that the change was not significant in the present study.
Based on the above mentioned studies, it could be concluded that Because diabetes is a multifactorial disease and various factors such as race, diet, and environment play a role in its development, and As the antioxidant capacity of saliva can be affected by various factors such as the potential level of antioxidants, free radical production, the genetic background of individuals, food intake, smoking, physical activity, stress and hormones, as well as various studies on saliva indicating that oral cavity conditions including decays and periodontal disease are involved in the amount of antioxidants, Therefore, in future studies , patients should be selected from same local and systemic conditions. About alpha-amylase, it is also recommended that future studies concurrent with evaluate salivary and pancreatic alpha-amylase and check the conformity of their results.
Conclusion
According to data obtained from this study and their analysis, it could be concluded that in women with gestational diabetes compared with non-diabetic pregnant women, levels of alpha amylase increased and salivary peroxidase decreased, but none of these changes are significant.
Reference
- Greenberg M, Glick MS. Burket’s oral medicine. 11 ed: BC Decker Inc 2008:191-200,509-20.
- Harrison J FA, Braunwald E,Kasper DL,Houser S, Jameson JL,et al. Harrison’S principles of internal medicine. 17 ed: U. S. A; 2008:2152-80.
- Little J, Falace D, Miller C, NL R. Dental management of the medically compromised patient. 7 ed: Lhnda Duncan,U.S.A; 2008:219-39.
- Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto Y, Kobayashi M, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes research and clinical practice. 2002;55(1):65-85.
- Association AD. Diagnosis and classification of diabetes mellitus. Diabetes care. 2010;33(Supplement 1):62-9.
- Azimi-Nezhad M, Ghayour-Mobarhan M, Parizadeh M, Safarian M, Esmaeili H, Parizadeh S, et al. Prevalence of type 2 diabetes mellitus in Iran and its relationship with gender, urbanisation, education, marital status and occupation. Singapore medical journal. 2008;49(7):571.
- Janghorbani M, Enjezab B. Review of Epidemiology of Gestational Diabetes in Iran. Journal of Isfahan Medical School. 2010;28(110):510-52.
- Wang Y, Tan M, Huang Z, Sheng L, Ge Y, Zhang H, et al. Elemental contents in serum of pregnant women with gestational diabetes mellitus. Biological trace element research. 2002;88(2):113-8.
- Sharma J, Sharma A, Bahadur A, Vimala N, Satyam A, Mittal S. Oxidative stress markers and antioxidant levels in normal pregnancy and pre-eclampsia. International Journal of Gynecology & Obstetrics. 2006;94(1):23-7.
- Chen X, Scholl TO. Oxidative stress: changes in pregnancy and with gestational diabetes mellitus. Current diabetes reports. 2005;5(4):282-8.
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine. 2005;352(24):2477-86.
- Idogun E, Odiegwu M, Momoh S, Okonofua F. Effect of pregnancy on total antioxidant capacity in Nigerian women. Pakistan Journal of Medical Sciences. 2008;24(2):292.
- Whitehead T, Thorpe G, Maxwell S. Enhanced chemiluminescent assay for antioxidant capacity in biological fluids. Analytica Chimica Acta. 1992;266(2):265-77.
- Nourmohammadi I, Atashbasteh M, Mehdiizade A, Shabani M. Assessment of antioxidant enzyme levels in mild and severe preeclampsia and normal pregnancies. Med Scie J of Is Azad Univ Tehran. 2009;19(4):49-53.
- Casanueva E, Viteri FE. Iron and oxidative stress in pregnancy. The Journal of nutrition. 2003;133(5):1700S-8S.
- Mufeed Ewadh J, Juda TM, Abbas Ali Z, Mufeed Ewadh M. Evaluation of amylase activity in patients with type 2 daibetes mellitus. American Journal of BioScience. 2014;2(5):171-4.
- Neergheen-Bhujun V, Seenauth-Beesoo V, Joonas N, Aruoma OI, Bahorun T. Alterations in the antioxidant status of patients suffering from diabetes mellitus and associated cardiovascular complications. Archives of Medical and Biomedical Research. 2014;1(2):35-46.
- Lurba E, Gratacós E, Martı́n-Gallán P, Cabero L, Dominguez C. A comprehensive study of oxidative stress and antioxidant status in preeclampsia and normal pregnancy. Free Radical Biology and Medicine. 2004;37(4):557-70.
- Homko C, Sivan E, Chen X, Reece E, Boden G. Insulin Secretion during and after Pregnancy in Patients with Gestational Diabetes Mellitus 1. The Journal of Clinical Endocrinology & Metabolism. 2001;86(2):568-73.
- Shahidi S, Oshagh M, Gozin F, Salehi P, Danaei S. Accuracy of computerized automatic identification of cephalometric landmarks by a designed software. Dentomaxillofacial Radiology. 2013;42(1):20110187-.
- Bakhtiari S, Bigom Taheri J, Bakhshi M, Mortazavi H, Shah Hoseini A, Vahid Dastjerdi E, et al. Effect of vitamin C on salivary total antioxidant capacity in smokers. Iranian journal of pharmaceutical research : IJPR. 2012;11(4):1045-9.
- Leonardi R, Giordano D, Maiorana F, Spampinato C. Automatic cephalometric analysis: a systematic review. The Angle Orthodontist. 2008;78(1):145-51.
- Bellamy L, Casas J-P, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. The Lancet. 2009;373(9677):1773-9.
- Albrecht SS, Kuklina EV, Bansil P, Jamieson DJ, Whiteman MK, Kourtis AP, et al. Diabetes trends among delivery hospitalizations in the US, 1994–2004. Diabetes care. 2010;33(4):768-73.
- Turok K, Ratcliffe S, Baxley E. Management of Gestational Diabetes Mellitus. Am Fam Physician. 2003;68(9):1767-72.
- Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth Cohort Kaiser Permanente of Colorado GDM Screening Program. Diabetes care. 2005;28(3):579-84.
- Myatt L, Cui X. Oxidative stress in the placenta Histochem Cell Biol. NCBI Google Scholar. 2004; 122:369-82.
- Ryu S, Kohen R, Samuni A, Ornoy A. Nitroxide radicals protect cultured rat embryos and yolk sacs from diabetic‐induced damage. Birth Defects Research Part A: Clinical and Molecular Teratology. 2007;79(8):604-11.
- Dennery PA. Effects of oxidative stress on embryonic development. Birth Defects Research Part C: Embryo Today: Reviews. 2007;81(3):155-62.
- Lappas M, Hiden U, Desoye G, Froehlich J, Mouzon SH-d, Jawerbaum A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxidants & redox signaling. 2011;15(12):3061-100.
- Kornhauser C, Garcia-Ramirez JR, Wrobel K, Pérez-Luque E-L, Garay-Sevilla M-E, Wrobel K. Serum selenium and glutathione peroxidase concentrations in type 2 diabetes mellitus patients. primary care diabetes. 2008;2(2):81-5.
- Chaudhary L, Tandon O, Vaney N, Agarwal N. Lipid peroxidation and antioxidant enzymes in gestational diabetics. Indian journal of physiology and pharmacology. 2003;47:441-6.
- Kinalski M, Sledziewski A, Telejko B, Kowalska I, Kretowski A, Zarzycki W, et al. Lipid peroxidation, antioxidant defence and acid-base status in cord blood at birth: the influence of diabetes. Hormone and metabolic research= Hormon-und Stoffwechselforschung= Hormones et metabolisme. 2001;33(4):227-31.
- Toescu V, Nuttall S, Martin U, Nightingale P, Kendall M, Brydon P, et al. Changes in plasma lipids and markers of oxidative stress in normal pregnancy and pregnancies complicated by diabetes. Clinical Science. 2004;106(1):93-8.
- Indira M, Chandrashekar P, Kattappagari KK, Chandra LPK, Chitturi RT, BV RR. Evaluation of salivary glucose, amylase, and total protein in Type 2 diabetes mellitus patients. Indian Journal of Dental Research. 2015;26(3):271.
- Yavuzyilmaz E, Yumak Ö, Akdoǧanli T, Yamalik N, Özer N, Ersoy F, et al. The alterations of whole saliva constituents in patients with diabetes mellitus. Australian dental journal. 1996;41(3):193-7.
- Panchbhai AS, Degwekar SS, Bhowte RR. Estimation of salivary glucose, salivary amylase, salivary total protein and salivary flow rate in diabetics in India. Journal of oral science. 2010;52(3):359-68.
- Nakajima K, Nemoto T, Muneyuki T, Kakei M, Fuchigami H, Munakata H. Low serum amylase in association with metabolic syndrome and diabetes: A community-based study. Cardiovascular diabetology. 2011;10(1):34.
- Dawood MH. Comparison of serum alpha amylase level between type one and type two diabetes mellitus among Sudanese in Khartoum state in 2015: Sudan University of Science & Technology; 2015.







