Mansour Zabihzadeh1, 2,3, Mojtaba Hoseini Ghahfarokhi1,3, Sasan Razmjoo-Ghalaei,Sholeh Arvandi2 and Zohreh Mashayekhi1,*
1 Department of Medical Physics, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. 2 Departments of Clinical Oncology, Golestan Hospital, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. 3Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
DOI : https://dx.doi.org/10.13005/bpj/937
Abstract
There is a concern about dose perturbation due to high-Z metallic port of temporary tissue expander (TTE) for patients with breast reconstruction undergoing to the postmastectomy radiation therapy (PMRT). The aim of this study is exactly determination the value of dose perturbation due to the presence of metallic port of TTE. The BEAMnrc code was used to simulate of a 6 MV-Primus Siemens Linac and to calculate the dose due to emerge of magnetic port (McGhan Style 133 model) at different depths in water phantom. The present depth dose and profile curves were calculated. A dose enhancement about 15% at front of the port and a dose reduction of about 10% at 5 cm distance from the backward direction of the port were resulted. The dose reduction at the shadow region of the magnetic port of TTE is significant and must be considered to calculate of accurate dose distribution.
Keywords
Breast cancer; Dose perturbation; Postmastectomy radiation therapy; Magnetic port; Temporary tissue expander
Download this article as:| Copy the following to cite this article: Zabihzadeh M, Ghahfarokhi M. H, Ghalaei S. R, Arvandi S, Mashayekhi Z. Dose Perturbation due to the Magnetic Port of Tissue Braest Expander in Patient undergoing the Postmastectomy Radiation Therapy. Biomed Pharmacol J 2016;9(1) |
| Copy the following to cite this URL: Zabihzadeh M, Ghahfarokhi M. H, Ghalaei S. R, Arvandi S, Mashayekhi Z. Dose Perturbation due to the Magnetic Port of Tissue Braest Expander in Patient undergoing the Postmastectomy Radiation Therapy. Biomed Pharmacol J 2016;9(1). Available from: http://biomedpharmajournal.org/?p=6522 |
Introduction
It has been known that breast cancer patients have risk to recurrence of the disease. This locoregional recurrence occursgenerally in the postmastectomy chest wall and/or regional nodal basins, including the axillary, supraclavicularand internal mammary regions. Many breast cancer patients have tumors with size >5 cm and almost with four or more captured axillary lymph nodes1. There are complications in more than 50% patients who underwent postmastectomy breast reconstruction and need postoperative radiotherapy for local control and survival advantages2, 3.Breast cancer patients with surgical mastectomy are recommended to breast reconstructions to enhance the women’s body symmetry, the positive aesthetic and psychological results and to gain the good feeling4. Tissue expanders provide excellent symmetry for breast reconstruction and also make color and tissue appearance like to the natural skin.
To use breast implants it is necessary that the quantity of the patient’sskin be enough for the placement of final prosthesis. Placement of a temporarytissue expander (TTE) in the patient’s breastfollowed by inflating of silicone’s bag by saline solution cause the distension of skin and pectoral muscle so that create a space to fill by a permanent implant5. Many patientswho have received breast reconstruction by TTE insitu, benefit from radiotherapy at 4-8 weeks after mastectomy.The tissue expander port has a magnetic valve located inside a membranewhich is equipped with a magnetic disk to determine its location inside the patient’s body.This magnetic port is often made of high atomic number material and density which is located inside the irradiated area. Therefore,it has a potential todisturb thedelivered dose to target and can create obstacle to access the optimal radiotherapy treatment planning6-8. Krueger et al (2001)announced a 68% complication rate in patients who received radiotherapy with expander/implant breast reconstruction3. Asena et al (2015) reported a dose reduction of 20% and 56% for photon tangent treatment and electron boost field at the downstream region of the implant, respectively9.Moni et al (2004) measured dose perturbation around the magnetic valvefor 6 MV photon beam using films and thermo-luminescent dosimeters (TLD) and reported an increase in the dose up to 40% in front of metallic port and a decrease about 25% directly under it5.The results reported by Damast et al (2006) showed a maximum dose reduction of 22 and 16% at 2 cm away from magnetic port for the 6 and 15 MV beams, respectively1. Kuske et al (1991) established a mammary breast phantom with silicone implants and reported that the presence of the prosthesis during radiation led to no hot or cold spots10. A dose reduction of 30% and difference from 80% to 140% were estimated betweenTPS and MC calculated dosefor 9–22 MeV electrons11. From literatures, different and even in-contrast reported data of dose perturbations due to the metallic ports oftissue expanders are confusing andconcerning parameter to optimize a perfect radiation treatment plan.
There is a concern that photon attenuation effect of metallic port may be caused a decrease indelivered dose to the targeted tissue beyond the port in its shadow region that is known clinically as cold spot. The aim of this studyis exactly determination of the amount of dose perturbationdue to the presence of metallic port oftissue expander for6 MV-PMRTby Monte Carlo (MC) method.
Materials And Methods
MC calculations
The MC radiation transport code used in this study was BEAMnrc which is built on the EGSnrc Code 12. The geometric and materialdata of components located in the beampath was based on the Siemens Linac data-sheet provided by manufacturer. The different parts of Linac head such as target, primary collimator, flattening filter, monitor chamber, mirror and jaws were modeled with proper component modules (CMs). A schematic figure of the Linac model and magnetic port inside the water phantom are shown in figure 1.
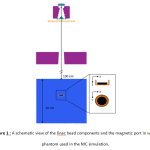 |
Figure 1 : A schematic view of the linac head components and the magnetic port in water phantom used in the MC simulation. |
It was assumed that the primary electron beam is parallel and has a Gaussian shape for energy distribution which is centered in 6.2 MeV with a 1 MeV full width at half maximum (FWHM). The lateral spread of electron fluence has also a Gaussiandistribution with 1 mmFWHM. A phase space file was defined under the lower jaw (X-jaw) used in all calculations. Directional Bremsstrahlung Splitting (DBS) was used with a splitting number of 1000 to improve the dose uncertainties. The electron and photon cut-off energies were set to 700 keV (ECUT) and 10 keV (PCUT), respectively. Electron range rejection was set to 2MeV (ESAVE). The threshold for secondary particle production was same as ECUT for charged particles and PCUT for photons.
The field size of 10×10 cm2on the surface of 30×30×30 cm3 water phantom and at a source to surface distance (SSD) of 100 cm was modeled. The PDD and the lateral dose profiles were scored in voxels with 0.2 cm resolution along the interested directions and compared with relatedmeasurements. In this study the McGhan Style 133 model of tissue expander (Inamed Aesthetics, Santa Barbara, CA) was used and simulated by EGS-imprz option from BEAMnrc code. It contains aninjection site as the Magna-Site, which is composed of a rare-earth magnet (samarium-cobalt, SmCo5) that is 20 mm in diameter and 2.7 mm thick encased in 0.4 mm thick titanium with a diameter of 35 mm and width 6.6 mm. The cylindrical voxels with diameter of 2 cm and height of 0.2 cm were used to score delivereddose in the front and back layers of the magnetic port located in different depths of phantom. The lateral dose profiles werescored in the concentric annuals with resolution of 2 mm at the front and back regionsof themagnetic port.
In the all calculation process dose uncertainties were <1% by choosing enough value for number of histories.
Measurements
In this study 6 MV photon beam from Primus-Siemens Linac was investigated. All dose measurements were performed by the calibrated Farmer type ionization chamber (0.125 cm3) with DOSE1 electrometer (FC65G, Scanditronix, Wellhofer, Germany) for field size of 10×10 cm2 at the source to surface distance (SSD) of 100 cm. The present depth dose (PDD) on the central beam axis and dose profile curves at depths of maximum dose, 5 and 10 cm weremeasured in 50 cm3 PTW-Blue water phantomand processed by RFAplus (Version 5.2, Scanditronix-Wellhofer, Germany). All dose measurements were followed by recommendations of IAEA, TRS-398protocol13. These data were used to validate of our MC model of 6 MV photon ofSiemens PrimusLinac.
Results
Validation of MC Linachead model
A good matching between the measured and simulated data was found for an incident electron beam with a mean energy of 6.2 MeV and a Gaussian energy spread with FWHM=1 MeV. The spatial FWHM was 1 mm in the bothcross-line and in-line directions. The agreement between the measurements and calculations (figure 2) were within 1% for depth dose profile beyond the depth of maximum dose and for the lateral profile inside the field.
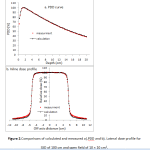 |
Figure 2.Comparisons of calculated and measured a).PDD and b). Lateral dose profile for SSD of 100 cm and open field of 10 × 10 cm2. |
The PDD was normalized to 100 at maximum dose depth. Lateral dose profile in depth of 10 cm was normalized to its central voxel value.
Dose perturbation due to the magnetic port of TTE
The maximum uncertainty of MC calculated data was better than 1%. The PDD curves with and without the magnetic port located in depth of 1.5 cm (depth of maximum dose), 10 and 20 cm were calculated. For better illustration, only the PDD curves from locating of port in depth of dmax and d=10 cm aredepicted in figure 3. It can be found that presence of port disturbs the dose at theupper depths close to the port as well as depths at shadow region behindit. A maximum dose enhancement factor, dose with port/dose without port, about 1.13,1.13 and 1.15at the upper surface of port were resulted for port located in depths of 1.5, 10 and 20 cm, respectively.In the shadow region of port, the beam attenuation effect of port caused amaximum PDDreduction ratioof 0.86, 0.81 and 0.87 at depth close to the port,respectively. The reductiontrend of PDD curve in the shadow region continues with increase in depths.
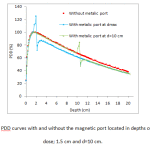 |
Figure 3. The PDD curves with and without the magnetic port located in depths of maximum dose; 1.5 cm and d=10 cm. |
The dose profiles at different distances from the magnetic port (5 cm from the lower surface of port, just under the port, just above the magnetic part of port and just above the port) were calculated when the magnetic port is located at different depths of dmax, 10 and 20 cm and compared with profiles from without port. Only dose profiles for positioning of port in depth of dmax (1.5 cm) were depicted in figure 4. The dose reduction of about 7.48, 5.98 and 10.23% on the beam central axis in the shadow regionwere calculated for 5 cm distance from the backward of the portlocatedin depth of 1.5, 10 and 20 cm, respectively. Additionally, the dose at the front face of magnet part of port increased about 14.8%, 14.83% and 14.51% for mentioned depths, respectively. The dose perturbations due to presence of port are limited to the lower surface in shadow region of the port.
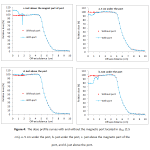 |
Figure 4: The dose profile curves with and without the magnetic port located in dmax (1.5 cm), a. 5 cm under the port, b. just under the port, c. just above the magnetic part of the port, and d. just above the port.
|
Discussion
In the current study, uncertainties of calculated doses were better than 1% in important regions. In order to validate the model, results indicated that after comparison of calculated and measured data, there is a good agreement between them (figure 2). As shown in figure 3, dose at regions above the port has risen up to a maximum value which is mainly occurred due to interaction of high atomic number components of the port with photons and subsequently production of backscattered electrons. The maximum of dose enhancement value was about 14% for three depths of port location. Range of backscattered electrons is short so that the dose enhancement rapidly falls by receding port surface and is restricted to near normal tissues about the port. A same effect was reported by Chatzigiannis et al (2011) who expressed that in the presence of a port in photon beam path an increasing dose about 9 and 12% is happened at 2 mm away from the magnet surface for 6 and 18 MV photons, respectively14. Moreover, Gossman et al (2009) have estimated that the ports containing titanium metal alloy can create up to 5 and 7% electron backscattering in 6 and 18 MV, respectively15. It should be noted that the results are in compliance with ours and the discrepancies between enhancement values may be due to variation of radiation beam arrangement, beam energy, port components, location of the port in the phantom, etc.
By evaluation of dose profiles at regions with different distances to the port, it was released that metal components of the port cause to more attenuation compared to the normal tissue and so the amount of beam transmission was decreased in the shadow region. After the falling of dose, forward scatter of electrons from the port and buildup of electrons cause to rise up the dose to a maximum value which is followed by a decreasing trend in dose with depth16. This perturbation can result in underdosage of tissues located at port shadow region. This effect was observed in other researches clearly. Thompson and Morgan (2005)reported an underdosage of the order of 10% using a tangential pair of parallel 6 MV opposed beams and up to 30% for a single 6 MV photon beam. In addition, Damast et al (2006) by film measurement and also Trombetta et al (2009) by MC calculation showed that in the presence of the port, an attenuation about 22 and 22% is happened for beam parallel to the port and 7 and 7% for beam perpendicular to the port, at 22 and 55 mm below the port end, respectively1, 17. Furthermore, another recent research found the value of underdosage of 6 MV photon due to existence of the port was 7% in the case of frontal irradiation of the chest wall. No significant changes were seen in their dose distributions for irradiation with an opposed pair of beams6.However, some researchers have declared that the presence of the port have no significant effect on dose distribution of organs at risk and soft tissues around the tissue expander. Moni et al (2014)have reported that it seems the metallic port in tissue expanders has a minor contribution to the high complication rate in patients who have tissue expander and undergoing radiation therapy5.Recently, study done by Liljegren et al (2014) showed the presence of breast implants during postmastectomy radiotherapy can’t result in increased doses to ipsilateral lung and heart as organs at risks18
Conclusion
ourresults shows that the magnetic port of TTE attenuates the absorbeddose of 6 MV beam. A maximum dose enhancement about 15% at front of the port and a dose reduction of about 10% at 5cm distance from the backward direction of the port were resulted. This dose reduction at shadow region of the port increase at closer clinical distances (< 5 cm) that must be considered to calculate of accurate dose distribution by TPS.Considering the challenging results about the perturbation effect of tissue expander port, definite conclusion entails further evaluation of the realistic clinical cases of the patients.
Acknowledgment
This study was funded by the research and technology deputy of Ahvaz Jundishapur University of Medical Sciences & Arvand international University of Medical Sciences, Ahvaz, Iran.
References
- Damast S, Beal K, Ballangrud Å, Losasso TJ, Cordeiro PG, Disa JJ, et al. Do metallic ports in tissue expanders affect postmastectomy radiation delivery? International Journal of Radiation Oncology* Biology* Physics.66:305-10 (2006).
- Anderson PR, Freedman G, Nicolaou N, Sharma N, Li T, Topham N, et al. Postmastectomy chest wall radiation to a temporary tissue expander or permanent breast implant—Is there a difference in complication rates? International Journal of Radiation Oncology* Biology* Physics.74:81-5 (2009).
- Krueger EA, Wilkins EG, Strawderman M, Cederna P, Goldfarb S, Vicini FA, et al. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. International Journal of Radiation Oncology* Biology* Physics.49:713-21 (2001).
- Girotto JA, Schreiber J, Nahabedian MY. Breast reconstruction in the elderly: preserving excellent quality of life. Annals of plastic surgery. 50:572-8 (2001).
- Moni J, Graves-Ditman M, Cederna P, Griffith K, Krueger EA, Fraass BA, et al. Dosimetry around metallic ports in tissue expanders in patients receiving postmastectomy radiation therapy: an ex vivo evaluation. Medical Dosimetry. 29:49-54 (2004).
- Trombetta DM, Cardoso SC, Alves VG, Facure A, Batista DV, da Silva AX. Evaluation of the Radiotherapy Treatment Planning in the Presence of a Magnetic Valve Tissue Expander. PloS one.10(2):e0117548 (2015).
- Al-Rahbi ZS, Al Mandhari Z, Ravichandran R, Al-Kindi F, Davis CA, Bhasi S, et al. Dosimetric comparison of intensity modulated radiotherapy isocentric field plans and field in field (FIF) forward plans in the treatment of breast cancer. Journal of medical physics.38:22 (2013).
- Wieslander E, Knoos T. Doseperturbation in the metallic implants: treatment planningsystem versus Monte Carlo simulations. Physics in Medical and Biology.48: 3295-305 (2003).
- Asena A, Kairn T, Crowe S, Trapp J. Establishing the impact of temporary tissue expanders on electron and photon beam dose distributions. Physica Medica.31:281-5 (2015).
- Kuske RR, Schuster R, Klein E, Young L, Perez CA, Fineberg B. Radiotherapy and breast reconstruction: clinical results and dosimetry. International Journal of Radiation Oncology* Biology* Physics.21:339-46 (1991).
- Srivastava SP, Cheng C-W, Andrews J, Das IJ. Dose perturbation due to metallic breast expander in electron and photon beam treatment of breast cancer. Journal of Radiation Oncology. 3:65-72 (2014).
- Rogers D, Faddegon B, Ding G, Ma CM, We J, Mackie T. BEAM: A Monte Carlo code to simulate radiotherapy treatment units. Medical physics.22:503-24 (1995).
- Andreo P, Burns DT, Hohlfeld K, Huq MS, Kanai T, Laitano F, et al. IAEA TRS-398: Absorbed dose determination in external beam radiotherapy: An International code of practice for dosimetry based on standards of absorbed dose to water. IAEA: International Atomic Energy Agency. 10A ed. Vienna2000. p. 46-80.
- Chatzigiannis C, Lymperopoulou G, Sandilos P, Dardoufas C, Yakoumakis E, Georgiou E, et al. Dose perturbation in the radiotherapy of breast cancer patients implanted with the Magna-Site: a Monte Carlo study. Journal of Applied Clinical Medical Physics.12: 3292 (2011).
- Gossman MS, Seuntjens JP, Serban MM, Lawson RC, Robertson MA, Christian KJ, et al. Dosimetric effects near implanted vascular access ports: an examination of external photon beam calculation. Journal of applied clinical medical physics.10:2886 (2009).
- Werner BL, Das IJ, Khan FM, Meigooni AS. Dose perturbations at interfaces in photon beams. Medical physics.14:585-95 (1987).
- Trombetta DM, Silva AXd, Cardoso SC, Facure A, da Rosa LA, editors. Attenuation of 6 MV beam in post-mastectomy breast radiotherapy due to metallic heterogeneity of tissue expanders. International Nuclear Atlantic Conference – INAC 2009, Rio de Janeiro,RJ, Brazil, September27 to October 2, 2009.
- Liljegren A, Unukovych D, Gagliardi G, Bjöhle J, Wickman M, Johansson H, et al. No difference in dose distribution in organs at risk in postmastectomy radiotherapy with or without breast implant reconstruction. Radiation Oncology.9:14(2014).








