Abdelaali Atmani and Lakhdar Sekhri*
Laboratoire de Dynamique Interaction et Réactivité des Systèmes, Process Engineering Department, Faculty of Applied Sciences, University Kasdi Merbah, Ouargla 30000, Algeria.
DOI : https://dx.doi.org/10.13005/bpj/904
Abstract
The antibacterial effect of some selected algerian plants like Cyndon dactylon (L) pers were evaluated on several bacterial strains : Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATTC 27853, Staphylococcus Coagulasse (ATTC 5118), Staphylococcus aureus ATCC 25923, Klebcsiella pneumonie and Enterococcus faecalis.The in vitro antibacterial activity was performed by agar disc diffusion method. The combination of Cyndon dactylon (L) pers with each of the standard antimicrobs E (Erythromycine), C (Chloramphenicol), CTX (Cefotaxime), AMX (Amoxicillin), CZN : (Cefazoline), CXN (Cefalexine) were most active and showed significant synergic effects. Moreover, Cyndon dactylon (L)/ other extracts of screened medicinal plants showed also high synergic effects. The results obtained in the present study suggest that Cyndon dactylon (L) pers can be used in treating deseases caused by the tested organisms. Further chemical and pharmacological investigations may be carried out to isolate and identify the chemical constituents in the selected plants responsible for the antimicrobial activity.
Keywords
Cyndon dactylon (L) pers; bacterial strains; synergic effect; extract
Download this article as:| Copy the following to cite this article: Atmani A, Sekhri L. A Comparative Study of the Antibacterial Activity of Cyndon Dactylon (L) Pers ; its Synergic Effect With Some of the Standard Antimicrobs and Extracts of Some Medicinal Plants. Biomed Pharmacol J 2016;9(1) |
| Copy the following to cite this URL: Atmani A, Sekhri L. A Comparative Study of the Antibacterial Activity of Cyndon Dactylon (L) Pers ; its Synergic Effect With Some of the Standard Antimicrobs and Extracts of Some Medicinal Plants. Biomed Pharmacol J 2015;9(1). Available from: http://biomedpharmajournal.org/?p=6465 |
Introduction
Since the 1940’s, chemists have developped all sorts of highly effective antibiotics (Sulfa drugs, penicillins, tetracyclines, and others that are effective) against bacterial and viral infections. In recent years there has been a flood of papers describing the synthesis of new antibacterial compounds and isolation of some natural products and study of their biological antimicrobial activities.1-6
Today there is an imperative necessity to find out new antibacterial compounds with various chemical structures and new mecanisms of action for new and re-emerging contagious syndoms.7 Therefore, researchers are increasingly turning their attention to folk medicine, looking for new leads to develop better drugs that are effective against bacterial infections and viral infections from infuenza and the common cold and even the more serious herbs infections and AIDS (Aquired Immune Defectious Syndrome). The viral infectious account for about 60% ilnesses, contrasted with about 15% for bacterial infections. However, due to the appearance of new strains of the bacteria and the weakness of chemotherapeutics and antibiotic resistance exhibited by pathogens has led to the screening of several medicinal plants for their potential antimicrobial activity.8-10
The medicinal plants have been used for ages as remedies for human diseases. Plant derived compounds are getting more and more interest owing to their adaptable applications. An increasing number of reports dealing with the assessment of antimicrobial effects of different extracts of various medicinal plants are frequently available.11-14
The aim of of this study was to evaluate the activity of extracts from 9 plants against several Gram-positive and Gram-negative bacterial strains in vitro.
Experimental
Materials and Methods
Fresh plant/plant parts : Cynodon dactylon (L) Pers, Malva parviflora, Mentha viridis Hort, Mentha pulegium L, Artemisia, Rosmarinus Officinalis were collected randomly from the mountain of Batna-Algeria in November 2013. The plants were deposited at Lab. Dynamique, Interaction et Réactivité des Systèmes, Department of Process engeneering, Faculty of Applied Sciences, University of Kasdi Merbah-Ouargla, Algeria. Fresh plant material was washed under running tap water, air dried under dark and then homoggenized to fine powder and stored in closed container away from light and moisture.
Preliminary Phytochemical Analysis
Qualitative Phytochemical analysis of the crude powder of th 9 plants collected was determined as follows :Resins,15 Coumarins,16 Terpenes and Steroids (Liebermann-Burchard reaction),17 Phenols,18 Tannins, Alkaloids, Saponins, Cardiac glycosides, Flavonoids.19
Extraction of plant material
The extracts were prepared by soaking 200g of the plant powder in a mixture of EtOH/H2O (70/30) evaporated under reduced pressure. The resulting extracts were diluted with distilled water and left overnight. The filtrates were subjected to extraction by various solvents with increasing polarity (petroleum ether, dichloromethane, ethyl acetate , and butanol). The organic phases were separated and evaporated. The rsulting residue was stored at 4°C.
Microorganisms
All bacterial standard strains : Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATTC 27853, Staphylococcus Coagulasse (ATTC 5118), Staphylococcus aureus ATCC 25923, Klebcsiella pneumonie, Enterococcus faecalis.
Preparation of the bacterial culture media
3.7 of muller Hilton agar was mixed with hot distilled water and autoclaved at 121°C and 2 atm for 15 minutes. After autoclavingit was allowed to cool to 45°C in a water bath. Then the medium was poured into sterillized petri dishes with a uniform depth of approximately 5 mm.20
Preparation of plant extract impregnated discs
Whatman N°1 filter paper was used to prepare discs of 6 mm in diameter. They were sterillized by autoclaving and then dried during the autoclaving cycle. The discs were then impregnated with extract of the plants.21
Disc diffusion method
Disc diffusion method for antimicrobial susceptibility test was carried out according to the standard method by Kirby-Bauer to assess the presence of antibecterial activities of plant extracts.22 A bacterial suspension adjusted to 0.5 McFarland standard (1.5×108 CFU/ml) was used to inoculate Mueller Hinton agar plates evenly using a sterile swab. The discs impregnated with the plant extracts were placed individually on the Mueller Hinton agar surface. The discs were spaced far enough to avoid both reflection waves from the edges of the petri disces and overlapping rings of inhibition. The plate was then incubated at 37°C for 18 hours in inverted position to look for zones of inhibition. Zones of inhibitions produced by the sensitive organisms were demarcated by a circular area of clearing around the plant extract impregnated discs. The diameter of the zone of inhibition through the center of the disc was measured to the nearest millimeter.
The rsulting residue of all extracts stored at 4°C were tested at a concentration of 10-3 g/ml and were prepared in DMSO.
Results and Discussion
The antibacterial activity of 9 species extract tested in vitro by agar disc difusion against 6 bacterial species. Table 1 summarizes the microbial growth inhibition of these extracts of the screened plant species.These extracts of nine plants showed significant bacterial activity against all the bacteria tested (Escherichia coli, Pseudomonas aeruginosa, Staphylococcus Coagulasse, Staphylococcus aureus, Klebcsiella pneumonie, Enterococcus faecalis). The maximum antibacterial activity was recorded against Staphylococus aureus and a maximum inhibition diameter of 16 mm with Cyndon dactylon (L) Pers, Nigella sativa and Camilia sinensis. Weak inhibition was recorded with the extract of medicinal plant Mentha viridis Hort against all the bacteria tested. As far as Rosmarinus officinalis is concerned, the maximum antibacterial activity was recorded against Staphylococus aureus and a maximum inhibition diameter of 14 mm. Similar results were obtained with Artemisia with a maximum inhibition diameter of 12 against Staphylococus Coagulasse.
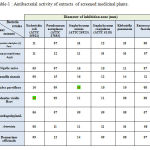 |
Table 1 : Antibacterial activity of extracts of screened medicinal plants. |
All the standard antimicrobs (C, CTX, CZN, CXN and AMX) exhibited a positive effect against Escherichia coli, Staphylococus and Klepsiela pneumonie. On the other hand E, CZN, CXN and AMX were ineffective against Pseudomonas and Staphylococus Coagulasse G. Table-2 summarized the microbial growth inhibition of these standard antimicrobics.
As far as the synergic effect is concerned the combination of Cyndon dactylon (L) pers with each of the standard antimicrobics, E, C, CTX, CZN and AMX were most active and showed the synergic effect. The maximum antibacterial activity was recorded against Esherichia coli, Pseudomonas aeruginosa, Staphylococcus coagulasse and Enterococcos faecale with a maximum synergic effect of ∆R = 02-04 mm, whereas Cyndon dactylon (L)/CXN Showed no synergic effect against Pseudomonas aeruginosa, and Staphylococus coagulasse. Table-3 summarizes the microbial growth inhibition of Cyndon dactylon (L)/standard antimicrobics.
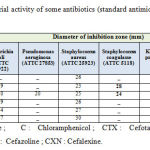 |
Table 2 : Antibacterial activity of some antibiotics (standard antimicrobs) |
The maximum antibacterial activity was recorded with Cyndon dactylon (L) Pers/ Nigella sativa and Cyndon dactylon (L) Pers/ Juncus maritimus asch against Pseudomonas aeruginosa and Enterococcos faecal with a maximum synergic effect of ∆R = 6 mm and 5 mm respectively. Table-4 summarizes the microbial growth inhibition of Cyndon dactylon (L)/ other extracts of screened medicinal plants.
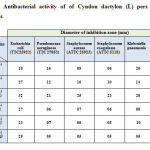 |
Table 3 : Antibacterial activity of of Cyndon dactylon (L) pers with some antibiotics |
In conclusion, Cyndon dactylon (L) pers with each of the standard antimicrobics, E, C, CTX, CZN and AMX were most active and showed the synergic effect against Esherichia coli, Pseudomonas aeruginosa, Staphylococcus coagulasse and Enterococcos faecale. Antibacterial activity of mixture of Cynodon dactylon (L) Pers and other extracts of screened medicinal plants possess a broad spectrum of activity against a panel of bacteria responsible for the moste common bacterial diseases. These promissssory extracts open the possibility of finding new clinically effective antibacterial compounds. Further chemical and pharmacological investigations may be carried out to isolate and identify the chemical constituents in the selected plants responsible for the antimicrobial activity.
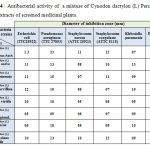 |
Table 4 : Antibacterial activity of a mixture of Cynodon dactylon (L) Pers and the other extracts of screened medicinal plants. |
Acknowledgment
The authors are thankful to Dr. Ouassila Mokhtari, Aris Hospital, Batna ; Dr. A. Khalil, Hakim Saadan Hospital, Biskra ; Dr. Ounissi and Mr. Berrah, Mohammed Bouthiaf Hospital, Ouargla 30000, Algeria for their assistance and providing the necessary facillities to carry out this work.
References
- Sekhri, L., Kadri, M. L., Chaoula, S. and Snigra, M., Biomedical & Pharmacology Journal, (2008), 1 (2) : 257.
- Babarbi, E., Haffas, M., Guerri, M. and Sekhri, L., Biomedical & Pharmacology Journal, (2010), 3 (2), 277.
- Thirupathaiah, Yoshihisha Takaishi and Venkateshwar Rao, G., Biomedical & Pharmacology Journal, (2008),1 (2) : 331.
- Jayashree, B. S., Sahu, A. R., Srinivasa Murthy, M. and Venugopala, K. N., Asian J. Chem., (2007), 19 (1) : 73.
- Singh, K., Gautam, J., Jain, A. K. and Mishra, P. K., Oriental Journal of Chemistry, (2007), 23 (2) : 641
- Suresh Kumar, S., Rajesh, R., and Siddiqui, Annes A. Oriental Journal of Chemistry, (2006), 22 (3) : 641.
- Rojas, R., Bustamante, B., Bauer, J. et al., Antimicrobial activity of selected Peruvian medicinal plants, J Ethnopharmacol, (2003), 88 : 199.
- Colombo, M. L., Bosisio, E., Pharmacological activities of Cheldonium majus L (Papaveraceae). Pharmacol Res., (1996), 33 : 127.
- Iwu, M.W., Ducan, A. R., and Okunji, C. O., New antimicrobials of plant origin. In ; Janick J. ed. Perspictives on new Crops and new Uses. Alexandria, VA ; ASHS Press, (1999), 457.
- Benkeblia, N., Antimicrobial activity of essential oil extracts of various onions (allium cepa) and garlic (Allium stivum). Lebensm-Wiss u-Technol (2004), 37 : 263.
- Babamer, Zohra Y., Sekhri, L., Al-Jaber, Hala I., Al-Qudah, Mahmoud A., and Abu Zarga, Musa H. Journal of asian Natural Products Research, (2012),1.
- Ashakkumar, R., and Ramaswamy, M. J., Chem Bio Physci Sec., (2013), 3(2) : 1279
- Bindu. J., Anjali. K., and Vibhor K.j., Asian Journal of Biochemical and Pharmaceutical research, (2011), 2(1) : 437.
- Mukundam, B., Shagufa, A., and Swarnamoni, D. A., Asian J Pharm Bio res., (2012), 2(3) : 183.
- Handa, Sukhder S., Decpak Mundkinajeddu, G. V. R., Joseph, J. and Gajendra, N., Indian Herbal Pharmacpoeia, Ajoint publication of Regional Research Laboratory and Indian Drug Manufacturers Association, India, Vol.II : (1999), 137-145.
- Geisman, T. A., Chemistry of Havonoids compounds. Macmillan Co. New York. (1962), 90-10.
- Al-Abid, M. R., Zurrzusamme mse turungder Abschla B membrane in Phoenix dactylifera. Wurzburg University. Wurzburg, F. R. of Germany, (1985),153-140.
- Winberg, J., Urinary tract infections in children curr. Opin. Inf. Dis., (1990), 3 : 55-6.
- Oguyemi AO. In : Sofowora A. ed. Prodeedings of a Conference on African Medicinal plants. Ife-Ife : Univ Ife, (1979), 20-22.
- Cappuccino, J. G., and Sherman, N., Micro-A Laboratory manual. Addison Wesley Longman Inc, ‘1999), 254-256.
- Swarnamoni, D., Mukundam, B., and Shagufa, A., Asian PharmClin Res., (2013), 6 (4) : 136-139.
- Bauer, A. W., Kirby, W. M. M., Sheris, J. S., and Turck, M., Amer J Clin. Pathol., (1966), 45 : 493-496.








