Daryoush Fatehi1, Farhad Naleini2, Mohammad Gharib Salehi2, Daryoush Afshari3, Sam Mirfendereski4, Mohammad Farzizadeh2 and Ayoob Rostamzadeh5*
1Department of Medical Physics, Faculty of Medicine, Shahrekord University of Medical Sciences, Shahrekord, Iran 2Department of Radiology, Faculty of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran. 3Department of Neurology, Faculty of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran. 4Department of Radiology, Faculty of Medicine, Shahrekord University of Medical Sciences, Shahrekord, Iran. 5Department of Anatomy and Neuroscience, Faculty of Medicine, Shahrekord University of Medical Sciences, Shahrekord, Iran
DOI : https://dx.doi.org/10.13005/bpj/840
Abstract
Imaging technology is an important part of the diagnosis and management of spinal trauma. However, many efforts have been made to develop new diagnostic biomarkers through advanced imaging techniques. Unfortunately, there is still no consensus for practical use of biomarkers in SCI patients The authors conducted an all-encompassing literature review and relevant images were included as examples. Spinal cord and soft-tissue injuries are best evaluated by magnetic resonance imaging (MRI). However, advanced MRI techniques provide researchers with a non-invasive approach that allows evaluation of physiological and biochemical condition of the spinal cord and the brain at cellular and molecular level. The advent of new rehabilitation and treatment strategies could demand more precise and advanced techniques to approach the pathophysiology and anatomy of the spinal cord, offering more accurate and non-invasive support to research and clinical follow up.
Keywords
Spinal trauma; Imaging techniques; Magnetic resonance imaging; Spinal cord injury; Imaging biomarker
Download this article as:| Copy the following to cite this article: Fatehi D, Naleini F, Salehi M. G, Afshari D, Mirfendereski S, Farzizadeh M, Rostamzadeh A. Traumatic Spinal Cord Injury; Theranostic Applications of Advanced MRI Techniques. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Fatehi D, Naleini F, Salehi M. G, Afshari D, Mirfendereski S, Farzizadeh M, Rostamzadeh A. Traumatic Spinal Cord Injury; Theranostic Applications of Advanced MRI Techniques. Biomed Pharmacol J 2015;8(2). Available from: http://biomedpharmajournal.org/?p=3161 |
Introduction
The spinal cord is located in the vertebral canal which starts from the medulla oblongata to the level of the second lumbar vertebrae. It is surrounded by cerebrospinal fluid (CSF) with an average length of about 45 cm in adults (1). Then, nerve roots from the lumbosacral segments descend in the vertebral canal in the form of cauda equina. The spinal cord divides into 31 segments, each of which has an emerging pair of spinal nerves that transmit information to and from the peripheral nervous system. The spinal cord is divided into; 8 cervical, 12 thoracic, 5 lumbar, 5 sacral and 1 coccygeal nerves (2). The butterfly-shaped gray matter is located in the inner part of the spinal cord and consists of mainly cell bodies, dendrites and axons, which are surrounded by the white matter; it is organized into three funiculi (anterior, lateral and posterior) in each half of the spinal cord (3). The descending and ascending axonal tracts running in these white matter funiculi convey sensory, motor, and autonomic information between the peripheral nervous system and cerebral regions. When damage occurs to the spinal cord, sensory input, movement of certain parts of the body, and involuntary functions such as breathing can be lost or greatly impacted. When temporary or permanent impairment occurs due to damage to the spinal cord, it is classified as a spinal cord injury (SCI) (4).
Spinal cord injury (SCI) is a major cause of morbidity due to related neurological conditions such as paraplegia and quadriplegia (5). Patients with inclusive spinal trauma and poor motor or sensory response may have little chance of improvement (6). However, patients with incomplete injury could have good prognosis if early diagnosis is made and treatment of fracture and/or hematomas immediately starts (7). It should be noted that the cause of fracture is different according to age and sex, for example young men mostly suffer from trauma; but elderly female’s fractures are due to osteoporosis (8). In addition to traffic accidents, there are other causes of trauma such as fall, sports or violence. Moreover, treatment of spinal injury may include stabilization and determination of the location and extent of SCI, most of the cases would undergo surgical operation followed by medications such as Methylprednisolone which could have potential side effects as well (9).
There are two general classes of SCI: Traumatic SCI (TSCI) and non-TSCI (nTSCI) (10). TSCI occurs when an external physical impact, such as the one resulting from a motor vehicle accident, a fall, or from violence, damages the spinal cord. TSCI as an acute, traumatic lesion of the spinal cord with varying degrees of motor and/or sensory deficit or paralysis results in motor, sensory and autonomic dysfunction could affect multiple body systems and may cause a life-long risk of various secondary complications (10). In addition, it has a considerable impact on the lives of injured individuals and their social surroundings. In this definition, injuries of the cauda equina, the most caudal part of the spinal cord, were also included. According to instructions from related SCI organizations, TSCI could include fractures, dislocations and contusions of the vertebral column (11).
Pathophysiology of TSCI
An acute tear, compression or distortion of the spinal cord by external forces could cause immediate death of the cells at the site of injury, resulting in a secondary injury that exacerbates the tissue damage through intricate mechanisms according to the neuropathological reviews(12). After a primary injury, immediate vascular damage may lead to hemorrhage, ischemic changes and edema. Moreover, an inflammatory response could arise with neutrophil and then macrophage infiltration (13). In fact, demyelination and death of oligodendrocytes may be associated with neuron necrosis. Macrophages are then responsible for removal of the damaged tissue, leading to the formation of cavities at the level of the lesion (14). Astrocytes form a glial scar, and collagenous fibrosis may also appear in the area of the injury. Some peripheral types of remyelination would occur through schwann cell activity, and varying amounts of spinal cord tissue may be replaced by schwannosis (15). Many destructive responses, such as excitotoxicity, the formation of free radicals and lipid peroxidation, would contribute to additional cell death during the acute and subacute phase following the initial trauma. As a consequence of local neuronal injury in SCI, secondary degeneration can also cause progressive and widespread changes in neural tracts at sites distant from the lesion over several years (16). Histologically, degeneration has been shown to be spread in both anterograde and retrograde directions after SCI in human. Furthermore, axonal changes, such as fragmentation and dying back, could characterize the acute phase of degeneration, as an active process triggered by rises in intracellular calcium levels (17). Moreover, the acute phase of degeneration could follow by slow and progressive myelin degradation, which can continue for a number of years after the initial trauma. Demyelination may also accompany by astrogliosis, which eventually leads to isotropic scarring in regions where degeneration has occurred (18). As an endpoint of neurodegeneration, the spinal cord becomes atrophic after SCI. Secondary degeneration has even been shown to reach cerebral regions, and there would be some histological evidences of the atrophy of corticospinal neurons in human after SCI (19). Furthermore, the grey matter volume and thickness of the sensorimotor cortex as well as the white matter volume in the corticospinal tract may be decreased after injury (20). Such structural changes were shown to be progressive during the first year after injury, although the rate of atrophy could decelerate in the area of the cranial CST after 6 to 12 months (21). These dynamic atrophic changes were associated with clinical scores, suggesting better clinical outcomes with low volume changes in the cerebral CST after injury (22).
nTSCI may occur when a pathological process other than external physical force damages the spinal cord such as motor neuron diseases, spondylotic myelopathies, infectious and inflammatory diseases, neoplastic diseases, vascular diseases, toxic, metabolic conditions, and congenital or developmental disorders (23) . Also, theranostic agents can be designed to
personalize treatment, and minimize damage to normal tissue (24).
Classification of SCI into traumatic and non-traumatic and by the severity of the injury is particularly important for the treatment, recovery and rehabilitation of the patients (25). When SCI occurs, it can be complete or incomplete, which, in turn, alters whether there is total or partial motor or sensory deficit (26). The neurological extent of the injury, or how much motor or sensory function is left intact after the SCI, is measured by a neurological examination. This examination could determine the level of impairment of the patient, reported as American Spinal Injury Association (ASIA) Impairment Scale grade (27).
Location of the injury can be diagnosed via imaging methods which can evaluate the spine integrity, or define the repercussion of the trauma on the diameters of the spinal canal and neural foramina (28). Radiography is usually used to make diagnosis; however, there are some instances of spinal damage due to fractures which are overlooked on radiography (29). Computed tomography (CT) or magnetic resonance imaging (MRI) can ensure diagnosis of spinal fractures in such cases as well as the time which the movement of spinal column is contraindicated due to the probability of neurological damage (Figures 1 and 2) (30). Moreover, MRI could play a crucial role in detection and evaluation of spinal trauma. In addition, subtle bone marrow, soft-tissue, and spinal cord abnormalities, might not be apparent in other imaging modalities, can also be readily detected by MRI (31). Early detection could lead to prompt and accurate diagnosis, expeditious management, and avoidance of unnecessary procedures, most of the time. Many advantages of MRI such as higher contrast resolution, multiplanar capability, choice of various pulse sequences, and absence of bony artifacts could make it possible to diagnose spinal trauma more accurately than other methods (32). In addition, other important findings about neural and extra neural injuries may require surgical interventions, for example, significant disc herniation and epidural hematomas can also be observed. In cases of spinal cord edema, contusion, hemorrhage and ischemia, MRI results may play a role as prognostic indicators too (33).
Why do we use advanced MRI techniques?
Severe central nervous system (CNS) injury is the most common cause of death and disability resulting from trauma. The important factors which could determine the prognosis of patients with CNS injury are the severity of the primary injury followed by secondary cord injury which may begin from the time of traumatic impact (34). Although mitigation strategies for the primary injury is not within the scope of this review; however, most treatments of SCI have been focused on accurate diagnosis of primary cord injury and the prevention of secondary, delayed cord injury. Therefore, many efforts have been made to develop new diagnostic biomarkers through advanced imaging techniques. Unfortunately, there is still no consensus for practical use of biomarkers in SCI patients (35). In fact, conventional medical imaging techniques are still unable to accurately predict patient’s prognosis after a SCI. Although patients with incomplete SCI are able to regain some loss of function, this can be disrupted by immediate physiological response that masks the true extent of the injury (25). It is important to fully understand the complexity of a SCI before being able to provide a way to recovery from the disability. Fortunately, physicians are able to offer some insight into the initial diagnosis of SCI; however, extent of injury is difficult to predict because of the initial inflammatory response. The extensive damage that occurs throughout entire axons following neuronal damage in the spinal cord is indeed not limited to the spinal cord (36).
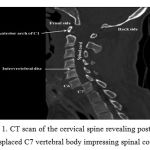 |
Figure 1: CT scan of the cervical spine revealing posteriorly displaced C7 vertebral body impressing spinal cord. |
A number of studies have demonstrated cortical changes including decreased number of neurons in the motor regions of the cortex as well as corticospinal tract as a result of SCI (37). On the other hand, currently advanced MRI techniques provide researchers with a non-invasive approach that allows evaluation of physiological and biochemical condition of the spinal cord and the brain at cellular and molecular level. Although conventional MRI is the imaging modality of choice for guiding management after injury and may appear to be of value in the prediction of prognosis, the provided information is mostly qualitative (38). In addition, in emerging clinical trials investigating experimental cell-based, pharmaceutical or rehabilitative interventions, there is a need for more specific and sensitive end-point measures that provide information about the condition of nerve fiber tracts using noninvasive techniques (39). To determine the efficacy of agents in a timely and economical manner, biomarkers are required that can be used as surrogate markers of patient`s outcome. Recent developments in quantitative neuroimaging of the spinal cord and brain have the potential to detect anatomical, physiological, biochemical, and molecular changes and functional reorganization following SCI (40). There is now a pressing need to validate the accuracy and sensitivity of these MRI biomarkers, in order to increase the understanding of underlying mechanisms of damage and consequent functional reorganization, to identify potential therapeutic targets, and to track potential treatment-induced changes (41).
Role of advanced MRI techniques in the evaluation of TSCI
Diffusion tensor imaging (DTI)
Diffusion or Brownian motion refers to the completely random displacement of molecules due to thermal energy. The diffusion coefficient, D, characterizes this thermal motion and is usually expressed in units of square millimeters per second (mm²/s) (42).This constant is dependent on the temperature, molecular weight and viscosity of the solution. When considering biological tissues, the apparent diffusion coefficient (ADC) is used as a diffusion constant to incorporate the effects of cellular structures and active processes within tissues on diffusion (39). If diffusion is equivalent in all directions, it is called isotropic, whereas the term diffusion anisotropy is used when diffusion is restricted in some directions. In an isotropic medium such as free water, diffusion can be represented as a sphere and characterized by a single scalar ADC that is equivalent in each direction (43). In some biological tissues, especially in the white matter of nervous tissue, the ADC depends on the direction in which it was measured. The ADC is higher in parallel with nerve fiber bundles than in the perpendicular direction, indicating anisotropic diffusion (39). This asymmetrical diffusion pattern, with different diffusion properties in different directions, can be modeled by a three-dimensional ellipsoid in which the orientation of the longest axis, which has the highest ADC, represents the local fiber orientation . Moreover, the concept of diffusion tensor could provide a simplified mathematical model to estimate microscopic diffusion properties, such as the magnitude, the degree of anisotropy and the principal orientation, by performing six or more diffusion-weighted measurements in independent directions (44).
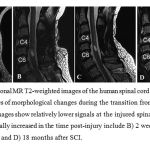 |
Figure 2 |
Diffusion tensor imaging (DTI) is a relatively new MRI modality with the advantageous feature of using diffusing water molecules to probe the tissue architecture (45). It can either non-invasively produce quantitative information about the direction and integrity of cerebral and spinal white matter tracts or reveal pathological changes in areas that may appear normal using conventional MRI. DTI has been developed from a technique known as diffusion-weighted imaging, which measures the attenuation of MR signals caused by diffusion, and was initially used for brain imaging. This method can give us unique quantitative information on the microstructural features of white matter in the CNS (45). Moreover, diffusion properties can be assessed using quantitative indices such as ADC, mean diffusivity (MD) and fractional anisotropy (FA) (46). The ADC could reflect the average diffusivity of water molecules in all directions; in fact, the stronger water molecules diffuse within a tissue, the larger the ADC is. However, the weaker water molecules diffuse within a tissue, the lower the ADC could be (47). Thus, tissues with high water mobility and few boundaries to water motion could have high ADC values, such as cerebrospinal fluid and vasogenic edema, whereas tissues with a high degree of complexity and boundaries to diffusion may have a relatively lower ADC, such as white matter fiber bundles and MD, representing the degree of diffusional motion of water molecules (regardless of direction), is measured in mm2/s. Furthermore, FA represents a rotationally invariant parameter, where 0 represents completely isotropic diffusion and 1 represents extremely limited diffusion in only one direction (48) (Figure 3).
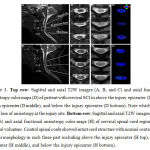 |
Figure 3: Top row: Sagittal and axial T2W images (A, B, and C) and axial fractional anisotropy color maps (D) of patient with cervical SCI in above the injury epicenter (D top), injury epicenter (D middle), and below the injury epicenter (D bottom). |
Since diffusion barriers within the tissue could change after injury, DTI could act as an invaluable tool to monitor histological changes to the spinal cord after a SCI. Although most researches have focused on diffusion measurements at or within a few segments adjacent to site of injury, DTI parameters of the spinal cord could be sensitive to histological changes occurring after a traumatic injury (40). However, in addition to the primary injury site, there might be a secondary damage to the spinal cord in regions distant from the (primary) injury site that may result in histological changes which could affect water diffusion, including degeneration of fiber tracts, ischemia, edema, and oxidative damage to the tissue membranes which may act as barriers to diffusion. This inflammatory response could result in impaired medullary circulation as well as changes into spinal cord structure, which may consequently develop necrosis that has been documented throughout the entire length of the spinal cord, up to the brainstem. These physical changes to the cellular microstructure, including axon number and volume changes, and alterations in intracellular and extracellular water balance, may be associated with changes in apparent diffusion, including both longitudinal diffusion (along the tracts) and transverse diffusion (across the spinal tracts) (49).
Moreover, axon morphometric parameters in various white matter tracts appear to underlie some differences in overall diffusion, which could be useful in the detection of injury to spinal white matter tracts. Thus, as a biomarker for injury severity, DTI measurements of diffusion throughout the spinal cord after an injury might be taken into account. In addition, mean diffusivity could significantly be decreased in regions away from the lesion site consistent with the secondary injury processes such as cytotoxic edema, chronic atrophy and axonal loss. Changes in mean diffusivity have also been reported in the high cervical spinal cord (rostral to an injury) in patients with chronic SCI (50). These observations could raise the question of whether changes in diffusivity in regions distant from the injury are correlated to injury severity. Furthermore, it is expected that severity of injury would change the secondary injury processes, as the extent of demyelination and remyelination could depend on the number of axons that are disrupted by the injury. One of the important applications of DTI could be the evaluation of SCI in animal models. In fact, DTI could demonstrate a significant decrease in anisotropy and increase in radial diffusivity at the level of injury and in the areas of the cord that are apparently normal on conventional T2-weighted images (51).
It should be noted that in hyperacute SCI (0-6 hours), diffusion measurements are able to distinguish SCI on the basis of severity. However, the unique feature of DTI may be its ability to detect changes in diffusion metrics at regions rostral and caudal to the lesion. In addition, a decrease in diffusivity remote from the lesion could be observed during recovery from SCI (50). These findings are possibly related to cytotoxic edema, axonal loss, or chronic atrophy. Interestingly, changes in DTI indexes away from the lesion could be correlated with injury severity, indicating that they may be used as surrogate markers of neural injury. Moreover, DTI of the spinal cord in human was initially inadequate due to the small area of the cord, susceptibility artifacts, and cardiac and respiratory motion artifacts (52).
However, adequate spatial resolution could still remain an important problem. It is difficult to visualize the individual funiculi on diffusion-weighted images, particularly in the lower thoracic cord. Moreover, the chronic phase of SCI shows wallerian degeneration, astroglial scar formation, and progressive cavitation of the cord with rostral-caudal spreading. Identifying specific changes in DTI metrics to characterize particular histological events during recovery from SCI remains a challenge too. Thus, the use of faster imaging techniques such as parallel imaging and single-shot echoplanar imaging and the use of cardiac pulse gating could help to reduce the artifacts. However, scan acquisition time is still a limitation for patients with acute SCI because these patients often cannot withstand additional scanning time in the MRI suite (53)
In general, the most important advantage of tensor imaging could be its ability to show changes in white matter tracts even in cases with normal routine imaging results In diffuse axonal injury, with normal routine CT scan and conventional MRI, there would be reduction in diffusion anisotropy after 24 h, suggesting axonal injury (54). It has also been well understood that signal changes seen on routine MRI may not be associated with neurological deficits and clinical findings, whereas DTI has been shown to be correlated with motor deficits. Moreover, in experimental studies, changes in axial diffusivity on DTI in the spinal cord injury as early as three hours after trauma were reported to be a potential predictor of long-term motor recovery, as DTI can detect early subclinical physiological changes in the cord (55).
Moreover, Wallerian degeneration above or below the injury level has also been presented on pathological examination. Buss and colleagues have found that there could be a sequential loss of myelin proteins during Wallerian degeneration after spinal cord injury which can be observed (several) years after injury. Similarly, tensor imaging in a rat model with spinal cord contusion has been shown to be evolve with changes in the ADC with recovery in ADC values with time, suggesting that recovery from spinal cord injury can be considered as a dynamic process coming after for years. In fact, stem cell therapy for spinal cord injury patients is being tried with the hope of achieving axonal regeneration and recovery. In the future, the use of stem cells in patients with spinal cord injury could probably prove to be a promising therapy. Thus, tensor imaging has the potential to noninvasively identify axonal regeneration after stem cell therapy (55, 56).
Magnetic resonance spectroscopy (MRS)
MR spectroscopy (synonymous terms for MRS, including Chemical Shift Imaging (CSI), Spectroscopic Imaging (SI) and Multivoxel Spectroscopy), enables the determination of metabolite concentrations in a predefined region of interest in neurological, psychiatric and metabolic disease, both noninvasively and in vivo. In contrast to other MR imaging techniques, such as diffusion-weighted imaging, blood oxygen level– dependent contrast imaging, or structural MR imaging methods, MR spectroscopy could provide information about the chemical microenvironment from atomic nuclei in a variety of functional groups. This allows detection of changes in the concentration of various metabolites for investigating healthy tissue and pathologic processes and exercise- or drug-induced changes. The most widely available MRS method, proton (1H; hydrogen) spectroscopy is an FDA-approved procedure in the US that can be ordered by clinicians for their patients if indicated. Other methods, such as phosphorous-31 (31P), carbon-13 (13C), or fluorine-19 (19F) MRS, have been successfully applied in humans. But with the ever-increasing importance of clinical MR imaging, these exotic and time-consuming applications have been push to the side and are only available at a few academic centers. In addition, 1H MRS does not require any additional hardware beyond what is already being used for MRI. Compared to DTI, the application of MRS to SCI may have more advantages. For instance, MRS can provide metabolic information about the cellular biochemistry and function of the neural structures within the cervical spinal cord. MRS also can be used to assay a series of pertinent biochemical markers, such as N-acetyl aspartate (NAA), lactate, choline (Cho), myo-inositol (Myo-I), glutamine-glutamate complex (Glx) and creatinine (Cr), with particular sensitivity to NAA and lactate (57).
Metabolites mentioned are including:
N-acetyl -aspartate (NAA)
produces a large resonance in a H2O suppressed 1H spectrum. The peak may contain up to 20% contributions from Aspartyl-glutamate (NAAG). NAA is generally associated with neurons and axons in the adult brain. It has received considerable interest in several disorders where there is neuron loss. However, its function is largely unknown (58).
The Choline (Cho) peak arises from a mixture of glycero-phosphoethanolamine and glycerophosphocholine. Both phospholipids are present in cellular membranes. This resonance can provide information about cell density and membrane integrity (or peroxidation) (59).
Myo-Inositol (MI) provides a relatively large resonance and is involved in osmotic regulation across the cellular membrane and could be specific for glial cells. The amino acid glycine may also contribute to the myo-inositol resonance. Scyllo-inositol, an isomer of inositol appears also as a singlet peak more downfield. Taurine resonates close to the scyllo-inositol region (59).
A glutamate and glutamine (Glu, Gln) peak can be detected in the human brain. Glutamine is a precursor of glutamate. Glutamate is involved in neurotransmission. Gamma-aminobutyric acid (GABA), also present but in lower concentrations during normal physiological conditions may overlap with the Glu, Gln resonance at 1.5T field strength (60).
The creatine (Cr), resonance originates from intracellular Cr and PCr. These are involved in the Creatine kinase reaction and consequently in energy metabolism (61).
Some studies have demonstrated that NAA can only be found in axons and neurons and may be considered an indicator of axonal integrity. Although little is known about specific mechanism, lactate is considered to play a central role in metabolic dysfunction after CNS injury and may be correlated to ischemia and mitochondrial dysfunction. In the early changes, the observation of a cervical stenosis patient without spinal cord signal changes showing slightly higher Myo-I and Glx compared to that of the control group suggested Myo-I as a potential early marker for spinal cord inflammation and early stage demyelination in cervical stenosis before neurological impairment (62). (Figure 4).
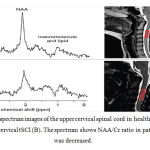 |
Figure 4: 1H-MR spectrum images of the upper cervical spinal cord in healthy volunteer (A) and patient with cervical tSCI (B). The spectrum shows NAA/Cr ratio in patients with tSCI was decreased. |
In the late changes, while the patient with spinal cord signal changes had a significantly higher Cho/Cr ratio than the control, the patient without spinal cord signal changes had no significant difference compared to the control. These results show that increased Cho levels appear later than the aforementioned cellular metabolic changes in SCI. MR Spectroscopy of the spinal cord is a promising tool for research and diagnosis because it can provide additional information complementary to other noninvasive imaging methods. Moreover, in neuroresearch MRS is definitely a revolutionary tool that will help understand the biochemistry of brain diseases. Metabolic information obtained from MR spectra is an emerging component in modern neurochemistry and standardized reproducible measurements (63).
However, the application of this promising technique to the spinal cord requires comprehensive expertise. The following methodological challenges, related specifically to the application of MR spectroscopy in the human spinal cord, have hindered its application in the clinical settings for many years:
- Strong susceptibility changes, related to the anatomic tissue heterogeneity (vertebral bodies, CSF, muscle tissue, and so forth) around the spinal cord, may lead to spatially periodic distortions of the static magnetic (B0) field along the spinal cord. This could distort the line shape of the spectra and reduces the signal to noise ratio (SNR).
- The small diameter of the spinal cord (approximately 1 cm in the cervical cord and even lower in the thoracic and lumbar cord6) limits the possible voxel size, resulting in a low SNR and potentially imprecise quantification of metabolite concentrations.
- The attainable SNR is also limited by the relatively great distance from the region of interest to the receive coils; for example, in the cervical spinal cord, the distance is approximately 5 cm in all directions and can reach >15 cm in the lower cord regions.
- The pulsatile flow of the CSF, caused by waves of arterial pressure transmitted via brain contractions due to the fixed total volume inside the skull to the CSF, can reduce the spectral quality (eg, impaired water suppression, distorted line shape and phase). The induced spinal cord motion does not seem to be critical because the maximum transversal displacement in the cervical region is approximately 0.6 mm in the anteroposterior direction.
- Subject motion is another severe problem because voxel dimensions are in the range of millimeters and are maximized to include as much spinal cord tissue as possible for increasing the SNR. Hence, patient motion in the range of millimeters (which is not unlikely considering the relatively long scanning times needed for spectroscopy) would already move the position of the voxels considerably out of the spinal cord. The result might be the contamination of the spectra with resonances of non-neural tissues, such as lipids, from nearby bone marrow or muscles and a further reduction of the SNR for the metabolites of interest.
- Anatomic variability, subject motion, or pathophysiologic changes of the electromagnetic tissue properties may affect transmit (B1) field and hence alter desired flip angles, localization profiles, and SNR.
- With conventional MR spectroscopy localization techniques such as the point-resolved spectroscopic sequence, the signal of different metabolites could stem from slightly different regions (chemical shift displacement artifact1). If one considers the small size of the spinal cord, the excitation volumes for some metabolites might be shifted partially out of the cord region. This has to be considered to minimize measurement errors with regards to relative metabolite concentrations (64).
Functional MRI (fMRI)
Functional MRI has quickly become the preferred technique for imaging normal structure activity, especially in the typically active tissues such as brain and nerve. The need for a noninvasive method of mapping neural function in the SC, such as fMRI, is related to the fact that clinical decisions about the best treatment course to take following trauma to the SC, or after the effects of diseases such as SCI, may require knowledge of how the cord is functioning according to the ASIA International Standards for Neurological Classification, which involves pin-prick tests across dermatomes and motor tests of various muscle groups. This method is a functional neuroimaging procedure using MRI technology which could measure brain activity by detecting associated changes in blood flow. This technique is based on the coupling of cerebral blood flow and activation of neurons. When an area of the brain is active, the flow of blood in the region also increases (65).
Two different approaches have been emerged for fMRI of the SC, one based on the established brain fMRI method employing blood oxygenation level-dependent (BOLD) contrast, and the other based on signal enhancement by extravascular water protons (SEEP). The physiological changes underlying the SEEP contrast mechanism are discussed in detail in a separate review in this issue and so are not discussed here. The BOLD contrast mechanism is well known from brain fMRI and also occurs in the SC. Each method has been shown to have specific benefits and drawbacks. SEEP contrast is based on detection of changes in tissue water content and can therefore be obtained with proton density-weighted spin-echo parameters which could provide high-quality images of the SC. Most spinal fMRI studies employing BOLD contrast to date has employed echo-planar spatial encoding to achieve the highest imaging speed. Previous studies indicated that there are some preserved white matter pathways spanning the injury level, and preserved sensory function below the injury, in agreement with clinical assessments based on the ASIA standard. Regardless of the stimulus or the health of the SC in question, spinal fMRI has been shown to be able to detect neuronal activity in the SC. Thus, without the need for invasive procedures or any changes to existing clinical MRI facilities, this method makes it possible to observe SC function in both patients and healthy subjects. Currently, fMRI is being developed to assess neurological function after surgery (66).
Conclusion
The extension and severity of the spinal trauma injury depends on several factors secondary not only to the features of the primary lesion but also to the treatment strategies and the degree of secondary lesions like demyelination and Wallerian degeneration. DTI as a feasible technique can detect Wallerian degeneration, which is not detected on routine or conventional imaging. Also, as documented in other studies, it may be correlated well with motor deficits and is a predictor of long-term motor recovery.
Currently, advanced MRI techniques could provide unique insight into the pathophysiology and microstructural alterations associated with spinal cord disorders (67). After SCI injury, the establishment of fast and efficient treatment strategy would depend on specific and detailed anatomical and pathological information that can be precisely provided by CT and MRI. Even in the more severe injuries, some pathways may be preserved and contribute to functional recovery, which can be achieved by regeneration, remyelination or by neural plasticity, sprouting undamaged pathways. Moreover, although it is well established that cortical reorganization occurs, it remains unclear whether reorganization involves time-dependent anatomical changes. Improving the understanding of the neuronal mechanisms which may subtend clinical recovery during the acute injury phase is the key to develop evidence-based rehabilitation therapy and the tracking of treatment-induced changes. Quantitative neuroimaging of the spinal cord is an advancing field that may increase the understanding of disease progression and facilitate the prediction and monitoring of individual patients following SCI. These new neuroimaging techniques exploit the physical water properties that could define the MR contrasts, which would provide multiple measures of underlying microstructural changes in myelin, iron deposits, and water. Ultimately, quantitative and qualitative advanced MRI techniques in longitudinal multicenter assessments in acute SCI are required to measure central effects and their impact on cortical reorganization as SCI patients recover. This should allow the identification of the most sensitive imaging biomarkers and their applicability in clinical trials. The advent of new rehabilitation and treatment strategies could demand more precise and advanced techniques to approach the pathophysiology and anatomy of the spinal cord, offering more accurate and non-invasive support to research and clinical follow up.
References:
- Heimer L. The human brain and spinal cord: functional neuroanatomy and dissection guide: Springer Science & Business Media; 2012.
- Snell RS. Clinical neuroanatomy: Lippincott Williams & Wilkins; 2010.
- Chen LM, Mishra A, Yang P-F, Wang F, Gore JC. Injury alters intrinsic functional connectivity within the primate spinal cord. Proceedings of the National Academy of Sciences. 2015;112(19):5991-6.
- Jia X, Kowalski RG, Sciubba DM, Geocadin RG. Critical care of traumatic spinal cord injury. Journal of intensive care medicine. 2013;28(1):12-23.
- Devivo M. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal cord. 2012;50(5):365-72.
- Barnes M, Good D. Traumatic spinal cord injury. Neurological Rehabilitation: Handbook of Clinical Neurology. 2013;110:411.
- Schwab ME, Strittmatter SM. Nogo limits neural plasticity and recovery from injury. Current opinion in neurobiology. 2014;27:53-60.
- Wang H, Zhang Y, Xiang Q, Wang X, Li C, Xiong H, et al. Epidemiology of traumatic spinal fractures: experience from medical university–affiliated hospitals in Chongqing, China, 2001–2010: Clinical article. Journal of neurosurgery: Spine. 2012;17(5):459-68.
- Morioka T, Honma T, Ogawa K. Incomplete Avulsion Fractures of the Scapular Spine Caused by Violent Muscle Contraction. The Keio journal of medicine. 2013;63(1):13-7.
- Fitzharris M, Cripps R, Lee B. Estimating the global incidence of traumatic spinal cord injury. Spinal cord. 2014;52(2):117-22.
- Kanwar R, Delasobera BE, Hudson K, Frohna W. Emergency Department Evaluation and Treatment of Cervical Spine Injuries. Emergency medicine clinics of North America. 2015;33(2):241-82.
- Vierck C, Baastrup C, Maersk‐Moller C, Roth M, Cannon R, Finnerup N, et al. A preclinical model of hyperalgesia following spinal stenosis/compression. European Journal of Pain. 2015.
- Losey P, Anthony DC. Impact of vasculature damage on the outcome of spinal cord injury: a novel collagenase-induced model may give new insights into the mechanisms involved. Neural regeneration research. 2014;9(20):1783.
- Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nature Reviews Neurology. 2012;8(11):647-56.
- Macaya D, Spector M. Injectable hydrogel materials for spinal cord regeneration: a review. Biomedical materials. 2012;7(1):012001.
- Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Wars). 2011;71(2):281-99.
- Lu P, Kadoya K, Tuszynski MH. Axonal growth and connectivity from neural stem cell grafts in models of spinal cord injury. Current opinion in neurobiology. 2014;27:103-9.
- Zhuo J, Xu S, Proctor JL, Mullins RJ, Simon JZ, Fiskum G, et al. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage. 2012;59(1):467-77.
- Nardone R, Höller Y, Brigo F, Seidl M, Christova M, Bergmann J, et al. Functional brain reorganization after spinal cord injury: systematic review of animal and human studies. Brain research. 2013;1504:58-73.
- Freund P, Weiskopf N, Ashburner J, Wolf K, Sutter R, Altmann DR, et al. MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: a prospective longitudinal study. The Lancet Neurology. 2013;12(9):873-81.
- Monti E, Pedoia V, Binaghi E, Balbi S. Study of the Prognostic Relevance of Longitudinal Brain Atrophy in Post-traumatic Diffuse Axonal Injury Using Graph-Based MRI Segmentation Techniques. Computational Modeling of Objects Presented in Images: Springer; 2014. p. 245-68.
- Belanger HG, Vanderploeg RD, Curtiss G, Warden DL. Recent neuroimaging techniques in mild traumatic brain injury. 2014.
- New P, Marshall R. International Spinal Cord Injury Data Sets for non-traumatic spinal cord injury. Spinal cord. 2014;52(2):123-32.
- Penet M-F, Chen Z, Kakkad S, Pomper MG, Bhujwalla ZM. Theranostic imaging of cancer. European journal of radiology. 2012;81:S124-S6.
- Wilson JR, Grossman RG, Frankowski RF, Kiss A, Davis AM, Kulkarni AV, et al. A clinical prediction model for long-term functional outcome after traumatic spinal cord injury based on acute clinical and imaging factors. Journal of neurotrauma. 2012;29(13):2263-71.
- Kirshblum S, Biering-Sorensen F, Betz R, Burns S, Donovan W, Graves D, et al. International standards for neurological classification of spinal cord injury: cases with classification challenges. The journal of spinal cord medicine. 2014;37(2):120-7.
- Shavelle RM, Paculdo DR, Tran LM, Strauss DJ, Brooks JC, DeVivo MJ. Mobility, continence, and life expectancy in persons with ASIA impairment scale grade D spinal cord injuries. American Journal of Physical Medicine & Rehabilitation. 2015;94(3):180-91.
- Stroman PW, Wheeler-Kingshott C, Bacon M, Schwab J, Bosma R, Brooks J, et al. The current state-of-the-art of spinal cord imaging: methods. Neuroimage. 2014;84:1070-81.
- Inaoka T, Ohashi K, El-Khoury GY, Singh H, Berbaum KS. Clinical role of radiography for thoracic spine fractures in daily practice in the MDCT era: a retrospective review of 255 trauma patients. Japanese journal of radiology. 2012;30(8):617-23.
- Gawor G, Biese K, Platts-Mills TF. Delay in spinal cord injury diagnosis due to sedation: A case report. The Journal of emergency medicine. 2012;43(6):e413-e8.
- Siemund R, Thurnher M, Sundgren P. How to image patients with spine pain. European journal of radiology. 2015;84(5):757-64.
- Forbes WSC. 10 Computed Tomography. Imaging Techniques in Orthopaedics. 2012:123.
- Kurland D, Hong C, Aarabi B, Gerzanich V, Simard JM. Hemorrhagic progression of a contusion after traumatic brain injury: a review. Journal of neurotrauma. 2012;29(1):19-31.
- Don ASA, Tsang CK, Kazdoba TM, D’Arcangelo G, Young W, Zheng XS. Targeting mTOR as a novel therapeutic strategy for traumatic CNS injuries. Drug discovery today. 2012;17(15):861-8.
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):263-9.
- Kaas JH, Merzenich MM, Killackey HP. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals. Annual review of neuroscience. 1983;6(1):325-56.
- Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nature neuroscience. 2012;15(4):528-36.
- Petersen E, Zimine I, Ho YL, Golay X. Non-invasive measurement of perfusion: a critical review of arterial spin labelling techniques. The British journal of radiology. 2014.
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of magnetic resonance. 2011;213(2):560-70.
- Freund P, Curt A, Friston K, Thompson A. Tracking changes following spinal cord injury insights from neuroimaging. The Neuroscientist. 2013;19(2):116-28.
- Farrar CT, Kamoun WS, Ley CD, Kim YR, Catana C, Kwon SJ, et al. Sensitivity of MRI tumor biomarkers to VEGFR inhibitor therapy in an orthotopic mouse glioma model. PloS one. 2011;6(3):e17228.
- Koskinen E. Traumatic Spinal Cord Injury-Current Epidemiology in Finland and Evaluation of Cervical Injury by Diffusion Tensor Imaging. 2015.
- Winston GP. The physical and biological basis of quantitative parameters derived from diffusion MRI. Quantitative imaging in medicine and surgery. 2012;2(4):254.
- Yamada I, Hikishima K, Miyasaka N, Kato K, Kojima K, Kawano T, et al. Gastric carcinoma: evaluation with diffusion-tensor MR imaging and tractography ex vivo. Magnetic resonance imaging. 2015.
- Walker L, Chang L-C, Nayak A, Irfanoglu MO, Botteron KN, McCracken J, et al. The diffusion tensor imaging (DTI) component of the NIH MRI study of normal brain development (PedsDTI). NeuroImage. 2016;124:1125-30.
- Sternberg E, Lipton M, Burns J. Utility of diffusion tensor imaging in evaluation of the peritumoral region in patients with primary and metastatic brain tumors. American Journal of Neuroradiology. 2014;35(3):439-44.
- Giandomenico V, Modlin IM, Pontén F, Nilsson M, Landegren U, Bergqvist J, et al. Improving the diagnosis and management of neuroendocrine tumors: utilizing new advances in biomarker and molecular imaging science. Neuroendocrinology. 2013;98(1):16-30.
- Pajevic S, Jones D. Statistical issues in diffusion tensor MRI. Diffusion MRI: Theory, Methods and Applications. 2011:331-53.
- Jirjis MB, Kurpad SN, Schmit BD. Ex vivo diffusion tensor imaging of spinal cord injury in rats of varying degrees of severity. Journal of neurotrauma. 2013;30(18):1577-86.
- Petersen JA, Wilm BJ, von Meyenburg J, Schubert M, Seifert B, Najafi Y, et al. Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. Journal of neurotrauma. 2012;29(8):1556-66.
- Grydeland H, Walhovd KB, Tamnes CK, Westlye LT, Fjell AM. Intracortical myelin links with performance variability across the human lifespan: results from T1-and T2-weighted MRI myelin mapping and diffusion tensor imaging. The Journal of Neuroscience. 2013;33(47):18618-30.
- Simon NG, Kliot M. Diffusion weighted MRI and tractography for evaluating peripheral nerve degeneration and regeneration. Neural regeneration research. 2014;9(24):2122.
- Vedantam A, Jirjis MB, Schmit BD, Wang MC, Ulmer JL, Kurpad SN. Characterization and limitations of diffusion tensor imaging metrics in the cervical spinal cord in neurologically intact subjects. Journal of Magnetic Resonance Imaging. 2013;38(4):861-7.
- Shenton M, Hamoda H, Schneiderman J, Bouix S, Pasternak O, Rathi Y, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain imaging and behavior. 2012;6(2):137-92.
- Kamble RB, Venkataramana NK, Naik AL, Rao SV. Diffusion tensor imaging in spinal cord injury. The Indian journal of radiology & imaging. 2011;21(3):221.
- Rostamzadeh A, Shabani A, Ahadi R, Farzizadeh M, Gharib A, Miraki S. Noninvasive Stem Cell Labeling Using USPIO Technique and their Detection with MRI. International Journal of Pediatrics. 2014;2(2.3):66-.
- Zhao L, Xiao H, Gao X, Wang R, Liu R, Zhang P. 1H-MRS Analysis for Prefrontal Cortex, Hippocampus and Thalamus of Adolescent Patients with Depression. Journal of Medical Imaging and Health Informatics. 2015;5(6):1229-32.
- Barker PB. Ultra-High Field MRSI (7T and Beyond). Functional Brain Tumor Imaging: Springer; 2014. p. 195-209.
- Tamraz JC, Outin C, Secca MF, Soussi B. MRI Principles of the Head, Skull Base and Spine: A Clinical Approach: Springer Science & Business Media; 2013.
- De Graaf RA. In vivo NMR spectroscopy: principles and techniques: John Wiley & Sons; 2013.
- DesRoches C-L, Patel J, Wang P, Minassian B, Salomons GS, Marshall CR, et al. Estimated carrier frequency of creatine transporter deficiency in females in the general population using functional characterization of novel missense variants in the SLC6A8 gene. Gene. 2015;565(2):187-91.
- Zhang C, Das SK, Yang D-J, Yang H-F. Application of magnetic resonance imaging in cervical spondylotic myelopathy. World journal of radiology. 2014;6(10):826.
- Wu X, Hanson LG, Skimminge A, Sorensen PS, Paulson OB, Mathiesen HK, et al. Cortical N-acetyl aspartate is a predictor of long-term clinical disability in multiple sclerosis. Neurological research. 2014;36(8):701-8.
- Hock A, Henning A, Boesiger P, Kollias S. 1H-MR spectroscopy in the human spinal cord. American Journal of Neuroradiology. 2013;34(9):1682-9.
- Devor A, Sakadžić S, Srinivasan VJ, Yaseen MA, Nizar K, Saisan PA, et al. Frontiers in optical imaging of cerebral blood flow and metabolism. Journal of Cerebral Blood Flow & Metabolism. 2012;32(7):1259-76.
- Smits M. Functional magnetic resonance imaging (fMRI) in brain tumour patients. European Association of NeuroOncology Magazine. 2012;2(3):123-8.
- Vedantam A, Jirjis MB, Schmit BD, Wang MC, Ulmer JL, Kurpad SN. Diffusion tensor imaging of the spinal cord: insights from animal and human studies. Neurosurgery. 2014;74(1):1-8.








