Manuscript accepted on :
Published online on: --
Plagiarism Check: Yes
Nazanin Shafeie¹, Abotorab Tabatabai Naini² and Hossein Kargar Jahromi3
¹Department of Nursing, Islamic Azad University, Firuzabad Branch, Firuzabad, Iran. ²Veterinary Department (Ph.D), Department of Clinical Studies, School of Veterinary Medicine, Shiraz University, Shiraz, Iran. 3Zoonoses Research Center, Jahrom University of Medical Sciences, Jahrom, Iran.
DOI : https://dx.doi.org/10.13005/bpj/850
Abstract
Lots of biological dressings and indigenous medicines have been reported to possess wound healing properties. Calendula officinalis (marigold) has many pharmacological properties. It is used for the treatment of skin disorders, pain and also as a bactericide, antiseptic and antiinflammatory (8). In this investigation, the effects of different concentrations of Calendula officinalis gel on histological and biomechanical changes of skin are studied. Seventy-five mature male rats were randomly divided into three groups (control, placebo, and treatment group). Under sterile conditions, a 2×2-cm piece of cervical skin for histopathological groups and a rectangular shape with a metal ruler from cervical to lumbar region for biomechanical groups, were excised in each animal. Treatment group received a daily topical application of 5%, 7%, and 10% C.officinalis gel, the placebo group received a daily topical application of the base gel, and the control group received no treatment during this experimental study. Fourteen and 21 days later, the rats were euthanized and biopsies were taken from the site of the initial incisions and samples were collected for histopathological and biomechanical investigation. Histopathological and biomechanical restorations in the group treated with 7% gel were significantly more than the placebo and control group. Upper and lower doses seem to be less effective, although the reasons for this remain unclear.
Keywords
calendula officinalis gel; Medicines; Histopathological and biomechanical restorations
Download this article as:| Copy the following to cite this article: Shafeie N, Naini A. T, Jahromi H. K. Comparison of Different Concentrations of Calendula Officinalis Gel on Cutaneous Wound Healing. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Shafeie N, Naini A. T, Jahromi H. K. Comparison of Different Concentrations of Calendula Officinalis Gel on Cutaneous Wound Healing. Biomed Pharmacol J 2015;8(2). Available from: http://biomedpharmajournal.org/?p=5849 |
Introduction
Tissue repair and wound healing are complex processes that involve inflammation,
granulation, and tissue remodeling. Skin damage can occur as a result of burns, cuts, abrasions and ulcers to varying degrees of severity. If not repaired, any breach of the skin may compromise its barrier function and expose the body’s tissues to microbial
infections and mechanical damage. The healing of cutaneous wounds requires complex interactions between the dermal and epidermal cells, the extracellular matrix (ECM), and the nervous and vascular components of the damaged and
surrounding skin (19, 36). Calendula officinalis, or pot marigold, is a common garden plant belonging to the Compositae family. Native to Southern Europe, Calendula grows up to 60 cm in height and produces large yellow or orange flowers (60). It is used topically as a natural anti-inflammatory medicine and for poorly healing
wounds and leg ulcers. The dosages cited are 2 mL of tincture diluted to 250–500 mL with water or 2–5 g of herb in 100 g of ointment (53). Other topical uses include treatment for 1st degree burns and scalds, bruises, boils, and rashes (21, 33).
Calendula officinalis has many pharmacological properties. It is used for the treatment of skin disorders, pain and also as a bactericide, antiseptic and anti-inflammatory. Butanolic fraction of Calendula officinalis possesses a significant free radical scavenging and antioxidant activity (7). Calendula officinalis flowers were believed to be useful in reducing inflammation, wound healing, and as an antiseptic, was used to treat various skin diseases, ranging from skin ulcerations to eczema. Internally Calendula officinalis has been used for stomach ulcers and inflammation. The flavonoids, found in high amounts in Calendula officinalis, are
responsible for its anti-inflammatory activity; triterpene saponins may also be important. Calendula officinalis also contains carotenoids(44).
Material and Methods
Preparation of the gel Dried calendula flower tops were used for extraction. Calendula flowers (263.7 g) were extracted with 1,200 cc ethyl alcohol 70%. For this,
the flowers were placed in an Erlenmeyer flask and the alcohol was added. This flask was stoppered and sealed and then placed in a dark room at room temperature and shaken every day for 1 week. The dark liquid was then decanted. A rotary device was used for the next step. This device can separate solvent faster and work with a vacuum. If the flask was left at room temperature to evaporate, it would have taken a long time. When the solvent separated, the rest of our mixture was ready and was called extract. This apparatus works with ice, and water always flows in it. Finally, the vacuum increased solvent separation. Ten days later, the oily extract of calendula flowers was available. To prepare the gel, at first the extract was dried with a freeze dryer and then the gel base was made. For this, 1 g carbopol was added to 95 cc distilled water (5% gel), 1 g to 93 cc distilled water (7% gel), and 1 g to 90 cc distilled water (10% gel); after 4 or 5 h, all of the carbopol powder dissolved and sodium hydroxide was added to make the gel base. Finally, 5 g (5% gel), 7 g (7% gel), and 10 g (10% gel) of extract was added to the gel base and used for all experiments. Experimental animals Seventy five white Sprague-Dawley male rats weighing between 180 to 220 g were divided into three groups that contained 25 rats, Two of them 50 rats for 14 , 21 days and histopathological testing, the other one 25 rats for 21 days and biomechanic testing (Table 1). Each one was housed individually in a separate standard cage and fed with normal mouse chow and water ad libitum. Their information was written on their cage. Temperature (25°C) and the ratio of daylight hours to non-daylight hours (12:12 h ratio of light to dark) were kept constant. The rats were divided into five groups as follows: group É control without any treatment (n=15), group ÉÉ gel base with placebo treatment (n=15), group ÉÉÉ 5% gel (n=15), group ÉV 7% gel (n=15), and group V 10% gel (n=15). Care was taken to avoid unnecessary stress to the animals throughout the experimental period. Surgical procedures The rats of all groups were anesthetized by injection of 0.8 cc ketamine (5%) and 0.2 cc xylazine intramuscularly in the hamstring muscles. Before making incisions, the dorsal aspect of the cervical or cervical to lumbar area was shaved and washed with a scrub solution of povidone–iodine. Under sterile conditions, a skin incision was made in a square shape 2×2 cm in the cervical region for histopathological groups, and a rectangular shape with a metal ruler from cervical to lumbar region for biomechanical groups, the long side of the defect was parallel to the vertebral column on both sides and the cranial border 1 cm caudal to the axis vertebrae. In all groups only the skin was removed. The same procedures were performed for both the experimental and control groups. The scalpel blade
used to create defects. After operation and treatment the area was left without a bandage. The duration of anesthesia was about 10 min for each rat. Treatment regimens In the control group, after making the incision on the cervical and cervical to lumbar region, no treatment was applied on the incision and the incisions remained intact. Fifteen rats in this group were followed 14 and 21 days later. All wounds during the 14 and 21 days were gently wiped with a sterile spatula without any gel. Daily observation was performed and any wound fluid or any evidence of infection or other abnormalities were noted. The rats from placebo group received the gel base in a thin, uniform layer on the wound daily for 14 and 21 days. In the treatment groups, the rats received 5%, 7%, and 10% calendula flower gel for 14 and 21 days. Sampling After 14 and 21 days post injury, the rats were euthanized by IV injection of 30 mg/kg thiopental sodium (nesdonal) via tail vein and sampling was done. Samples (2×2cm) for histopathological studies were taken from the edges of the wound, so that the sample contained both the lesion and its periphery including normal skin. Samples from the rest of animals (cervical to lumbar region) were collected for tensile testing. Biomechanical studies Skin from five animals in each group were dissected for biomechanical tensile testing and stored at ×20ÚC prior to testing. They were then thawed at room temperature before tensile testing. Throughout the testing period, the specimens were kept moist in saline soaked gauze. The specimens were mounted in a tensile testing machine controlled by a personal computer. The proximal section was clamped to the skin using a serrated interdigitating mechanical clamp. The distal section of the specimen was mounted in a clamp, so that a constant specimen length of 1.5cm was used in the test. Tensile testing to failure was performed by applying 10 kg force at a strain rate of 50 mm min•. The ultimate strength was recorded by the computer. Histopathological studies Skin samples were taken from both the wound and adjoining normal skin and fixed in 10% neutral buffered formalin. After fixation, the tissues were embedded in paraffin, and 5μm thickness sections were stained using hematoxylin and eosin. Five zones were examined from the sample morphometrically through a calibrated ocular on a Nikon light microscope (Nikon, Tokyo, Japan) at magnification of ×40, ×100 and×400. The criteria that were studied in the histopathological sections consisted of hemorrhage, mononuclear cell infiltration, reepithelialization of the epithelium, fibroblast content, present of fibrocytes, collagen content and neovascularization. Statistical analysis One-way analysis of variance and Duncan’s multiple range tests were used to
evaluate the differences of biomechanical parameters between the experimental, placebo and control groups. Differences were considered significant when pÂ0.05, using computer software SPSS version 11.5 for windows (SPSS, Chicago, IL, USA).
Results
Observations during daily care On the day postoperative care all of the wounds appeared clean. On the 2nd and 3rd days of treatment all the untreated wounds developed a moderately purulent-appearing exudate and inflammation. The treated wounds had no exudate during this time and over the 14 and 21 days of follow-up, but a slight inflammation was seen. On day 14 the wound size in all groups was reduced,
but it seemed the treated animals had smaller wound size. On day 21 post-injury, the wound size in the treated lesions was smaller than in the control one.
Biomechanical properties As is shown in Table 2 and figure 1 the ultimate tensile strengths of the 7% and 10% gel groups were significantly higher than those of the untreated group at 21 days post-injury (p<0.05).
This value for the 5% gel group was also higher than that of the control and placebo groups; however, the difference was not significant. The ultimate tensile strength of the 7% gel group was significantly greater than the 10% gel group respectively (p<0.05). The ultimate strength of the control and placebo group was similar and showed no significant difference.
Table 1: Test group arrangements
| 14 days | 21 days | 21 days | |
| Histopathological test | Histopathological test | Biomechanic test | |
| Control | 5 rats | 5 rats | 5 rats |
| Gel base | 5 rats | 5 rats | 5 rats |
| 5% gel | 5 rats | 5 rats | 5 rats |
| 7% gel | 5 rats | 5 rats | 5 rats |
| 10% gel | 5 rats | 5 rats | 5 rats |
Histopathological findings At microscopic level, however, the treated tissues at 14 days post-injury were not properly organized, but re-epithelialization was complete and the epithelium was thicker than the control and placebo group. Some of the treated lesions did not show any inflammatory cells and in others very few
macrophages and lymphocytes were present. No neutrophils were present at the treated lesions in this stage. Many fibroblasts and loops of new growing blood vessels were seen in the repaired area, while in the untreated animals the tissue was more unorganized and many inflammatory cells including macrophages and lymphocytes were present at the site of injury. Fewer fibroblasts and loops of new regenerating blood vessels were seen in the sections of the untreated animals. These changes were more apparent in the 7% gel group and the lesions showed more re-epithelialization
and positive changes than the other gel groups. The 10% and 5% gel groups showed no significant differences to each other, but there were differences to the control and placebo groups (Table 3). After 21 days of treatment, the thicknesses of the injured
treated skins decreased and were not significantly different from the control and placebo groups. At this stage, the treated tissues were more organized, the epithelium was thinner, and very few inflammatory cells were observed in the lesion. The number of the fibroblasts decreased and most of them changed to mature organized fibrocytes and were aligned along the reparative connective tissue. The number of blood vessels decreased but their calibre was greater than those of the 14 days postinjuries. In the untreated lesions, however, still some unorganized areas were present, and the macrophages and lymphocytes were observed. Fibroblasts were more numerous in comparison to those of the treated lesions. The blood vessels were more numerous and with a smaller calibre than those of the treated ones. At this stage the lesions in 7% gel groups showed more positive changes than the other gel groups. Actually the rate of healing in this group was much faster than the other groups (Table 4).
Table 2: Comparison of ultimate tensile strength of treated lesions with different Concentrations of calendula officinalis flower gel with control and placebo groups (p<0.05).
| Gel | Control | placebo | 5% gel | 7% gel | 10% gel |
| Ultimate tensile strength (kg) | 0.91±0.047 | 1.16±0.1 | 1.21±0.13 | 2.94±0.31* | 1.71±0.02* |
(*) means, groups which are significantly better.
Discussion
Wound healing is a dynamic, interactive process involving soluble mediators, blood cells, extracellular matrix, and parenchymal cells (55). Though the healing process takes place by itself and does not require much help, various risk factors such as infection and delay in healing have been mentioned to promote this process (48). Calendula officinalis flowers have anti-inflammatory effects (8), and so this can promote wound healing. Also, several components that affect the healing of burn
wounds (5), promote anti-tumor (62) and antiedematous activity (67) and result in the treatment of venous leg ulcers (12) are isolated from this flower. Adnan Hasanoglu et al., demonstrated that microionized flavonoid fractions can be used in the treatment of venous leg ulcers (20). The drugs tanus- improving effects on blood vessels, its regulation of lymphatic drainage and edema reducing effect may partly explain the healing of ulcers (1, 18). It has been shown in vitro and in vivo that micronized flavonoid fraction reduces the activation of the complement system (9). It has been thought that this mechanism of action constitutes the gel’s anti inflammatory effects and consequently, may contribute to the healing of ulcers. In a study conducted by Lonchampt et al., in vitro and in vivo effects of the drug on oxygen radicals were examined and it was proved to have a protective effect against active oxygen radicals (32). Recently, some studies demonstrated the favorable effects of micronized flavonoid fraction on microcirculation (20). The importance of regular microcirculation in wound healing is well-known. Delayed healing of wounds bound up tightly has been attributed to the development of ischemia due to impaired microcirculation (20). In the present study, it was observed that the use of calendula officinalis in wounds is beneficial, based on measurements and histopathological evaluations done at days 14 and 21 (p < 0.05). Its effectiveness in wounds is thought to be due to its contributions to the reduction of edema and the regulation of microcirculation in the environment. Adnan Hasanoglu et al., investigated whether systemic or topical usage of flavonoids fraction produces better and faster healing of
infected wounds in guinea pigs (20). The fact that it produced good results in infected wounds rather than in clean wounds has suggested that it might also have an antibacterial property, and as Calendula officinalis has much flavonoid in its structure, all of these properties can lead us to the results of this research.
Many plant species accumulate a wide range of triterpenic oleanolic acid (OL) derivatives, mainly glycosides (58). There is an array of medical products derived from some of these plants, which are used for the treatment of various diseases and
ailments (3). Marigold (Calendula officinalis), family Asteraceae, well known for its pharmaceutical and cosmetic use, contains two series of OL glycosides, i.e. glucosides (derivatives of 3-O-monoglucoside) and glucuronides (derivatives of 3-Omonoglucuronide) (24, 64). All of these compounds have biological activity: glucosides are mainly allelopathic and hemolytic agents, and glucuronides are potent fungistatics. It has also been shown that all glycosides of OL are synthesized in leaves (25, 59). So we used flowers but no allelopathic or hemolythic effects were seen.
Table 3: Cells and Vessels numbers in different groups after 14 days (significant difference between groups p<0.05).
|
Cells and Vessels number |
Gel groups | |||||
| 5% gel | 7% gel | 10% gel | Gel base | Control | ||
| Angiogenesis | 7±0.54 | 8±0.7* | 6±0.31 | 3±0.54 | 4.8±0.37 | |
| Lymphocyte | 16±1.78 | 12.2±1.49* | 20.2±2.7 | 21.4±2.6 | 22±1.51 | |
| Plasma cell | 0.8±0.2* | 0.8±0.2* | 1.6±0.24 | 1.6±0.24 | 2±0.00 | |
| Macrophage | 2.4±0.74* | 1.8±0.2* | 1.6±0.24 | 8.4±1.8 | 8.8±2.35 | |
| Fibroblast | 40±9.9 | 60.6±8.69* | 24.4±1.66 | 22.4±3.7 | 17.6±1.8 | |
| Fibrocyte | 9.4±0.74 | 14.2±2.8* | 3.4±0.67 | 2.8±.37 | 4±0.31 | |
(*) means, groups which are significantly better.
Nikiema et al., also showed that triterpenoids can promote keratinocyte proliferation due to their antiinflammatory effects (38). This study was designed to determine
whether or not the topical application of different concentrations of Calendula officinalis gel in two different days is an effective means of prompting wound healing. Significant positive effects on wound healing were detected by the three different
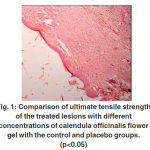 |
Figure 1: Comparison of ultimate tensile strength of the treated lesions with different concentrations of calendula officinalis flower gel with the control and placebo groups. (p<0.05) |
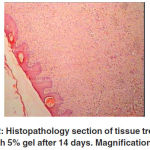 |
Figure 2: Histopathology section of tissue treated with 5% gel after 14 days. Magnification: 4 |
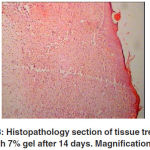 |
Figure 3: Histopathology section of tissue treated with 7% gel after 14 days. Magnification: 4 |
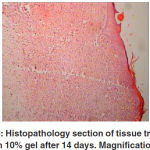 |
Figure 4: Histopathology section of tissue treated with 10% gel after 14 days. Magnification: 4 |
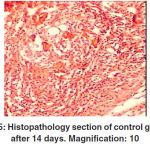 |
Figure 5: Histopathology section of control group after 14 days. Magnification: 10 |
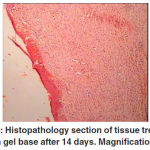 |
Figure 6: Histopathology section of tissue treated with gel base after 14 days. Magnification: 4 |
concentrations used. 7% concentration of this gel had significant positive effects on wound healing on days 14 and 21, and especially on the 14th day. This may be because of the covering of the wound after 21 days by scar tissue, so if we can use this flower flos extract parenterally it will be more effective. Walter B. Mors et al., showed that some of the components of this flower can be used parenterally to neutralize snake venom (37). TRAUMEEL is an anti-inflammatory, antiedematous, anti-exudative combination formulation of 12 botanical substances including calendula officinalis and 1 mineral substance. It is officially classified as a homeopathic combination remedy (56). This flower can be used parenterally for homeopathic therapy and the recommended maximum parenteral dose for large animals is 10ml per animal. In human homeopathy, lower concentrations and topical forms should be used, as it can be toxic for humans and the studies are not enough (11). Polyphenolic-polysaccharide compounds in the Asteraceae family including Calendula have chemical characterization and blood anticoagulant activity (41). All of these publications show that this product can, perhaps, be used parenterally.
Most of the other publications focus on the topical application of this gel, perhaps because of the probability of intoxication and/or it will be easier to use this product topically. Also, sterility and solubility are not important when we use a product externally, so because of the probability of intoxication and to find out which of the concentrations may be toxic, three different concentrations were used. The 7% gel was more effective than the others (Tables 3 and 4).
In Histopathological studies, lymphocytes and plasma cells were more apparent after 14 and 21 days when the 10% gel was used. These cells are the responses for allergy or inflammation, and so we can conclude that this concentration is irritant to cells. Rea et al., showed some of the components of the Calendula flower and Calendula flower extracts used in cosmetics and personal care products were not safe and could irritate the skin (53), but nothing was mentioned about the safe doses. Yoshikawa et al., also mentioned that some of the components have gastroprotective effects, but did not name any dose dependent effects (66). Fiume et al., indicated that acute toxicity studies in rats and mice showed the extract is relatively nontoxic (13). Animal tests showed at most minimal skin irritation, and no sensitization or phototoxicity. Minimal ocular irritation was seen with one formulation and no irritation with others. Six saponins isolated from Calendula officinalis flowers were not mutagenic in an Ames test, and a tea derived from this flower was not genotoxic in Drosophila melanogaster, the use of Calendula officinalis by oral route in humans needs a safety evaluation for this route of administration. Available data are insufficient to support the safety of Calendula officinalis extract by oral route. Lagarto et al., have studied the acute and subchronic oral toxicities of Calendula officinalis extract in male and female Wistar rats (30). They have dissolved a single acute Calendula officinalis extract dose of 2000 mg/ kg in distilled water and administered it by oral gavage for acute toxicity. Subchronic doses of 50, 250 and 1000 mg/kg/day were administered in drinking water. The major toxicological endpoints examined included animal body weight, water and food intake, selected tissue weights, and histopathological examinations. In addition, they examined blood elements: hematocrit, hemoglobin concentration, erythrocyte count, total and differential leukocyte count and blood clotting time. Blood chemistry: (glucose, total cholesterol, urea, total proteins, alkaline phosphatase, alanine aminotransferase (ALT) and aspartate aminotransferase (AST)) was examined too. In the acute study, no mortality and signs of toxicity were found. In the subchronic study, several of the blood elements were significantly affected in males and females after 90 days; hemoglobin, erythrocytes, leukocytes and blood clotting time. For blood chemistry parameters, ALT, AST and alkaline phosphatase were affected.
Histopathological examination of tissues showed slight abnormalities
in hepatic parenchyma that were consistent with the biochemical variations observed. These studies indicate that the acute and subchronic toxicities of Calendula Officinalis extract are low. Bashir et al., indicated that the crude extract of Calendula officinalis flowers contains both spasmolytic and spasmogenic constituents, exhibiting these effects through calcium channel blocking and cholinergic activities and so provided a scientific base for its traditional use in abdominal cramps and constipation (2), so perhaps if some of the useful components were extracted and worked to produce our gel, the results could be different. Flavonoids and terpenoids have protective properties; these are the two important components of this flower. Aqueous and aqueous-ethanol extracts of Calendula officinalis have fewer toxic effects (43), so because aqueous-ethanol extract was used, this flower gel has had fewer toxic effects. Hexaneextracted Calendula meal was tested in an acceptance trial with eighty 8–13 week old crossbred pigs to determine their response to diets containing 0, 2, 6, 10 or 20% Calendula meal. Performance parameters included feed intake, daily growth and post mortem histopathological examination of vital organs. Pigs fed a diet containing 2% calendula meal consumed significantly more feed than the ones fed a Calendula-free diet. As the Calendula meal content of the diet increased, feed intake tended to decrease. Post mortem examination of the vital organs showed statistically significant (P < 0.01) between-treatment differences in heart, kidney, thyroid and pancreas size expressed as percentage of bodyweight. The results of blood and blood serum
analyses for haematocrit, haemoglobin, oxygenated haemoglobin, aspartateaminotransferase, alanine-aminotransferase, lactate dehydrogenase, creatinine and zinc remained within the reference values for young pigs.
Although Calendula meal showed potential as a ration ingredient for young pigs, it is
not advised to include more than 10% of it in the diet (23). Compared with those of the untreated animals, the injured area of the treated group showed a gross decrease in the wound thickness at 14 days post-injury. This was relevant to the decrease in the inflammatory cells population at the site of the lesions, together with better maturation of the reparative cells and improved alignment of the fibroblasts, collagen fibers and blood vessels. Dovi et al., investigated whether neutrophils in diabetic mice are much higher in injured tissue than in healthy mice. They concluded that more neutrophils in the wound delay healing and, as can be seen, this gel can decrease neutrophils in the wound due to its anti-inflammatory effects, and can accelerate healing (10).
This supplement has prompted the proliferation of the fibroblasts, endothelial cells and epidermal cells. With oral administration of calendula officinalis slight histological changes were observed in the liver, exhibiting increased mononuclear cells in portal triads with the prevalence of lymphocytes. Activated Kupffer cells, inflammatory cells and focal necrosis, lymphocytes aggregation and focal hepatocellular atrophy were observed in females. Perivascular aggregation of lympho-reticular cells in the portal triad was also
observed (30).
These effects were also seen in the 10% gel group in this study. Histopathological results showed more aggregation of lymphocytes and plasma cells and decreased in fibroblast cells. Hexane and ethanolic extracts of Calendula officinalis stimulated the proliferation and migration of fibroblasts at low concentrations, e.g. 10 ìg/ml enhanced cell numbers by 64.35% and 70.53%, respectively (16). Regarding the mode of action of plant polysaccharides as wound-healing promoters, the so-called mucilagineous effect; the bioadhesion of polysaccharides to epithelia was demonstrated for some traditionally used herbs
such as Calendula officinalis and it was seen that the wounds of the treated groups healed considerably sooner than the untreated ones (54). It may be due to the bioadhesion of polysaccharides to epithelia. Mors et al., used in vitro scratch assays to examine the relative contribution of dermal fibroblasts in the wound repair process and they showed that this process will accelerate by fibroblast production (37). Experiments of Fonseca et al., demonstrated that small concentrations of marigold extracts are capable of stimulating the proliferation of mouse fibroblasts, with an approximately 27% increase of viability observed in cells treated with 11.25 and 15 mg/mL of this extract (14). These results support the observations of Matysik et al., who showed that marigold extract in small concentrations can stimulate the proliferation of human fibroblasts, but at high concentrations it can be toxic (35).
There is almost unanimous agreement that collagen performs a major role in restoring strength and remodeling scar tissue (34). Though the major function of collagen is to provide strength and integrity to the wound (63), it also plays a role in other functions such as hemeostasis, reepithelialization, cell–cell and cell–matrix interactions (6, 50). Not only collagen, but its degradative products also function in the healing process. For example, they are chemotactic to blood
monocytes (45).
Since inflammatory cells need to cross the extracellular matrix during skin repair following UV irradiation, it may be that the increase in gelatinases, which degrade most matrix extracellular molecules, may be beneficial for skin healing. Furthermore, the increase in both gelatinases (MMP-9 and MMP-2) induced by the ME in irradiated skin may be beneficial for procollagen synthesis, regulation of the inflammatory response and rearrangement of damaged skin. However, future studies addressing these possibilities are required (14). So the toxic effects of high concentrations could be due to the exorbitant production of these gelatinases, which
can destroy all pro-collagen fibers in the tissue. Radioemulsions containing trolamine were hoped to be “radioprotective” because they are macrophage cell stimulators that remove necrotic tissue, promote fibroblast proliferation, and, ex vivo, reduce vascular alterations, promote epithelial cell proliferation, and collagen secretion. The oil-inwater formulation also softens nonviable tissues. Although controlled studies show no clinical radioprotective effect over the control agents, many patients express satisfaction with these ointments and find them soothing. Pommier et al., compared the topical application of Calendula officinalis with Trolamine for the prevention of acute dermatitis during irradiation for breast cancer (44).
They concluded Calendula is highly effective for the prevention of acute dermatitis of grade 2 or higher and should be proposed for patients undergoing postoperative irradiation for breast cancer. We can conclude from these two studies that calendula can be used to treat radiation dermatitis because of prompting fibroblast production and collagen secretion. Preethi et al., investigated there was a significantly higher amount of hydroxyproline in the granuloma tissue excised from the burned skin of
drug treated animals compared to controls on both time periods (47). The hexosamine content was found to be decreased during thermal burn but was significantly higher in drug treated groups on both the 5th as well as the 10th day of burning. This could be due to increased synthesis or the decreased catabolism of collagen due to the presence of flavonoids in the extract which can produce artificial
cross linkage between collagen molecules. Also, there is a significant increase in the glutathione content in all animals in the treatment with the Calendula extract (47), so this flower can increase the antioxidant activity of injured tissues and accelerate their healing. The phytochemical constituents of Calendula officinalis include flavonoids like lupeol, quercetin, protocatechuic acid etc. and many alkaloids and triterpinoids (35).
Flavoxanthin, luteoxanthin, lycopene, auroxanthin, lutein, â-carotene etc. are the major carotenoids present in this flower (27). Most of these constituents are reported as free radical scavengers and enhance wound healing by producing artificial cross linkage (29). The production of free radicals at or around the wound bed may contribute to delays in wound healing through the destruction of lipids, proteins, collagen, proteoglycan, and hyaluronic acid (65). Agents that demonstrate significant antioxidant activity may, therefore, preserve viable tissue and facilitate wound healing. Given that the butanolic extract of Calendula demonstrates free radical scavenging activity against superoxide radicals and hydroxyl radicals in vitro in a dose dependent manner; that the same extract inhibits iron ascorbate-induced lipid peroxidation in rat liver microsomes (7), and that several organic solvent extracts of Calendula inhibit lipid peroxidation of liposomes in vitro (26),
it is argued that Calendula may facilitate wound healing via an important antioxidant effect. However clinical research is needed to validate these findings. The most important clinical endpoint in wound management is wound closure or 100% epithelialization. Given that wound closure is critically important, it is argued that any agent demonstrating significant wound-healing activity should be seriously considered in conventional practice. Calendula, for example, may facilitate wound healing by increasing both wound angiogenesis (17) and collagen, nucleoprotein, and
glycoprotein metabolism (4, 28), leading to improvements in both local circulation and granulation tissue formation (22). Several experimental studies lend support to these claims demonstrating that the daily application of a 1:10 alcoholic extract of Calendula or Calendula cream to paravertebral incisions in rats facilitates collagen maturation and epithelialization within 10 to 25 days (31). Preethi et al., investigated that oral and topical application of Calendula officinalis flower extract affects excision wounds made in rats (46). The parameters assessed with them were the days needed for re-epithelization and percentage of wound closure. The hydroxy proline and hexosamine content in the granuloma tissue of the wound was also measured. It was found that the percentage of wound closure was 90.0% in the extract-treated group, whereas the control group showed only 51.1% on the eighth day of wounding (p <0.01), and also the days needed for reepithelization in their research were 17.7 for the control animals; extract treatment at a dose of 20 or 100 mg/kg b.wt reduced the period to 14 and 13 days, respectively. They observed a significant increase in the hydroxy proline and hexosamine content in the extract-treated group compared with the untreated animals. This data indicate the potent
wound healing activity of Calendula officinalis extract. As seen, 20 mg/kg and 100mg/kg body weight of the extract have similar effects, so this research can confirm our findings as well. E.A. Torres Vargas et al., used calendula officinalis flower extract to produce hyperbranched polyglycerol electrospun nanofibers for wound dressing applications and observed that this flower can act on collagen deposition, and so, can affect wound healing (63). To summarize the results of this study, C. officinalis gel can affect collagen deposition and wound healing, but not all of its concentrations because there is both a dual and an opposite effect in different concentrations of this gel. Low concentrations have no effects and high concentrations have cytotoxic effects. All these criteria resulted in improved
biomechanical properties of the injured treated tissues compared with those of the untreated ones. We assume that reduction of the inflammatory cells and tissue oedema, in addition to improved maturation and alignment of the connective tissue
at the site of injury at 21 days post-injury resulted in an increase of tissue ultimate strength (40).
Increasing the tissue ultimate strength showed an enhancement in the collagen quantity and quality at the site of injury of the treated lesions (40). This criterion also showed that the inflammatory phase or possibly the beginning of the fibroplasia stage in the lesions of the treated group was shorter than those of the untreated animals. The presence of neutrophils and numerous macrophages and lymphocytes in the unorganized untreated tissues 14 days post injury showed that this drug may have been effective, mostly in the inflammatory phase or at the beginning of the fibroplasia.
Maximum load, which is the functionally most important parameter for characterizing healing wounds (49), reflects the ultimate tensile strength of the specimen, at which complete failure occurs rapidly, and load supporting ability of the tissue is substantially reduced (39). This happens as the intermolecular cross links are broken and the collagen fibrils pass each other or as collagen fibrils lose contact with basic substance (15). Higher ultimate strength of the treated tissues confirmed our Histopathological observations regarding more fibroblasts, fewer epithelial gaps and increased collagen synthesis in the treatment group. Calendula officinalis can precipitate wound healing by its anti-inflammatory effect. The faster course of inflammatory phase can induce the earlier initiation of collagen production (61), and this may explain the increase of wound tensile strength on the 21th day. However, there are also other mechanisms of the effect of this herb on wound healing. Antioxidant activities can improve the proliferation of cells into the injured area and so accelerate the synthesis of collagen (57). Other authors report the acceleration of the healing process by contraction of the wound area and increasing the wound tensile strength (52). Rane et al., refers to the increase in the amount of
hydroxylproline in granulation tissue in excisional wounds, indicating rapid collagen turnover and leading to accelerated healing (51).
From these findings it can be concluded that the application of calendula officinalis flower gel has negligible positive effects on the early stages of wound healing in experimentally induced cutaneous wound healing in rats. However, the 7% gel resulted in a better tissue alignment, collagen fibrils differentiation and maturation, while the 10% and 5% gel showed better results to the controland placebo groups, so the 7% gel is more effective, especially for the first fourteen days because the wound has less cover and this gel has fewer toxic effects.
References
- Armstrong DJ, Hienvu CN. Improvement in healing with aggressive edema reduction after debridement of foot infection in persons with diabetes. Arch Surg 2000; 135: 1405-1409.
- Bashir S, Janbaz KH, Jabeen Q, Gilani AH. Studies on spasmogenic and spasmolytic activities of Calendula officinalis flowers. Phytotherapy research (2006); 20: 906-910.
- Bisset NG, Wichtl M. Herbal Drugs and Phytopharmaceuticals. London: Medpharm Scientific Publishers, Stuttgard, CRC Press; (2001).
- Brown DJ, Dattner AM. Phytotherapeutic approaches to common dermatologic conditions. Arch Dermatol (1998); 134: 1401–1404.
- Chandran PK, Ramadasan K. Effect of Calendula officinalis flower extract on acute phase proteins, antioxidant defense mechanism and granuloma formation during thermal burns. J Clin Biochem Nutr (2008); 43: 58–64.
- Coban YK, Kalender AM. Treatment of gun-shot defect of the foot with bovine collagen matrix application. The Foot (2009); 19: 222-223.
- Cordova C, Siqueira I, Netto C, Yunes R, Volpato A, Filho VC, Curi-Pedrosa R, Creczynski-Pasa T. Protective properties of butanolic extract of the Calendula officinalis (marigold) against lipid peroxidation of rat liver microsomes and action as free radical scavenger. Redox report (2002); 7: 95-102.
- Della Loggia R, Tubaro A, Sosa S, Becker H, Saar S, Isaac O. The role of triterpenoids in the topical anti-inflammatory activity of Calendula officinalis flowers. Planta Med (1994); 60: 516–520
- Di Perri T, Auteri A. Action of S 5682 on the complement system (in vitro and in vivo study). Inter Anglo (1998); 7: 11-15.
- Dovi JV, Szpaderska AM, DiPietro LA. Neutrophil function in the healing wound: adding insult to injury? Thromb Haemost (2004); 92: 275-280.
- Dreyer LR. Homeopathic Formulations Useful for treating pain and/or inflammation. USA: Nutrition Research, Inc; (2007).
- Duran V, Matic M, Jovanovc M, Mimica N, Gajinov Z, Poljacki M, Boza P. Results of the clinical examination of an ointment with marigold (Calendula officinalis) extract in the treatment of venous leg ulcers. Int J Tissue React (2005); 27: 101-106.
- Fiume MZ. Final report on the safety assessment of Calendula officinalis extract and Calendula officinalis. International Journal of Toxicology (2001); 20: 13-20
- Fonseca YM, Catini CD, Vicentini F, Nomizo A, Gerlach RF, Fonseca MJ. Protective effect of Calendula officinalis extract against UVB- induced oxidative stress in skin: Evaluation of reduced glutathione levels and matrix metalloproteinase secretion. Journal of ethnopharmacology (2010); 127: 596-601.
- Freeman LJ, Hegreberg GA, Robinette JD, Kimbrell T. Biomechanical properties of skin and wounds in Ehlers-Danlos syndrome. Veterinary Surgery (1998); 18: 72-102.
- Fronza M, Heinzmann B, Hamburger M, Laufer S, Merfort I. Determination of the wound healing effect of calendula extracts using the scratch assay with 3T3 fibroblasts. Journal of ethnopharmacology (2009); 126: 463-467.
- Guba R. Wound healing, a pilot study using an essential oil-based cream to heal dermal wounds and ulcers. International Journal of Aromatherapy (1999); 9: 67-74
- Guilhou JJ. Fevrier F, Debure C, Dubeaux D, Gillet-Terver MN, Gulllot B, Levesque H, Marzin L, Mignot J, Ouvry P, Pillion G, Van Landuyt H, Zuccarelli F, Nicolaides AN. Benefit of a 2-month treatment with a micronized, purified flavonoidic fraction on venous ulcer healing. A randomized, double-blind, controlled versus placebo trial. Int J Microcirc Clin Exp (1997); 17: 21-26
- Harding KG, Morris HL, Patel GK. Healing chronic wounds. BMJ 2002; 324: 7330.
- Hasanoglu A, Ara C, Ozen S, Kali K, Senol M, Ertas E. Efficacy of micronized flavonoid fraction in healing of clean and infected wounds. International Journal of Angiology (2001); 10: 41-44.
- Henry T, Arnica Calendula Cantharis as external remedies in accidents. India: B. Jain publishers Pvt. Ltd; 1999
- Hey, B. The Illustrated Book of Herbs. England: New Holland Publishers; 1996.
- Hindle VA, Mathijssen-Kamman AA, Stockhofe N, Cone JW. The performance of young pigs fed different amounts of marigold (Calendula officinalis) meal; a pilot study. NJAS- Wageningen journal of life sciences (2002); 50: 83-94.
- Kasprzyk Z, Wojciechowski Z. The structure of triterpenic glycosides from the flowers of Calendula officinalis. Phytochemistry (1967); 6: 69-75
- Kasprzyk Z, Wojciechowski Z, Janiszowska W. Incorporation of 1-14C-acetate into glycosides of oleanolic acid in calendula officinalis. Phytochemistry (1970); 9: 561-564.
- Kaurinovic B, Popovic M, Cebovic T, Mimica-Dukic N. Effects of Calendula officinalis and Taraxacum officinale Weber (Asteraceae) extracts on the production of OH• radicals. Fresenius Environ. Bull (2003); 12: 250-253
- Kishimoto S, Maoka T, Sumitomo K, Ohmiya A. Analysiis of carotenoid composition in petals of Calendula (Calendula officinalis). Bioscience, Biotechnology, and Biochemistry (2005); 69: 2122-2128.
- Klouchek-Popova E, Popov A, Pavlova N, Krusteva S. Influence of the physiological regeneration and epithelialization using fractions isolated from Calendula officinalis. Acta Physiol Pharmacol Bulg (1982); 8: 63–67.
- Kuppast IJ, Nayak PV. Wound-healing activity of Cordia dichotoma Forst. f. Fruits. Nat. Pro. Rad (2006); 5: 99–102.
- Lagarto A, Bueno V, Guerra I, Valdes O, Vega Y, Torres L. Acute and subchronic oral toxicities of Calendula officinalis extract in wistar rats. Experimental and toxicologic pathology (2010); Article in press, corrected proof doi: 10.1016/j.etp.2010.02.015.
- Leach MJ, BN(Hons) ND, Matms RN. Calendula officinalis and Wound Healing: A systematic review. Wounds (2008); No: 8.
- Lonchampt M, Guardiola B, Sicot N, Bertrand M, Perdrix L, Duhautt J. Protective effect of a purified flavonoid fraction against reactive oxygen radicals. In vivo and in vitro study. Arzneimittelforschung (1989); 39: 882-885.
- Lueng AY, Foster S. Encyclopedia of Common Natural Ingredients used in Food Drugs and Cosmetics. New York: John Wiley & Sons; (1996).
- Madden JW, Erle E, Peacock JR. Studies on the biology of collagen during wound healing: Dynamic metabolism of scar collagen and remodeling of dermal wounds. Ann Surg (1971); 174: 511-520.
- Matysik G, W´ojciak-Kosior M, Paduch R. The influence of Calendulae officinalis flos extracts on cell and the chromatographic analysis of extracts. Journal of pharmaceutical and biomedical analysis (2005); 38: 285–292
- Metcalfe AD, Ferguson MWJ. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. Journal of the Royal Society Interface (2007); 4: 413-437
- Mors WB, Nascimento M, Ruppelt Pereira BM, Alvares Pereira N. Plant natural products active against snake bite – the molecular approach. Phytochemistry (2000); 55: 627-642.
- Nikie´ma JB, Vanhaelen-Fastre R, Vanhaelen M, Fontaine J, De Graef C, Heenen M. Effects of antiinflammatory triterpenes isolated from Leptadenia hastata Latex on keratinocyte proliferation. Phytotherapy Research (2001); 15: 131-134
- Nordin M, Frankle VH. Basic Biomechanics of the Musculoskeletal System. USA: Lippincott Williams and Wilkins; (2001).
- Oryan A, Khalafi-Nezhad A, Toloo N, Soltani Rad MN. Effects of 4-chloro-2,6-bis-(2-hydroxyl-benzyl)-phenol on Healing of Skin Wounds and Growth of Bacteria. J. Vet. Med (2007); 54: 585-591.
- Pawlaczyk I, Czerchawski L, Pilecki W, Lamer-Zarawska E, Gancarz R. Polyphenolic-polysaccharide compounds from selected medicinal plants of Asteraceae and Rosaceae families: Chemical characterization and blood anticoagulant activity. Carbohydrate polymers (2009); 77: 568-575.
- Pecking AP, Fevrier B, Wargon C, Pillion G. Efficacy of Daflon 500 mg in the treatment of lymphedema (secondary to conventional therapy of breast cancer). Angiology (1997); 48: 93-98.
- Pérez-Carreón JI, Cruz-Jiménez G, Licea-Vega JA, Arce Popoca E, Fattel Fazenda S, Villa-Treviño S. Genotoxic and anti-genotoxic properties of Calendula officinalis extracts in rat liver cell cultures treated with diethylnitrosamine. Toxicology in vitro (2002); 16: 253-258.
- Pommier P, Gomez F, Sunyach MP, Hombres A, Carrie C, Montbarbon X. Phase III randomized trial of Calendula officinalis compared with Trolamine for the prevention of acute dermatitis during irradiation for breast cancer. Journal of clinical oncology (2004); 22: 1447-1453.
- Postlethwaite AK, Kang AH. Collagen and collagen peptide-induced chemotaxis of human blood monocytes. J Exp Med (1976); 143: 1299–1307.
- Preethi K, Kuttan R. Wound healing activity of flower extract of Calendula officinalis. Journal of basic and clinical physiology and pharmacology (2009); 20: 0792-6855.
- Preethi K, Kutan C, Kutan R. Effect of Calendula officinalis flower extract on acute phase proteins, antioxidant defense mechanism and granuloma formation during thermal burns. J Clin Biochem Nutr (2008); 43: 58-64.
- Priya sK, Gnanamani A, Radhakrishnan N, Babu M. Healing potential of Datura alba on burn wounds in albino rats. J Ethnopharmacol (2002); 83: 193-199.
- Quirinia A, Viidik A. Freezing for postmortal storage influences the biomechanical properties of linear skin wounds. Journal of Biomechanics (1991); 24: 819-823
- Raghow R. The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J (1994); 8: 823–831.
- Rane MM, Mengi SA. Comparative effect of oral administration and topical application of alcoholic extract of Terminalia arjuna bark on incision and excision wound in rats. Fitoterapia (2003); 74: 553-558.
- Rashed AN, Afifi FU, Disi AM. Simple evaluation of the wound healing activity of a crude extract of Portulaca oleracea L. (growing in Jordan) in mus musculus JVI-1. J. Ethnopharmacology (2003); 88: 131-136.
- Re a TA, Mooney D, Antignac E, Dufour E, Bark I, Srinivasan V, Nohynek G. Application of the threshold of toxicological concern approach for the safety evaluation of calendula flower (Calendula officinalis) petals and extracts used in cosmetic and personal care products. Food and Chemical Toxicology (2009); 47: 1246-1254.
- Schmidgall J, Schnetz E, Hensel A. Evidence for bioadhesive effects of polysaccharides and polysaccharide-containing herbs in an ex vivo bioadhesion assay on buccal membranes. Planta Med (2000); 66: 48-53.
- Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med (1999); 341: 738–746.
- Stokman MA, Spijkervet FKL, Boezen HM, Schouten JP, Roodenburg JLN, de Veries EGE. Preventive Intervention Possibilities in Radiotherapy- and Chemotherapy-induced Oral Mucositis: Results of Meta-analyses. Journal of Dental Research (2006); 85: 690-700.
- Suguna L, Singh S, Sivakumar P, Sampath P, Chandrakasan G. Influence of Terminalia chebula on dermal wound healing in rats. PhytotherapyResearch (2002); 16: 227-231.
- Szakiel A, Grzelak A, Dudek P, Janiszowska W. Biosynthesis of oleanolic acid and its glycosides in calendula officinalis suspension culture. Plant Physiology and Biochemistry (2003); 41: 271-275.
- Szakiel A, Kasprzyk Z. Distribution of oleanolic acid glycosides in vacuoles and cell walls isolated from protoplasts and cells of Calendula officinalis leaves. Steroids (1989); 53: 501-511.
- Thomsen M. Phytotherapy Desk Reference. Denmark: Institute for Phytotheries; (2001).
- Toporcer T, Grendel T, Vidinsky B, Gal P, Sabo J, Hudak R. Mechanical properties of skin wounds after Atropa Belladonna application in rats. Journal of Metals, Materials and Minerals (2006); 16: 25-29.
- Ukiya M, Akihisa T, Yasukawa K, Tokoda H, Suzuki T, Kimura Y. Anti-inflammatory, anti-tumor-promoting and cytotoxic activities of constituents of marigold (Calendula officinalis) flowers. J Nat Prod (2006); 69: 1692–1696.
- Vargas T, do Vale Baracho NC, de Brito J, de Queiroz AAA. Hyperbranched polyglycerol electrospun nanofibers for wound dressing applications. Acta Biomaterialia (2010); 6: 1069-1078.
- Wojciechowski Z, Jelonkiewicz-Konador A, Tomaszewski M, Jankowski J, Kasprzyk Z. The structure of glycosides of oleanolic acid isolated from the roots of calendula officinalis. Phytochemistry (1971); 10: 1121-1124.
- Yeoh S. The influence of iron and free radicals on chronic leg ulceration. Primary Intention (2000); 8: 47–55.
- Yoshikawa M, Murakami T, Kishi A, Kageura T, Matsuda H. Medicinal Flowers. III. Marigold. (1): hypoglycemic, gastric emptying inhibitory, and gastroprotective principles and new oleanane-type triterpene oligoglycosides, calendasaponins A, B, C, and D, from Egyptian Calendula officinalis. Chemical and Pharmaceutical Bulletin (2001); 49: 863-870.
- Zitterl-Eglseer K, Sosa S, Jurenitsch J, Schubert-Zsilavecz M, Della Loggia R, Tubaro A, Bertoldi M, Franz C. Anti-oedematous activities of the main triterpendiol esters of marigold (Calendula officinalis). Journal of ethnopharmacology (1997); 57: 139-144.
(Visited 6,977 times, 3 visits today)







