Manuscript accepted on :
Published online on: 26-12-2015
Plagiarism Check: Yes
Palfilov Victor Fedorovich
Federal State Educational Institution of Higher Education
DOI : https://dx.doi.org/10.13005/bpj/534
Abstract
The process of fruit set of 10 apple varieties (Malusdomestica) having a different percentage of flavonols and water-soluble substances (WSS) in the pollen was studied. It was determined that the cultivars having higher content of flavonols and WSS in the pollen have a greater tendency to self-pollination; they are also better pollinators while cross-pollination. The percentage of phlorizin level in the styles of flower pistils of the same apple varieties was detected. It was also identified that varieties containing higher level of phlorizin in pistils have less pronounced tendency to self-pollination; their fruit set is weak while cross pollination. The conclusions about the possibility of using the flavonols percentage level in the pollen and phlorizin one in the styles of pistils as parameters for evaluation of the propensity to self-pollination and cross-fertility of apple varieties were done.
Keywords
Malusdomestica; Apple varieties; Flavonols content in pollen; Phlorizin content in the styles of pistils; Self-pollination (self-fertility);Cross-fertility (compatibility during cross-pollination)
Download this article as:| Copy the following to cite this article: Fedorovich P. V. Fruit Set of Apple Cultivars with Variouse Content of Flavonols in the Pollen and Phlorizin in the Styles of Flower Pestils. Biomed Pharmacol J 2014;7(2) |
| Copy the following to cite this URL: Fedorovich P. V. Fruit Set of Apple Cultivars with Variouse Content of Flavonols in the Pollen and Phlorizin in the Styles of Flower Pestils. Biomed Pharmacol J 2014;7(2). Available from: http://biomedpharmajournal.org/?p=3231 |
Introduction
The selection of varieties for industrial plantations or for hybridization during the selective processes requires a deep awareness of the relationship in between pollen and pistils of the same or different varieties of fruit cultivars. When forming the monosort garden plantations, the main task is to choose self-fertility cultivars (self-compatible ones, those, initiating the fruit set when pollinated with pollen of the same kind). The varieties prone to cross-fertility have permanently (no periodicity) abundant yields comparing to non-cross-fertility varieties [1]. It is necessary to select cross-fertile varieties for the non-monosort plantations in order to receive the fruits after their pollination. In order to increase the fertility of each cultivar, it is necessary to select compatible pollinator varieties.
However, hitherto the evaluation of self-fertility and selection of varieties pollinators was based on artificial pollination and self-pollination field experiments, but not on biochemical studies. The evaluation of compatibility based only on pollination and fruit set is not sufficiently reliable because it depends a lot on the environmental conditions [2].
The scientists think that the molecular genetic identification of allelic diversity of S-gene by DNA marker analysis, using the method of polymerase chain reaction (PCR), can help to identify the properties of self-compatibility during pollination (i.e. self-fertility). However, this method is not widely accessible, and the algorithm of different cultivars self-compatibility feature is not clear yet [3].
The cytological studies have revealed that the main reason of plants incompatibility (self-pollination and cross-pollination) is the trespassing of the program stage of fertilization, i.e. pollen tubes do not reach the ovary. In incompatible combinations the pollen of pollinator varieties usually either do not germinate on the stigma of pollinated varieties or, more commonly, the pollen tubes stop to grow in the styles of pistils, not reaching the ovules [4, 5]. But the reaction of the incompatibility between the pollen tube and the style can be reduced by pre-infiltration of the pistil which will be pollinated with some water [6] in order the pollen tubes reach the ovules. On the other hand the growth of pollen tubes depends on their own pollen elements. After washing with water, the pollen does not germinate neither on the stigma nor on the nutrient media [7,8]. It proves the fact that pollen tubes stop of growth can be defined by the substances contained in the reproductive structures of flowers. Concerning the nature of these substances, and the formation of self-sterility and cross-incompatibility during pollination, they were associated with the qualities of protein, enzyme, amino acid and nucleic acid composition of the pollen and pistil [9,10]. But this idea has not been widely acknowledged.
In the second half of the last century, the researchers have paid attention to the phenolic substances contained in significant quantities in the reproductive structures of plants. The fact of existence and overcoming incompatibilities with pollination fertilization under the influence of physiologically active phenolic substances contained in the pollen and pistil was established [11-15]. And the authors have concluded that the process of fertilization of plants can be influenced by the flavonoids in reproductive structures.
In [16] it is shown that the flavonols are the constant components of the flowering plants pollen. And depending on belonging to the botanical family, the pollen may preferably contain quercetin either kaempferol, or isorhamnetin types of flavonols. The Rosaceae family’s (the apple is a representative of this family) pollen flavonols is isorhamnetin.
Not only is the pollen of flowers saturated with flavonoids, but the pistils are as well. So the study of pistils of different propensity to self-fertility apple varieties revealed that the varieties prone to self-fertility contain a lower percentage of flavonoids phlorizin [17]. When in large concentration it can be the inhibitor of growth processes [26].
In order to improve the knowledge about the factors that affect the fertility of varieties while self-and cross-pollination, it is important to continue the studies about the impact of the quantitative content of flavonoids in the reproductive structures. It is necessary to search for new ways of express-breeding self-pollinated varieties and selection of the best pollinators for varietal plantations of fruit crops. The flavonoid content in the reproductive structures of the flowers can be determined by its extraction out of pollen and pistils.
The purpose of this research is to identify the role of pollen and pistils extracted flavonoids in determination of the propensity of apple varieties to self-fertility and cross-fertility.
Materials And Methods
The object of the research is the flowers of the following apple varieties: Spartan, Lobo, Orlik, Sinap orlowskiy, Green May, Martovskoe, Bessemyanka Michurinskaya, Melba, Antonovka ordinary and Uelsi, having different predilections to self-fertility and to cross-compatibility to each other. The trees of the mentioned varieties have been grafted onto the middle-sized clonal rootstock 54-118 and grew in industrial plantations of the I.V. Michurin All-Russian Research Institute of Genetics and Selection of fruit plants set in 2001.
The study included field experiments on pollination and laboratory analysis of reproductive structures of apple blossoms. Field experiments on pollination, flowers selection, pollen and pistils collection, as the objects of study, were carried out in accordance with common program and methods of fruit, berry, and nut crops studies [18]. The organization of experimental data obtaining and their subsequent mathematical processing was performed according to the rules adopted for analytical chemistry [19] and agricultural research [20]. The determination of water-soluble substances in the pollen of apple varieties was carried out by the mass (gravimetric) method [21]. The flavonols in the pollen were determined according to the photometric method [22]. Quantitative determination of phlorizin in the tissue pieces of apple flowers pistils was also carried out by the extracts photometry [23]. The program stage of fertilization, i.e. germination of pollen tubes in the pistil, was investigated by the fluorescence microscopy [24]. The tables represent the average results ± deviation from the averages obtained during the years of research. Significant figures are mostly rounded to integer values.
Results and Discussion
The comparison of apple varieties based on their content of water-soluble substances and flavonols in the pollen.
A comparison was carried out on samples of pollen kept at room temperature over calcium chloride to a constant weight [22]. The measurements showed that the pollen of different apple varieties can differ substantially in content of water-soluble substances (WSS), flavonols (flavonols glycosides) and consequently, in viability, i.e. germination on artificial medium (Figure 1).
 |
Figure 1: Pollen germination of the apple varieties during self-pollination, after 4 hours on 15% sucrose: 1 – Orlik (3% of flavonols); 2 – Melba (9% of flavonols); 3 – Lobo (11% of flavonols); 4 – Spartan (13% of flavonols) (2010, t0= 200C).
|
Figure 2 diagrammatically shows the results of these measurements. The abscissa indicates the apple varieties listed in ascending order of their pollen germination. The diagram shows that the germination of pollen is directly proportional to its content of water-soluble substances and flavonols. The content of water-soluble substances (WSS) and flavonols, in particular pollen varieties during the different years was in the typical for this cultivar range. It proves that the content of WSS and flavonols in the pollen is a variety feature, i.e. it is genetically determined. So these indicators can be used in the preparation of pomology characteristics of apple varieties. Apple varieties can differ substantially in content of water-soluble substances and flavonols in the pollen; particularly significant differences are in the content of flavonols in the pollen (figure 2). The utility of flavonols’ content for the pollen is that they are able to polymerize, i.e. to form “polyhydroxylated and polyethoxylated compounds.” This property is considered to be an important condition for the formation of pollen tubes by pollen [25]. For the growth of pollen tubes in the apple pistils, the ability of flavonols to activate the oxidation of other more recovered flavonoids, such as dihydrochalcone – phlorizin [26] contained in pistil apple is very important.
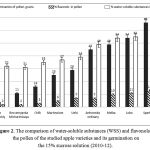 |
Figure 2: The comparison of water-soluble substances (WSS) and flavonols in the pollen of the studied apple varieties and its germination on the 15% sucrose solution (2010-12).
|
Flavonols, being the activators of phlorizin oxidation, contribute to its participation in the formation of polymer films [26] presumably needed when building the walls of the pollen tubes. The main flavonol of Rosaceae family is isorhamnetin [27]. Isorhamnetin is also needed as a donor of methyl (-CH3) group required during the final stages of pectin biosynthesis; pectin is one of the elements of growing pollen tube. Methyl groups are located on the outer side of the shell. If pectin is getting demethyled, it becomes less plastic. As a result, the germination of pollen tube is getting more difficult [28].
The comparison of apple varieties based on their content of phlorizin in the styles of flower pistils.
Apple pistils contain phlorizin flavonoids [17]. In the scientific literature this substance can be also called phloridzin. However, its more correct name is phlorizin (phlorhizin) [29]. The analyses of pistil styles parts indicate that phlorizin is distributed along the length of the style, according to the scheme shown on Figure 3.
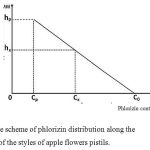 |
Figure 3: The scheme of phlorizin distribution along the length of the styles of apple flowers pistils.
|
The regularity of phlorizin distribution along the length of the styles of apple flowers pistils can be expressed by the following formula (1):
Сх= Со – hx(Со – Ср)/hp (1), where:
Cx is a phlorizin content in any part of the length of the flower pistil style at the distance hx from the base of the style, %; Cp is a phlorizin content in the stigma, %;
Co is a phlorizin content in the base of the pestle’s style %; hp is the maximum length of the style, mm.
Indicator (Cp) corresponding to the content of phlorizin in the pistil’s stigma is defined as the projection of the upper points of the graphs on the x-axis (C%). The point of intersection of the graphs with the horizontal axis (C%) corresponds to the percentage of phlorizin in the bottoms of the dry styles (C0). Along the length of the styles the phlorizin content in the tissue of apple pistil increases from stigma (Cp) to the bottom of the style (C0), according to the gradient (2):
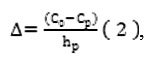
where Δ is a gradient value of phlorizin content along the length of the style, % mm. The rest of the notions are identical to the equation (1).
Taking into account that the gradient is a value of ascending or descending of some physical quantity in the space, in our case the gradient indicates the value which describes the percentage changes of phlorizin content in the dry tissues of pistil’s styles, considering the length changes from the stigma to the base by one millimeter.
 |
Figure 4: Phlorizin distribution along the length of the styles on the apple flower varieties pistils: a – Spartan (h – 10 mm); b – Antonovka ordinary (h – 14 mm); c – Orlik (h – 12 mm); d – Bessemyanka Michuriniskaya (h – 5 mm) (2010).
|
For example, the particularities of phlorizin distribution in the apple styles are shown graphically on Figure 4 for the four varieties. The scheme of the style division into parts for phlorizin analysis and numerical designator of the parts styles are listed on Figure 4 (b, c, d). The average length of the styles was hp ± 0.5 mm. The average values of Cp and Co were calculated according to the similar graphs, and the gradients were calculated for the seven varieties of apples. The average values during 2010-2012 years of studies are presented and compared with indicators of fruit set of open pollination and self-pollination in Table 1. The comparison based on phlorizin content was carried out for styles samples aged to a constant mass over sodium hydroxide [23].
Table 1: The parameters of phlorizin distribution along the length of pistils of the studies apple varieties and the percentage of their fruit set while free and self-pollination (2010-2012).
| Apple variety | hр,
mm |
Ср, % phlorizin in the stigma | Со, % phlorizin in the base of style | Phlorizin gradient, ∆, %/mm | % fruits set while free pollination | Fruits set while self- pollination % |
| Spartan | 10 | 5±1 | 13±2 | 1.0 | 32±3 | 10±2 |
| Lobo | 10 | 6±1 | 14±2 | 0.6 | 27±4 | 8±2 |
| Melba | 11 | 3±1 | 15±2 | 0.9 | 24±3 | 7±2 |
| Sinap orlovskiy | 13 | 12±2 | 32±2 | 1.6 | 9±2 | 0 |
| Antonovka ordinary | 14 | 8±1 | 32±2 | 1.6 | 8±2 | 0 |
| Orlyk | 12 | 7±1 | 32±2 | 1.7 | 14±2 | 0 |
| Bessemyanka michurinskaya | 5 | 10±2 | 30±2 | 2.6 | 2±1 | 1±1 |
The effect of phlorizin in pistil’s styles onto the fruits set of apple varieties.
The results mentioned in Table 1 indicate that the apple varieties, with a gradient of phlorizin in pistils more than 1.6% / mm (i.e., the level of phlorizin in the bottoms of the styles was (Co) 30-32%), reduced dramatically the percentage of fruit set to a level of 2-9% while free pollination and to 0% while self-pollination. The cultivars having the phlorizin gradient less than 1.0% / mm (i.e., the (Co) is less than 15%) had the fertility level within 24-32% of the total number of free-pollinated flowers while free pollination, and 5-10% while self-pollination. The larger gradient corresponds to a higher content of phlorizin in the styles tissues. In high concentrations phlorizin is a natural inhibitor of growth processes [26]. During the pollination process it restricts the growth of pollen tubes in the pistil’s cavities (table 3). As a result, the decrease of the fruit set ability can be expected for these varieties. It also means that the phlorizin distribution gradient in the styles can be considered as one of the factors determining the activity of apple varieties to fruit set during free- and self-pollination. However, the technology of this indicator obtaining is complex and time consuming.
Table 2: The content of phlorizin in the bases of flower pistils’ styles of the studied apple varieties during 2009-2012.
| Apple variety | 2009 | 2010 | 2011 | 2012 | Average |
| % of phlorizin in the bases of flower pistils’ styles (Со) | |||||
| Bessemyanka michurinskaya | 27 | 30 | 29 | 32 | 30±3 |
| Sinap orlovskiy | 29 | 28 | 28 | 27 | 28±2 |
| Оrlyk | 29 | 26 | 26 | 29 | 27±2 |
| Antonovka ordinary | 28 | 32 | 30 | 33 | 31±3 |
| Green May | – | 17 | 19 | 18 | 18±1 |
| Мartovskoe | – | 17 | 19 | 18 | 18±1 |
| Lobo | 13 | 13 | 11 | 12 | 12±1 |
| Spartan | 12 | 11 | 11 | 10 | 11±1 |
| Melba | 14 | 14 | 13 | 15 | 14±1 |
| Uelsi | – | 15 | 14 | 16 | 15±1 |
In order to obtain the breeding information it is sufficient to know the phlorizin content in the bottoms of styles (Co), because these parts of flowers contain the highest gradient of phlorizin. One millimeter of style base is enough for the test. It should be cut delicately with a blade. The results of these analyzes are presented in Table 2. Within the years the phlorizin percentage remains constant. It is one of the constant endogenous varietal characteristics of apple cultivars which, as well as the content of flavonols in pollen, should be used for the development of variety pomological characteristics.
The program interaction during pollen and pistil self-pollination for the apple cultivars having the various content of flavonoids.
The fertilization program stage of the varieties with the most contrast phlorizin content in pestles and flavonols in pollen was studied using the methods of fluorescent microscopy [24]. This study revealed (Table 3) that the varieties with a high level of phlorizin in styles’ bases (26-30 dm.%) such as: Bessemyanka Michurinskaya, Sinap Orlowskiy, Orlyk, while pollination by their own pollen, containing small quantities of flavonols (2-3 dm.%), have the lowest percentage of growth of pollen tubes to the ovaries (0-4%). Only a few pollen tubes of these varieties could germinate towards the ovary (figure. 5.B). The stopping of the pollen tubes growth in the pistil tissues is a major reason of incompatibility during self-pollination. The pollen tubes of Lobo and Spartan varieties, having a low phlorizin level in the bases of styles (12-13 dm.%), massively germinated towards the ovary (figure 5.A).
Table 3: Results of fluorescent microscopy of pollen germination in the pistils’ tissues during self-pollination of studied varieties (2011-2012)
| Variety | Pollen flavonols content,% (dm.) | Style bases phlorizin content,% (dm.) | Time of exposition, t (hour) | a/b | % of pollen tubes that reached the bases of pistils styles |
| Bessemyanka michurinskaya | 2±0,5 | 30±3 | 46-48 | 0/39 | 0 |
| Sinap orlowskiy | 2±0,5 | 28±3 | 46-48 | 0/40 | 0 |
| Orlyk | 3±0,5 | 28±3 | 46-48 | 2/40 | 0.5±0.3 |
| Lobo | 11±1 | 13±2 | 46-48 | 16/39 | 41±3 |
| Spartan | 13±2 | 12±2 | 46-48 | 19/40 | 48±3 |
Note.1. a / b- ratio is a number of pistils, where the pollen tube (a) has grown up to the base of pistil styles, to (b) the total number of microscope examined pistils.
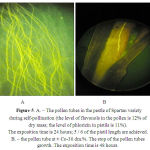 |
Figure 5: A. – The pollen tubes in the pestle of Spartan variety during self-pollination (the level of flavonols in the pollen is 12% of dry mass; the level of phlorizin in pistils is 11%). The exposition time is 24 hours; 5 / 6 of the pistil length are achieved. B. – the pollen tube at ± Co-30 dm.%. The stop of the pollen tubes growth. The exposition time is 48 hours.
|
The diagnostics of apple varieties self-fertilization.
The percentage of fruit set during the self-pollination is the indicator of the possibility of self-fertilization for those varieties [18]. In order to identify the potential impact of parthenocarpic processes onto the experimental results during the fruits formation, 150 flowers of the studied varieties were castrated. However, there were no fruits found among them.
Table 4: The fruit set of apple varieties with different content of flavonols in pollen and phlorizin in the bases of pistil’s styles during the self-pollination and free pollination (2009-2011)
| Apple variety | Pollen flavonols content,% (dm.) | Style bases phlorizin content,% (dm.) | Fruit set | ||
| Self-pollination
% (absolute.) |
Self-pollination
% (relatively to free pollination) |
Free pollination, % | |||
| Spartan | 13±2 | 11±2 | 10±2 | 63±6 | 16±3 |
| Lobo | 11±1 | 13±2 | 7±2 | 58±6 | 12±2 |
| Melba | 9±2 | 14±2 | 5±2 | 56±5 | 9±2 |
| Martovskoe | 5±1 | 18±2 | 2±1 | 15±3 | 13±3 |
| GreenMay | 4±1 | 20±2 | 1±1 | 10±2 | 10±2 |
| Orlyk | 3±1 | 25±3 | 0 | 0 | 8±2 |
| Sipan orlovskiy | 2±0.5 | 28±3 | 0 | 0 | 6±2 |
| Bessemyanka michrinskaya | 2±0.5 | 30±3 | 0 | 0 | 9±2 |
The data indicated in Table 4 show that the propensity of apple cultivars for fruit set during the self-pollination increases with the growth of flavonols content in the pollen and the decrease with phlorizin level in the bases of pistils’ styles. As a result, the highest percentage of the fruit set during the self-pollination (5-10% abs.) was observed for Lobo, Spartan, and Melba varieties, having the highest content of flavonols in pollen (10-12 dm.%) and the lowest content of phlorizin in pestles (11-14 dm.%). The varieties with a high content of phlorizin in a pestle (18-30 dm.%) and low content of flavonols in pollen (2-5 dm.%), such as: Sinap orlowskiy, Bessemyanka Michuriniskaya, Orlik, Green May, Martovskoe had an extremely low or zero level of the fruit set during the forced self-pollination; they had no inclination to self-fertilization.
The mentioned results of the quantitative content of flavonols in pollen and phlorizin in the bases of pistil’s styles can be used as markers while selecting the best apple varieties capable to self-pollination and self-fertility. So the best self-pollinating varieties should contain a high level of flavonols in the pollen (preferably more than 7% of its dry mass) and a low level of phlorizin in the bases of pistil styles (preferably less than 12% of their dry mass). The varieties prone to the elf-fertility are the best pollinators during the cross-pollination.
The evaluation of apple varieties cross-fertility.
The quantitative indicator of cross-compatibility during pollination is, as well as in the case of self-pollination, the fruit set percentage of pollinated varieties, by the pollen of another varieties [18]. The graphs of Figure 6 show the direct connection between the fruit sets and the content of flavonols in pollen of pollinator varieties. The fruit set increases if pollinator’s pollen contains more than 7% of flavonols out of its dry mass. Weakly compatible (absolute fruit set was 0 – 3%) are the varieties combination, where pollinated cultivars have phlorizin at 26 – 32% of its dry mass in the bases of styles; where the pollinator pollen contains minor amounts of flavonols: 2 – 4% of the dry mass. The highest fruits set percentage (absolute fruit set 28 – 31%), i.e. the best compatibility was observed when the pollinated apple trees varieties had a minimum content of phlorizin (11 – 13% of dry mass) in styles bases, while the pollen of the pollinator varieties contained the maximum amount of flavonols (10 – 12% of dry mass).
In combinations with high (25-30%) content of phlorizin in the styles of pollinated varieties and high (10-12%) content of flavonols in pollen of pollinator varieties grade, a high percentage of fruit set (absolute. 8 – 23%) was also observed. In these cases the high content of flavonols has the activating the role.
The mentioned peculiarities were observed during all the years of experimental work (2009 – 2012).
In general, according to the result of the research, the pollen of the best pollinator varieties contains the flavonols (more than 7% of its dry mass). The higher level of flavonols is, the stronger pollination properties of the variety are. The pollen of the bad apple pollinators contains less than 7% of flavonols.
 |
Figure 6: The dependence of the apple varieties fruit set on the level of flavonols in pollen of pollinating varieties. Pollinated varieties of apple trees are: a – Lobo; b – Spartan; c – Martovskoe; d – Green May; e – Orlik; f – Sinap orlowskiy. The phlorizin level in the bases of pistil’s styles is indicated in the brackets above the graphs, after the names of the pollinated varieties.
|
To conclude, the quantitative content of flavonols in the pollen can be used as an endogenous parameter in selecting the best pollinator varieties for apple orchards and it should be included in the catalog of the variety characterization.
References
- Sedov, E.N. and Z.M. Serova, 1980. About inbreeding apple production. In Science to Industry, Vol. X, Part.I, Eds., Osipov, Y.P. Orel: Priokskoe publishing, pp: 9-19.
- Ulyanovskaya, E.V. and N.V. Mozhar, 2005. Self-incompatibility system at pollination and its genetic control of the species of the Rosaceae family. Nauka Kubani, 4: 172-175.
- Suprun, I.I, E.V. Ulyanovskaya and Y.V. Ushakova, 2010. The use of DNA labeling to identify self-incompatibility alleles of apples. Research of the Kuban State University, 1(22): 57-59.
- Kobel, F., 1957. The physiological bases of fruits. Moscow: GISL, pp: 376.
(Kobel F., 1954. Lehrbuch des obstbaus auf physiologischergrundlage. ZweiteAuflage.) - Bannikova, V.P. and O.A. Khvedinich, 1982. The basis of plants embryology. Kiev: Naukova Dumka, pp: 164.
- Linskens, H.F., 1955. Physiologsche Untersuchungen der Pollenschlauch-Hemmungselbst-steriler Petunien. Z.Bot., 43: 1-44.
- Bransocheidt, P., 1930. Zur Physiologie der Pollen Keimung und ihrerexperimentellen Beeinflussung. Planta., 2: 368.
- Hecht, A., 1960. Growth of pollentubes of Oenotherea rganenis through the rwisein compatible styles. Amer.J.Bot., 47: 32.
- Heslop-Harrison, J. and Pollen, 1971. Development and Physiology. London: Butterworths, pp: 250.
- Hesloparrison, J., 1975. Incompability and the pollen stigma interaction Rev. Plant Physiol., 26: 403-425.
- Moewus, F., 1950. Zur Physiologie und Biochemie der Selbststerilitätbei Forsythia. Biol. Zentralbl., 69: 181.
- Milovanova, L.V., V.D. Kondratiev and A.M. Juravel, 1974. Biochemical characteristics of different ages stigmas and pollen in the selection of plum and apple. biophysical and physiological and biochemical studies of fruit and berry crops. Moscow: Kolos, pp: 35-43.
- Pashkar, S.I., 1974. By biochemical characteristics of heterosis, cytoplasmic male sterility in maize polyploidy in the process of selection. Fab. “Plant Physiology for breeding aid.” Moscow: Nauka, pp: 161-177.
- Minaeva, V.G., 1978. Flavonoids in the ontogenesis of plants and their practical use. Novosibirsk: Nauka, pp: 256.
- Ostreyko, S.A., 1988. The study of the role of phlorizin and chlorogenic acid in the generative processes of apple. Coll. Fruit in non-chernozem zone. Moscow, pp: 81-88.
- Wiermann, R., 1973. Über die Beziehundzwieschen flavonol auf bauenden Enzymeneinem flavonol um wandelnden Enzym und der Akkumulation phenylpropanoider Verbindung en wärendder Antherenentwichlung. Planta, Vol. 151, 2: 103-108.
- Palfitov, V.F., 2003. Diagnosis of self-fertility and growth vigor of apples. Michurinsk: Scientific Publications, pp: 198.
- The program and method of fruit berry and nut crops varieties study, 1999. Orel: VNIISPK, pp: 606.
- Doerfel, K., 1969. Statistics in Analytical Chemistry. Moscow: Mir, pp: 248.
(Doerffel, K., 1966. Statistik in der analytischenchemie. Leipzig) - Dospekhov, B.A., 1985. Methodology of the field experiment. Moscow: Kolos, pp: 418.
- Ermachkova, T.V. and V.F. Palfitov, 2008. Determination of water-soluble substances by weight method. Bulletin of the Michurinsk State Agricultural University, 2: 65-69.
- Palfitov, V.F., N.I. Saveliev, N.E. Kozlov and Molodtcov M.A., 2011. Biochemical selection of the best varieties of pollinators for apple. Horticulture and Viticulture, 4: 29-33.
- Palfitov, V.F., V.A. Potapov and A.M. Tarasov, 1998. A method for quantifying phlorizin: Patent №2115291 (RF). cl. A 01G7 / 00, A01N1 / 04. 1998.22.
- Litvak, A.I., 1978. Fluorescent macro- and microscopy in studies of fruit crops and grapes. Kiev: Shtiintsa, pp: 11.
- Minaeva, V.G. and G.N. Gorbaleva, 1967. On the effect of flavonoids on pollen germination and pollen tube growth of useful plants. Siberian flora. Novosibirsk: Nauka, pp: 231-235.
- Sarapuu, L.P., 1965. Physiological effect of phlorizin as β- inhibitor with growth and rest in apple. Plant Physiology, 12: 134-140.
- Wiermann, R., 1968. Untersuchungenzumphenylpropanstoffwechsel des pollens. Berichte der Deutschen Botanischen Gesellschaft, Vol. 81, 1‐2: 3-16.
- Bardinskaya, M.S., 1964. Plant cell walls and their formation. Moscow: Nauka, pp: 159.
- The Dictionary of Organic Compounds, Vol.3, 1949. Eds., Heilbronn, I. and G.M. Banbury. Moscow: IL, pp: 471.







