Manuscript accepted on :
Published online on: 21-12-2015
Plagiarism Check: Yes
Huda A. Qari1,2* and Ibrahim A. Hassan2,3
1Faculty of Science, King Abdulaziz University, Jeddah, KSA.
2Centre of Excellence in Environmental Studies, King Abdulaziz University, Jeddah, KSA.
3Faculty of Science, Alexandria University, 21526 El Shatby, Alexandria. Egypt.
DOI : https://dx.doi.org/10.13005/bpj/465
Abstract
The toxicity and biosorption of heavy metals by the green unicellular flagellate sp. were investigated. We found that Dunaliella algae are able to biologically remove heavy metals from wastewater at concentration (about 85 mg/L). The Dunaliella cells were first immersed for seven days in wastewater samples collected from different sources in Jeddah, KSA, and their growth rates were monitored determined visibly at wavelength of 560 nm. It was observed that at the initial stage (0–12 hours) the adsorption rate was so rapid that 74% of the metal was biologically adsorbed. The maximum biosorption capacity of live Dunaliella was estimated to be 0.79 mg lead per 75 alga cells. Other elements were adsorbed at relatively lower rates Both toxicity and biosorption are very important in developing Dunaliella for the treatment of wastewater containing heavy metals. Dunaliella algae could be used as phytoemediators to decrease toxicity of heavy metal from polluted wastewater for human health.
Keywords
Waste water; Heavy metal pollution; removal; adsorption; Dunaliella algae
Download this article as:| Copy the following to cite this article: Qari H. A, Hassan I. A. Removal of Pollutants from Waste Water Using Dunaliella Algae. Biomed Pharmacol J 2014;7(1) |
| Copy the following to cite this URL: Qari H. A, Hassan I. A. Removal of Pollutants from Waste Water Using Dunaliella Algae. Biomed Pharmacol J 2014;7(1). Available from: http://biomedpharmajournal.org/?p=2882 |
Introduction
The presence of heavy metals in the environment is of major concern because of their toxicity, bioaccumulating tendency, and threat to human life and the environment (Igwe & Abia, 2006). As human populations have expanded, Earth’s atmosphere and natural waters have become dumps for agricultural and industrial wastes. Heavy metals are among the conservative pollutants that are nonbiodegradable (El-Nady & Atta, 1996; Walter et al., 2011). As a result of this, their concentrations often exceed the permissible levels normally found in soil, water ways and sediments (Bhatnagar & Kumari, 2011). Hence, they find their way up the food pyramid. When they accumulate in the environment and in food chains, they can profoundly disrupt biological processes (Hassan & Basahi, 2013, Hassan et al., 2013; 2014).
The chemistry and toxicology of these heavy metals are complex and interesting (Hassan et al., 2014). Metals can be toxic to microbial population at sufficiently high concentrations. However, some metals such as silver, mercury, cadmium and copper are markedly more toxic even at very low levels (Puranik & Paknikar, 1997)
Remediation methods of the last half century have been largely unsuccessful2. Adsorptive removal of heavy metals from aqueous effluents which have received much attention in recent year is usually achieved by using activated carbon or activated alumina (Abdel-Raouf et al., 2012; Igwe & Abia, 2005, Igwe et al., 2005a;b; 2006) . Many other biosorbents of algal, fungal and bacteria biomass have been utilized (Mclean & Beveridge, 2001; Fedrickson et al., 2000; Vijayaraghan et al., 2005).
Bio-treatment with algae is particularly attractive because of their photosynthetic capabilities, converting solar energy into useful biomasses and incorporating nutrients such as nitrogen and phosphorus causing eutrophication (De la Nou & Basseres, 1989; De la Nou & De Pauw, 1988). Moreover, compared to physical and chemical treatment processes, algae based treatment can potentially achieve nutrient removal in a less expensive and ecologically safer way with the added benefits of resource recovery. Recently, Bhatnagar and Kumari (2013) stated that algae are significantly efficient in treating more than one problem at a time, which is not possible by conventional process of chemical treatment. The phycoremediation shows advantage over other chemical methods as the removal of algal mass from the treated effluents is easy and economic (De la Nou & De Pauw, 1988; Igwe & Abia, 2006; Bhatnagar & Kumari, 2013).
The aim of this study was to examine the possibility to biologically purify wastewater from heavy metals using the green unicellular flagellate Dunaliella sp.
Materials and Methods
A pure culture of Dunaliella sp. was supplied by Department of Botany and Microbiology, Alexandria University. The algae were centrifuged (5000 rpm for 20 min) and then stored in liquid medium for 7 d at 20 °C under light (60 W white fluorescent lamp) (Vijayaraghan et al., 2005). Algae were grown in a standard growth medium (Ting et al., 1989, Chen 2005) with little modifications (Table 1).
Table 1: Composition of growth medium
| Constituents | Concentration (gL-1) |
| EDTA | 0.10 |
| K2HPO4 | 0.50 |
| NaNO3 | 3.10 |
| NaCl | 1.20 |
| NaHCO3 | 19.90 |
| K2SO4 | 1.20 |
| FeSO4·7H2O | 0.05 |
| MgSO4·7H2O | 0.30 |
| CaCl2·2H2O | 0.08 |
Heavy Metal Sorption and Analysis
100 ml of wastewater was added to 250 ml flask containing 10 mg L-1Dunaliella cells and were shaked at 25°C for 48 h. At the designed period of 12, 24, 36, 48, 60, 72, 84, 96, 108 and 120 h, 10 ml of the solution were collected for analysis.
Dunaliella in the solutions was removed by filtration and the filtrates were analyzed to determine the concentration of the remaining metal ions.
Concentrations of heavy metals (Cd, Pb, Ni, Cr, Zn and Cu) in water samples were determined before and after inoculating with algae using Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES) (Hassan & Basahi, 2013; Al- Dhaibani et al., 2013). All safety rules were applied (COST, 2008).
Data analysis
The sorption of metals onto microbial surface is described by the following model (Chen, 2005);
1/q = 1/(qmax bc) + 1/qmax
where c is the final metal concentration (mg L-1), q is the metal uptake (mg/105 cells), b is the sorption binding constant (L/mg), qmax is the saturation capacity (mg/105 cells), from the slope and intercept of a 1/q vs 1/c linear plot such that qmax =intercept−1 and =intercept/slope.
Statistical Analysis
Each treatment was made in 10 replicates to ensure statistical validity. One –way ANOVA was applied to log-transformed data (to ensure they were normally distributed) using STATGRAPHICS statistical Package (STAT. 4). The differences between means were analyzed by Student T- Test at the P<0.05 significance level.
Results and Discussion
The analyses of heavy metal concentrations in wastewater samples are presented in Table 2.
Table 2: Mean (+SD) heavy metal concentration (mg L-1) in wastewater. (n = 10).
| Element | Concentration |
| Cd | 80.25 + 6.76 |
| Pb | 53.97 + 3.27 |
| Ni | 19.14 +1.72 |
| Cr | 34.87 + 4.98 |
| Zn | 179.12 + 21.25 |
| Cu | 62.17 + 7.04 |
Zinc (Zn) was found the most abundant element in wastewater (179.12 mg L-1), while Ni was less abundant (19.14 mg L-1). Other elements showed relatively high concentrations 34.87, 53.97, 62.17 and 80.25 mg L-1, for Cr, Pb, Cu and Cd, respectively. The concentrations recorded are higher than those recommended by WHO24. Moreover levels recorded in our study were much higher that that recorded in wastewater in other semi-arid regions of the world such as Iran (Mansouri, & Ebrahimour, 2011; Qishlaqi et al., 2008), India (Shama et al., 2006; Vijayaraghan, 2005) and Jordan (Al-Khashman, O.A. (2013).
Figure 1 indicated that Dunaliella has removed 95% of Zn and Cd after 108 hours, and 90% of Cu after 60 hours of incubation. Moreover, 93% of Pb, Ni and Cr were removed after 36 hours of incubation. This indicates that biosorption efficiency towards Pb, Cr and Ni is higher than other elements (Puranik & Paknikar, 2011).
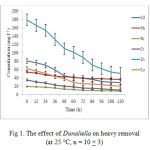 |
Figure 1: The effect of Dunaliella on heavy removal (at 25 °C, n = 10 + 3)
|
The dependence of heavy metals biosorption by Dunaliella on time is shown in Fig.2. Generally, it is reported that the uptake of metal ions can be divided into two stages: rapid and slow stage (Walterv et al., 2011). In the ‘rapid’ stage, the metal ions are adsorbed onto the surface of microorganism. In the ‘slow’ stage, the metal ions transport across the cell membrane into the cytoplasm.
It was reported that lead phosphate precipitated on the cell wall and inside the cell of cyanobacteria (Anabaena cylindrical). Their results confirmed a very fast uptake in the cell envelope and then a longer uptake period inside the cell envelope and then a longer uptake period inside the cell (Sa’idi, 2010).
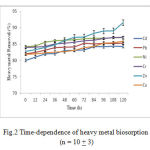 |
Figure 2: Time-dependence of heavy metal biosorption (n = 10 + 3)
|
In this study, rapid biosorption was observed at the beginning (0–12 min, with 74% of metal adsorbed), then reached the equilibrium within 24 h with 95% of metal ions adsorbed. Rangsayatorn et al. (2004) and Horikoshi et al. (1979) reported that cadmium was rapidly adsorbed by S. platensis during the first 5 min and by C. regularis within 6 min, respectively. Such rapid uptake of heavy metals by living cells is very significant when the cells are used in bioremediation process (De la Nou, 1989; WHO, 2006; Abdel-Raouf et al., 2012).
In conclusion, the results of the present study showed that Dunaliella has substantial for phytoremediation of heavy metals from the wastewater. However, further study is required before considering this plant Species for phytoremediation
Acknowledgements
This work is supported partially with a grant from CEES. We would like to thank Prof. A.F Khaleafa and Prof. Samir Khalil (Department of Botany & Microbiology, Alexandria University, Egypt) for supply of a pure culture of Dunaliella algae and help in analysis.
References
- Abdel-Raouf, N. Al-Homaidan, A.A. &. Ibraheem, I.B.M. (2012). Microalgae and waste water treatment. Saudi J. of Bio. Sci., 19: 257–275.
- Al-Dhaibani, A.A., Nakhlawy, F.S. Alsolaimani, S. G. & Almehmadi, F. M. (2013). Phytoremediation of Cadmium Contaminated Soil by Sunflower. Aust. J. Bas. App. Sci., 7(7): 888-894.
- Al-Khashman, O.A. (2013). Assessment of heavy metals contamination in deposited street dusts in different urbanized areas in the city of Ma’an, Jordan. Environ. Earth Sci., 70: 2601 – 2612. Doi:10.1007/S12665-013-2310-6.
- Bhatnagar, S. & Kumari, R. (2013). Bioremediation: A Sustainable Tool for Environmental Management. A Review. Annual Review & Research in Biology. 3(4): 974 – 993.
- De la Nou, E. & Basseres, J. (1989). Biotreatment of anaerobically digested swine manure with microalgae. Biological Wastes 29: 17–31.
- Christenson, L. & Sims, R. (2011). Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnology Advances 29:686–702.doi:10.1016/j.biotechadv. 2011.05.015.
- COST 859, (2008). European Cooperation in the field of Scientific and Technical Research. Fondation européenne de la science) est une association à but non-lucratif de droit français (Alsace) 1 quai Lezay-Marnésia, B.P. 90015, 67080 Strasbourg cedex, France http://w3.gre.a
- Chary, S., Kamala, C.T. & Raj, D. (2013). Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxico. Environ. Safety, 69(3): 513 – 524.
- Chen, S. (2005). Bioremediation potential of spirulina: toxicity and biosorption studies of lead. J Zhejiang Univ. SCI, Biology. 6B(3) 171- 174.
- De la Nou, E, J. & De Pauw, N. (1988). The potential of microalgal biotechnology. A review of production and uses of microalgae. Biotechnol. Adv. 6: 725–770.
- El-Nady, F. & Atta, M.M. (1996).Toxicity and bioaccumulation of heavy metals to some marine biota from the Egyptian coastal waster .J. Environ. Sci. Health. A-31 (7): 1529-1545.
- Fedrickson, J.K. Kostandarithes, H.M. Li, S.W. Plymale, A.E. & Daly, M. J. (2000). Reduction of Fe (iii), Cr(vi), U(vi), and Tc(vii) by Deinococcus radiodurans R1. Appl. Environ. Microbol. 66(5): 2006-2011.
- Hassan, I.A. & Basahi, J. (2013). Assessing roadside conditions and vehicular missions using roadside lettuce plants. Polish J. Environ. Studies, 22 (2), 75 – 81.
- Hassan, I.A., Basahi, J.M. & Ismail, I.M. (2013). Gas exchange, chlorophyll fluorescence and antioxidants as bioindicators of airborne heavy metal pollution in Jeddah, Saudi Arabia. Current World Environment, 8(2) 203 – 213,
- Hassan, I., Basahi, J. Ismail, I. & Zahran, A. (2014). Effects of airborne heavy metal pollution on physiological and biochemical processes in lettuce (Lactuca sativa L. Romaine) Plants. Advances in Env. Biology.). (in press)
- Igwe, J.C. & Abia, A.A. (2005). Sorption kinetics and intraparticulate diffusivities of Cd, Pb and Zn ions on maize cob, Pb and Zn ions on maize cob. Afr. J. Biotech. 4(6): 509-512.
- Igwe, JC. Okpareke, O.C. & Abia, A.A. (2005a) Sorption kinetics and intraparticulate diffusivities of Co, Fe and Cu ions on EDTA-modified maize cob, Intern. J. Chem. India 15(3): 187-191.
- Igwe, J.C. Ogunewe, D.N. & Abia, A.A. (2005b). Competitive adsorption of Zn(ii), Cd(ii) and Pb(ii) ions from aqueous and non-aqueous solution by maize cob and husk. Afr. J. Biotechnol. 4(10): 1113-1116.
- Igwe, J.C. Nwokennaya, E.C. & Abia, A. A. (2006). The role of pH in heavy metal detoxification by biosorption from aqueous solutions containing chelating agents. Afr. J. Biotechnology 5(7): 1113-1116.
- Igwe, J.C. & Abia A.A. (2006). Bioseparation process for removing heavy metals from wastewater using biosorbents. African J. of Biotechn. 5 (12), 1167-1179.
- Mansouri, B & Ebrahimour, M. (2011). Heavy Metals characteristics of wastewater stabilization ponds. American-Eurasians J. Agric. & Environ. Sci., 10(5): 763 – 768
- Mclean, J. & Beveridge, T.J. (2001). Chromate reduction by a psendomonad isolated from a site contaminated with chromate copper arsenate. Appl. Environ. Microbiol. 67: 1076-1084
- Puranik, P.R. & Paknikar, K.M. (1997). Biosorption of lead and zinc from solutions using streptoverticillium cinnamoneum waste biomass. J. Biotechnol. 55: 113 – 124.
- Qishlaqi, A., Moore, F & Forghani, G. (2008). Impact of untreatedwastewater irrigation on soils and crops in Shiraz suburban area, SE Iran. Environ. Monit. Assess. 141: 257–273. DOI: 10.1007/s10661-007-9893-x
- Sa’idi M. (2010). Experimental studies on effect of Heavy Metals presence in Industrial Wastewater on Biological Treatment. . INT. J. ENVIRON. SCI. 1 (4): 666 –
- Sharma R. K. Agrawal M. & Marshall. F. (2006). Heavy Metal Contamination in Vegetables Grown in Wastewater Irrigated Areas of Varanasi, India. Bull. Environ. Contamin. Toxicol. 77: 312–318. doi: 10.1007/s00128-006-1065-0
- Ting, Y.P, Lawson, F. & Prince, I.G. (1989). Uptake of cadmium and zinc by the alga Chlorella vulgaris: part 1. Individual ion species. Biotech. Bioeng., 34: 990 – 996
- Vijayaraghan, K. Jegan, J.R. Palanivela, K. & Velan, M. (2005). Copper removal from aqueous solution by marine green alga ulva reticula. .Electronic J. Biotecnol. 7(1): 22 -23
- Walter, H.A. Kangas, P.C. & Walter, M. (2011). Algal turf scrubbing: cleaning surface waters with solar energy while producing a biofuel. BioScience, 61(6), 434 – 441
- World Health Organization Water Guidelines. (2006). Appendix 2, Water Wells and Borehole s, John Wiley & Sons, Ltd. htttp://onlinelibrary.wiley.com/doi/10.1002/0470031344.app2/pdf.







