Manuscript accepted on :
Published online on: 16-12-2015
Plagiarism Check: Yes
Su Meihua1*, Zhang Shuilian1 and Yang Duoduo2
1School of Physical Education , Minnan Normal University, Zhangzhou Fujian - 363 000, China.
2College of P. E. and Health Sciences, Bijie University, Guizhou - 551 700, China.
DOI : https://dx.doi.org/10.13005/bpj/394
Abstract
The aim of the present study was to observe the effect of Spirulina supplementation on DNA damage in mice blood cell and blood redox status after acute exercise. 40 male Kunming mice was randomly assigned into four groups: Control group (CG), exercise group without Spirulina supplementation (EG), Spirulina supplementation group without exercise (SG) and Spirulina supplementation group with exercise (SEG). The exercise fatigue model was built up for mice through the protocol of repeated exhaustive treadmill running for seven days and the single cell gel electrophoresis (SCGE) was used to detect the DNA damage of blood cells in different groups. Blood samples were drawn from different groups and the changes of SOD and GSH which stand for anti-oxidative level in plasma. Also, Malondialdehyde (MDA), a marker of lipid peroxidation, was measured in the plasma of mice.This studies showed that the DNA damage of blood cell in SEG group was significantly lower than that of EG group (P<0.01) and there was no statical difference between CG and SG groups. And the level of GSH in SEG group were significantly lower than that of EG group (P<0.01).This results showed that plasma concentrations of malondialdehyde (MDA) were significantly decreased after supplementation with spirulina (P < 0.001). Those results leaded to conclusion that acute exercise induced more free radical to change the level of SOD, GSH and MDA and also occurred more DNA damage. It was suggested that DNA breakage occurs in blood cell after exhaustive exercise as a consequence of oxidative stress and the Spirulina supplementation has obvious protective effect to prevent exercise-induced DNA damage.
Keywords
Spirulina supplementation; blood cell; DNA damage; Single cell gel electrophoresis(SCGE); Oxidative stress
Download this article as:| Copy the following to cite this article: Meihua S, Shuilian Z, Duoduo Y. Protective Effect of Spirulina Against Cell DNA Damage and Oxidative Stress Induced by Exhaustive Exercise. Biomed Pharmacol J 2013;6(2) |
| Copy the following to cite this URL: Meihua S, Shuilian Z, Duoduo Y. Protective Effect of Spirulina Against Cell DNA Damage and Oxidative Stress Induced by Exhaustive Exercise. Biomed Pharmacol J 2013;6(2). Available from: http://biomedpharmajournal.org/?p=2649 |
Introduction
Oxidative damage of biomolecules such as DNA and lipids has been implicated in the modification of aging and degenerative diseases. Reactive oxygen species (ROS) play an important part as mediators of tissue injury and inflammation after strenuous exercise and exercise oxidative stress induced lipid peroxidation may be one of the mechanisms causing DNA damage[1]. It has been experimentally confirmed antioxidant supplements could prevent or alleviate the DNA damage [2].Antioxidant supplements are commonly used by athletes as dietary nutrition to counteract the oxidative stress of exercise to increase exercise performance[3].A study in 2007 found that spirulina was used as a dietary supplement to improve the antioxidant potential of many geriatric patients who had taken it for 16 weeks and the plasma of these patients showed a measured increased level of total antioxidant status[4]. Spirulina supplementation significantly reduced the increased lipid peroxidation level in HCD-fed rabbits[5]. Some studies have reported that Spirulina supplementation induces an obvious increase in exercise performance, the mechanism of which may be through an increase in β-oxidation pathway rate and increases the reducing levels of
glutathione[6]. Spirulina contains vitamin B1 (thiamine), B2 (riboflavin), B3 (nicotinamide), B6 (pyridoxine), B9 (folic acid), vitamin C, vitamin D, vitamin A, and vitamin E[7][8]. So spirulina can also acts as vitamin E which could prevent the DNA damage of toxic effect to the body, and prevent or reduce the form of free radical during human exposure to ionizing radiation , smoking, food additives in the daily environment[7][9]. However, the current studies about the effect of Spirulina on preventing DNA damage after exhaustive exercise are still rarely reported. Whether exhaustive exercise does increase the need for additional antioxidants in the diet is not clear. This experiment used single cell gel electrophoresis (single cell gel electrophoresis, DNA SCGE)[10] to detect the DNA damage induced by exhaustive exercise in mice blood cells, and the protective effect of Spirulina on mice blood cells were observed , so as to provide a reference basis on how to supplement Spirulina in human body. Furthermore, it aimed to observe the effect of Spirulina to reduce the oxidative stress and whether could it enhance the DNA repair capacity to increase exercise ability.
Materials and Methods
Animals and Exercise protocol
Healthy male mice (KM, supplied by animal center of medicine department of Peking University), 7-8 weeks, the mean weight were 27.3±2.1g. The animal room was kept at 20–24℃and 40–60% humidity with a 12/12 hr dark/light cycle. 40 mice were randomized into four groups: Control group(CG), exercise group without Spirulina supplementation(EG),Spirulina supplementation group without exercise(SG)and Spirulina supplementation group with exercise(SEG),each group had 10 mice. All the mice in the SG and SEG was fed with diet containing 25% Spirulina dry power and those mice was fed for seven days.
The exercise protocol was done as Marra et al. [11], but it was repeated for seven days on the exercised group, once per day. It consisted of an acute exhaustive exercise bout of treadmill running. Mice were run at 28m/min on a 28 slope for 90 min following a brief (10min) warm-up. Exhaustion was determined by failure to run after continued prodding and splaying within the treadmill lane. Control group mice were exposed to the noise and vibration of the treadmill for the same duration as the exercised mice.
Supplementation of Spirulina
The mice of SG and SEG group were fed with diet containing 25% spirulina dry power, all these mice was fed spirulina for 1week pre-exercise and 1week during the training period. Spirulina was purchased from Fuzhou wonderful Biological Technology Co., Ltd..
Alkaline comet assay
The alkaline comet assay was done as described by Godard et al.[10]. Fully-frosted clean microscope slides were covered with 1% normal melting point (NMP) agarose and allowed to polymerize at room temperature to allow agarose to dry. After solidification, the gel was scraped off from the slide. The slides were further coated with 0.6% NMP agarose. When this layer had solidified a second layer containing the whole blood sample (0.5μL) mixed with 0.5% low melting point (LMP) agarose was placed on the slides. After 10 min of solidification on ice, slides were covered with 0.5% of LMP agarose. An amount of 100 μL of this agarose cell suspension was layered on the top of the second layer. Finally, the fourth layer of 0.5% low melting point (LMP) agarose was added to cover the third layer and allowed to solidify for 10 min at 4℃.
Afterwards the slides were immersed for one hour in ice-cold freshly prepared lysis solution (2,5M NaCl, 100 mM Na2EDTA, 1% Na-sarcosine, 1% 10 mM Tris–HCl, pH 10 with Triton X-100 and 10% DMSO added fresh to lyse cells and allow DNA unfolding). After lysis, slides were placed in the freshly prepared electrophoresis buffer (300 mM NaOH, 1 mM Na2EDTA, pH 13.0) to remove salts. The slides were set in this alkaline buffer for 10 min to allow DNA unwinding and expression to alkali labile sites. Denaturation and electrophoresis was performed at 4℃ under dim light at 25 V (300 mA). After electrophoresis, the slides were washed three times at 5 min intervals with buffer (1% Triton X-100, 10%DMSO) to remove excess alkali and detergents. Each slide was stained with ethidium bromide (20 μg/ml) for 10 min and covered with a cover slip. Slides were stored at 4℃ in humidified sealed containers until analysis. To prevent additional DNA damage, handling with blood samples and steps included in the preparation of slides for the comet analysis were conducted under yellow light or in the dark.
Slides were examined at 100× magnification on an Olympus fluorescence microscope (Olympus Optical Co, Ltd, Tokyo) with excitation at 520 nm green barrier filter. Twenty five randomly selected cells were submitted to image analysis system. After automatic delimitation of nucleus head and tail as well as elimination of background fluorescence and touching cells, different parameters are calculated. Olive tail moment (OTM) was used to evaluate DNA damage. The parameter was calculated automatically using the caps image analysis system: Olive tail moment = (tail mean − head mean) × tail%DNA/100.
Activity of antioxidant systems
Blood samples were obtained from different groups immediately after exercise. Blood was drawn into two 5 cc green-top Vacutainer tubes (containing 143 USP units sodium heparin) and one 5 cc purple-top Vacutainer tube (containing 1 mg/ml EDTA). Aliquots of whole blood were taken immediately for the comet assay analysis. Remaining blood was centrifuged at 2500×g for 10 min; plasma was then aliquoted to cryotubes for various assays. Samples were flash frozen in liquid nitrogen and stored at -80℃until time of analysis (within 6 months of collection).The activity of SOD, GSH and MDAwas determined spectrophotometrically according to the method of the Nanjing Jiancheng Bioengineering Institute(China) with a spectrometer.
Statistical analysis
The data are presented as means ± SD of six independent experiments. They were analyzed using the software of statistical package for the social sciences (SPSS) version 11.0 for Windows. The statistical difference between groups was determined with a two-way ANOVA followed by Dunnett’s test (two-sided) as multiple comparisons. The minimum level of significance was considered to be P<0.05.
Results
DNA damage o of mice blood cells in different groups induced by exhaustive exercise
DNA damage is visualized at the individual cell level as an increased migration of genetic material (‘‘comet tail’’) from the nucleus (‘‘comet head’’). As presented in Figure1, the control group(CG) and the Spirulina supplementation group without exercise(SG) did not have vivid ‘‘comet tail’’ (A and B respectively); the exercise group with Spirulina supplementation (SEG) showed out a little ‘‘comet tail’’ (C); the exercise group (EG) has obvious ‘‘comet tail’’(D).
In Figure 2, the comet assay parameter was used to express the DNA damage of blood cell induced by repeated exhaustive exercise. Olive tail moment is the product of tail length and percentage DNA in tail, thus tail moment represents both the amount of DNA migrated into the tail and the distance migrated (Fig. 1). The olive tail moment is commonly reported [10] as a valid marker of single-strand DNA breakage. The results showed that the higher this values, the greater the damage that has occurred to the nuclear DNA. Statistically significant differences (P<0.001) in DNA damage were found in the blood cells of mice in EG as compared to CG and SG, the DNA damage of blood cells in SEG show significant lower than EG(P<0.01). There were no significant differences between CG and SG(P>0.05). The CG and SG cells sustained the least background damage. Figure 1 and Figure 2 summarize the different levels of DNA damage in different group of mice.
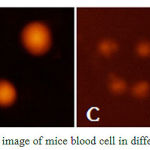 |
Figure 1: DNA comet image of mice blood cell in different group(×100).
|
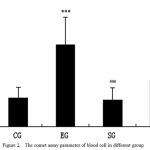 |
Figure 2: The comet assay parameter of blood cell in different group
|
All the data was expressed by x ±s , compared with CG, *P<0.05,**P<0.01,***P<0.001;compared with EG, #P<0.05, ##P<0.01,###P<0.001.
Measurement of SOD, GSH and MDA
From the table1, it showed that the SOD activities in plasma of mice were largely altered in all groups. The SOD activities of EG group were significantly lower than the other three groups, in contrast, the level of GSH and MDA of EG group were significantly higher than the other three groups. The SOD activities in SG group increased obviously than CG group(P<0.001). The level of GSH in SG group decreased obviously than EG group(P<0.001), and it did not showed out statistical difference compared with CG group. However, the level of MDA reduced significantly(P<0.05) in SG group than CG group. Compared with EG, the SOD activities in SEG group increased(P<0.01) ,and GSH level in SEG group reduced in varying degree(P<0.05) , also the level of MDA in SEG group reduced significantly(P<0.001) .
Table 1: The changing of SOD, GSH and MDA on blood tissue in different group
| Group | SOD(NU/mL) | GSH(mg/L) | MDA(nmol/ mL) |
| CG | 203.67±18.61 | 191.51±25.16 | 10.76±3.21 |
| EG | 171.72±27.26** | 268.78±45.71** | 18.48±4.47*** |
| SG | 215.86±26.62***## | 187.98±30.63## | 8.93±3.94*### |
| SEG | 194.78±25.17## | 223.12±34.81# | 15.12±5.51***## |
All the data was expressed by x ±s ,compared with CG, *P<0.05,**P<0.01,***P<0.001;compared with EG, #P<0.05, ##P<0.01, ###P<0.001.
Discussion
It is well known that the consequences of severe exercise are soreness and stiffness. Both consequences develop in the days following severe exercise[12]. Moreover, there would be an increase in oxygen consumption during exercise, which would result in the production of oxygen radicals[13]. DNA damage are physical abnormalities in the DNA, such as single- and double-strand breaks[14]. Comet assay has been widely used in various studies to detect the DNA damage connected with kinds of diseases due to its rapid, simple, and sensitive technique for measuring DNA breaks and repair in single cells[15]. DNA damages can be recognized by enzymes, and, thus, they can be correctly repaired if redundant information, such as the undamaged sequence in the complementary DNA strand or in a homologous chromosome, is available for copying[16]. Moreover, DNA damaging agents can damage other biomolecules such as proteins, carbohydrates, lipids, and RNA. Generally, DNA damage is the leading basis in the carcinogenic process, however DNA can be repaired, for the body has some repair mechanism after injury , so as to avoid the occurrence of tumor[17][18][19]. Blood mononuclear cell DNA oxidative damage is found to increase in human body after intense exercise[20]. It was also found that the DNA of skeletal muscle cells, lymphocytes and blood cells occurred damage to some extent[21]. In the present study, the SCGE technique was used to detect DNA damage in mice blood cell ,the results showed that the parameter of olive tail moment was significantly increased post exhaustive exercise, suggesting that DNA of mice blood cells appeared serious injury, this result supported previous reports[22][23][24].
It is well known that most of athletes have to run to exhaustion to achieve wonderful exercise performance ,so it would beneficial for our health , especially for the athlete , to find out the protective supplements to reduce muscle damage. Reactive oxygen species (ROS) cause lipid peroxidation and oxidation of some specific proteins, thus affecting many intra- and intercellular systems [25]. Antioxidants work together in animal cells against toxic reactive oxygen species [26]. SOD plays a key antioxidant role, which protects against oxidative damage especially mediated by free radicals and lipid perioxidation. Glutathione (GSH )is an antioxidant that has been used as a measure of oxidative stress. GSH plays an important role in antagonizing exogenous poison,oxygen free radical injury, adjusting the immune function, maintaining cell protein and inhibiting cell apoptosis, which is the main material cells against reactive oxygen damage. And it is sensitive and can comprehensively reflect the capacity of cells against damage[27]. The present study also investigates the status of lipid peroxidation. Malondialdehyde(MDA ) is the product of lipid peroxidation and its level is a marker of lipid oxidation, also its content can reflect the degree of lipid peroxidation in vivo and indirectly reflect the extent of the damage of cells[28]. The results of the present experiments showed that SOD activity decreased significantly after acute exercise, in contrast, GSH content increased as well as DNA damage in mice blood cell. However, the Spirulina supplementation reduce the MDA level and DNA damage, which was suggested that DNA damage was related with tissue lipid peroxidation, and the high intensity exercise caused oxidative damage in the blood and eventually lead to DNA damage of blood cells. The fact that, in the present study, a significant level of DNA damage was detected after exhaustive running in the mice probably shows that DNA might be a weak link in a cell’s ability to tolerate oxygen free-radical attack. It is conceivable that the levels of exercise attained in our experiments could be associated with oxidative stress, and perhaps the deleterious effects associated with such stress. It is possible that a depression in the running performance of the mice could be attributed to disruption of the oxidant/antioxidant balance consequently resulting in oxidative stress.
Spirulina is a popular nutritional supplement that is accompanied by claiMSS for antioxidant and performance-enhancing effects[29]. Spirulina has been used as a complementary dietary ingredient of feed for poultry and increasingly as a protein and vitamin supplement to aquafeeds[30]. Spirulina as an antioxidant one hand can inhibit the oxidase enzymes system, activate and protect anti-oxidase system, on the other hand, it can directly react with lively free radical, change lipid peroxides into hydroxyl resin, and it is a strong free radical scavenger[30][31]. The study found that DNA damage of SEG group were significantly lower than that of EG in mice blood cell, indicating that Spirulina can effectively alleviate DNA damage of blood cell caused by high intensity exercise , the reason may be related to antioxidant effect in vivo on Spirulina. The results also showed that GSH activity and MDA content in SEG group were significantly lower than those of EG in plasma of mice, suggesting the supplement of Spirulina can alleviate the oxidative damage effect on the blood induced by high intensity exercise , which reduce the free radicals attacking the blood nuclei and nuclear genetic material. Therefore DNA damage in SEG mice was significantly lower than that of EG mice. However, the activity of SOD in SEG mice plasma were significantly higher than those of EG, the results may be related with the different repair mechanism of Spirulina supplementation . Therefore, the protective mechanism of Spirulina supplementation on the protection of DNA damage remains to be further studied.
Conclusion
In conclusion, the current data indicated that strenuous exercise induced elevated DNA damage due to oxidative stress in these animals. Spirulina supplementation inhibit lipid peroxidation and have free radical scavenging activity, which can be beneficial for the protection against oxidative stress and reduced the DNA damage on mice blood cell during exercise. So it is beneficial for the athletes to take Spirulina supplementation to relieve oxidative stress to prevent from more DNA damage.
Acknowledgment
This work was financed by the research fund of by Grant No.2013J05055 and also the Grant No.JA13199 from Fujian Province Science Foundation and Fujian Education Department Science Foundation of China .
References
- Jennifer M. Sacheck, Jeffrey B. Blumberg.Role of vitamin E and oxidative stress in exercise[J]. Nutrition,2001,17(10):809-814
- Etsuo Niki .Assessment of Antioxidant Capacity in vitro and in vivo[J].Free Radical Biology and Medicine. 2010, 49(4):503-515.
- Maria L. Urso,Priscilla M. Clarkson. Oxidative stress, exercise, and antioxidant supplementation[J].Toxicology,2003,189(1-2):41-54.
- Mark F. McCarty.Clinical Potential of Spirulina as a Source of Phycocyanobilin[J].Journal of Medicinal Food, 2007,10(4): 566-570.
- Mi Yeon Kim, Sun Hee Cheong, Jeung Hee Lee, et al. Spirulina Improves Antioxidant Status by Reducing Oxidative Stress in Rabbits Fed a High-Cholesterol Diet[J]. Journal of Medicinal Food. 2010, 13(2): 420-426.
- Kalafati M, Jamurtas AZ, Nikolaidis MG, et al.Ergogenic and antioxidant effects of spirulina supplementation in humans[J].Med Sci Sports Exerc 2010, 42(1):142-51.
- Chamorro G, Salazar M, Favila L, et al. Pharmacology and toxicology of Spirulina alga[J]. Rev Invest Clin,1996,48(5):389–399.
- Babadzhanov A.S., Abdusamatova N., Yusupova F. M.,et al. Chemical Composition of Spirulina platensis Cultivated in Uzbekistan[J].Chemistry of Natural Compounds.2004,40(3):276-279.
- Maret G. Traber, Jeffrey Atkinson.Vitamin E, antioxidant and nothing more[J].Free Radical Biology and Medicine, 2007, 43(1):4-15.
- Godard T, Fessard V, Huet S, et al. Comparative in vitro and in vivo assessment of genotoxic effects of etoposide and chlorothalonil by the comet assay[J]. Mutation Research, 1999, 444(1):103-116.
- Marra S, Burnett M, Hoffman-Goetz L. Intravenous catecholamine administration affects mouse intestinal lymphocyte number and apoptosis[J]. J Neuroimmunol, 2005, 158(1-2):76-85.
- BOFFI FM, MURIEL MG, LÓPEZ RA,et al.DNA DAMAGE INDUCED BY EXERCISE IN MIDDLE GLUTEAL MUSCLE OF THOROUGHBREDS HORSES[J].Journal of Basic & Applied Genetics, 2007,18 (1): 1-10.
- Davies K.J.A., Quintanilha A.T., Brooks G.A.,et al. Free radicals and tissue damage produced by exercise[J]. Bioch.and Biophysical Res. Com., 107(4): 1198-1205, 1982.
- Lynch, MD. How does cellular senescence prevent cancer?[J]. DNA Cell Biol , 2006, 25 (2): 69–78.
- Collins A., Harrington V. Repair of oxidative DNA damage: assessing its contribution to cancer prevention[J]. Mutagenesis, 2002,17(6):489–493.
- Stuart JA, Karahalil B, Hogue BA, et al. Mitochondrial and nuclear DNA base excision repair are affected differently by caloric restriction[J]. FASEB J , 2004,18 (3): 595–597.
- Spindler, SR. Rapid and reversible induction of the longevity, anticancer and genomic effects of caloric restriction[J]. Mech Ageing Dev , 2005, 126 (9): 960–966.
- Best, Benjamin P. Nuclear DNA damage as a direct cause of aging[J].Rejuvenation Research ,2009, 12 (3): 199–208.
- Powers SK, Jackson MJ. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production[J]. Physiol Rev. 2008, 88(4):1243-1276.
- Mastaloudis A, Yu TW, O’Donnell RP, et al. Endurance exercise results in DNA damage as detected by the comet assay[J].. Free Radical Biology and Medicine, 2004, 36(8):966-975.
- Selman C, McLaren JS, Collins AR, et al. Antioxidant enzyme activities, lipid peroxidation, and DNA oxidative damage: the effects of short-term voluntary wheel running[J]. Arch Biochem Biophys, 2002, 401(2): 255-261.
- Tsai K, Hsu TG, Hsu KM, et al. Oxidative DNA damage in human peripheral leukocytes induced by massive aerobic exercise [J]. Free Radic. Biol. Med, 2001, 31(11):1465-1472.
- Demirbag R., Yilmaz R, Güzel S, et al. Effects of treadmill exercise test on oxidative/ antioxidative parameters and DNA damage[J]. Anadolu Kardiyoloji Dergisi. 2006, 6(2):135- 140.
- Aniagu SO, Day N, Chipman JK,et al. Does Exhaustive Exercise Result in Oxidative Stress and Associated DNA Damage in the Chub (Leuciscus cephalus)?[J]. Environmental and Molecular Mutagenesis,2006, 47(8):616-623.
- Child RB, Wilkinson DM, Fallowfield JL, et al. Elevated serum antioxidant capacity and plasma malondialdehyde concentration in response to a simulated half-marathon run[J]. Med Sci Sports Exerc, 1998, 30(11):1603-1607.
- Aldred S. Oxidative and nitrative changes seen in lipoproteins following exercise[J]. Atherosclerosis, 2007, 192(1): 1-8.
- Richi B, Kale RK, Tiku AB. Radio-modulatory effects of green tea catechin EGCG on pBR322 plasmid DNA and murine splenocytes against gamma-radiation induced damage[J]. Mutat Res, 2012, 747(1):62-70.
- Tomic S, Brkic S, Maric D,et al. Lipid and protein oxidation in female patients with chronic fatigue syndrome[J]. Arch Med Sci , 2012, 8(5):886-891.
- Kalafati M, Jamurtas AZ, Nikolaidis MG, et al. Ergogenic and antioxidant effects of spirulina supplementation in humans[J].Med Sci Sports Exerc. 2010,42(1):142-51.
- Habib M.AB., Huntington TC. , Hasan MR. A review on culture, production and use of Spirulina as food for humans and feeds for domestic animals and fish [J].Fisheries and Aquaculture Circular.2008,33(1034):4-41.
- R John Aitken and Shaun D R. Antioxidant systems and oxidative stress in the testes[J]. Oxid Med Longev, 2008,1(1):15-24.







