Manuscript accepted on :
Published online on: 18-12-2015
Plagiarism Check: Yes
Nguyen Tu Hoang Khue* and Doan Thi Thanh Vinh
School of Biotechnology, Hochiminh City International University, Hochiminh, Vietnam.
DOI : https://dx.doi.org/10.13005/bpj/403
Abstract
Lactobacillus strainsare commonly used as probiotics. Formulation for the probiotics is essential. Thestudy has studied the effects of carbon sources including in glucose, fructose, maltose, lactose, saccharose at the different concentrations as 5, 10, 15, 20, 30, 40, 50 g/L on the cell differentiation of Lactobacillus rhamnosusPN04.As results, L. rhamnosusPN04formed differentiated cells those were called minicells with highly significantnumber as 7870minicells in one ml starting culture with the density of cells of 2.57×109 cellsin modified MRS broth with lactose 50 g/l.The minicells incubated with paclitaxel (10µg/mL) and cephalosporin (10µg/mL) in 10 hours were detectedon antimicrobial activities onStaphylococcusaureus ATCC 25923,Escherichia coli ATCC 9637, Salmonella typhiATCC 19430, Candidaalbicans ATCC 14053, Pseudomonasaeruginosa ATCC 27853.By scanning electron microscope, the minicell size was less than 400 nm. The study reported theL. rhamnosusPN04 differentiated cells produced in lactose containing MRS which will play a role in probiotic preparation and drug delivery.
Keywords
Lactobacillus rhamnosus; Cell differentiation; Carbon sources; Probiotic formulation; Drug package
Download this article as:| Copy the following to cite this article: Khue N. T. H, Vinh D. T. T. Effects of Carbon Sources on Cell Differentiation of Lactobacillus rhamnosus PN04 and Applications. Biomed Pharmacol J 2013;6(2) |
| Copy the following to cite this URL: Khue N. T. H, Vinh D. T. T. Effects of Carbon Sources on Cell Differentiation of Lactobacillus rhamnosus PN04 and Applications. Biomed Pharmacol J 2013;6(2). Available from: http://biomedpharmajournal.org/?p=2685 |
Introduction
Probiotics play an significant role in immunological, digestive and could have a important effect in alleviating infectious disease in children. Currently, members of the genera Lactobacillus and Bifidobacterium are mainly applied (Sanders, 1993). Probiotics are used very popular but the approach may have some difficulties that are able to survive in the intestinal ecosystem and the host animal. To survive inside body conditions, microbes must overcome a number of physical and chemical fences in the gastrointestinal tract. These include gastric acidity and bile acid secretion. They are also usually used to study the antibacterial and anti-cancer discovery besides being effect on human immune system (Choi et al., 2006).
The effective cancer therapy continues to be a daunting challenge due mainly to considerable tumor cell heterogeneity, drug resistance of cancer cells, dose-limiting toxicity of chemotherapeutics, and difficulties of targeted delivery to tumors (MacDiarmid et al., 2011). Consequently, over the past decade a significant global effort has focused on the discovery and development of molecularly targeted drug delivery systems (DDSs) (MacDiarmid et al., 2009). Molecularly targeted drugs such as cell cycle inhibitors are being developed to achieve a higher degree of tumor cell specificity and reduce toxic side effects. A potential strategy to limit toxicity is to encapsulate the drug and target it directly to cancer cells. However, current strategies that used liposomes (Medina et al., 2004), nanoparticles (Brannon et al., 2001; Ferrari et al., 2005) or polymer therapeutics (Duncan, 2003) are hampered by shortcomings such as drug leakage lack of versatility in terms of packaging a diverse range of different drugs, thereby reducing drug potency, and difficulties in production scale-up, particularly for nanoparticles (MacDiarmid et al., 2007a). Recently, a promising new technology for targeted and intracellular delivery of chemotherapeutic drugs relies on using bacterially derived nano sized (100 – 400nm in diameter)particles (termed as minicells) to package a range of different chemotherapeutic drugs. This technique has been experimented successfully for both Gram-positive (Listeria monocytogenes strain) and Gram-negative bacteria Salmonella typhi (S. typhimurium), Escherichia coli (E. coli), Shigellaflexneri, Pseudomonas aeruginosa (P. aeruginosa); drug-packaged nano-sized particles effect apoptosis of tumor cells both in vitro and in vivo; and they also targeted to cancer cells in vivo with high specificity and, thus, delivered in high concentration in vivo without toxicity (MacDiarmid et al., 2007b). Besides, antibiotic resistance is increasing due to the target for treatment.It is necessary to develop new molecularly targeted drug delivery systems from Lactobacillus. Recently, there were studies on detection of minC (Nguyen et al., 2012) and minDfrom L. acidophilus for minicell study (Nguyen et al., 2013a; Nguyen et al., 2013b).
Questions regarding reliability, quality of those commercial products as well as safety issues have been raised. It is necessary to investigate the effects of carbon sources on the cell differentiation for further formulation and drug delivery. The investigation in L. rhamnosusstrainis to understand the effects of carbon source on different Lactobacillus strains. These points will be applied for the probiotic formulation and in vitro drug delivery using biocells, a bacterially-derived lactic acid bacteria carrier.
Material and Methods
Bacterial strains and growths conditions
Astrain Lactobacillus rhamnosusPN04isolated from HottuyniacordataThunb.(Nguyen et al., 2013a)was used in all experiments of this study.L.rhamnosusPN04 was grown in Lactobacilli MRS broth (Difco, Detroit, Mich., U.S.A) at 37°C in aerobic condition.
The preparation of inoculums started with transferring the stock cultures of L. rhamnosusPN04 into liquid MRS medium and incubated overnight at 37°C in aerobic condition. After the growth of culture, the microorganisms were transferred to the plate of solid MRS medium. The plate incubated at 37°C for 48h in order to allow sufficient growth of colonies. All plates were then stored at 4°C. Prior to each experiment, the Lactobacillus inoculums were individually prepared by inoculating a single colony of them into 10 mL media which was then incubated overnight at 37°C.
Biochemical tests forLactobacillusrhamnosus PN04
The strain was firstly isolated and determined by 16S rDNA (Nguyen et al., 2013a). In order to study the effects of carbon source on LactobacillusrhamnosusPN04, the API 50CHL kit (BioMerieux) was used to test the biochemical properties of this strain.
Screening the carbon source on the cell differentiation of LactobacillusrhamnosusPN04
According to biochemical reactions of Lactobacillus with sugar and to study the effectsof culturing,L. rhamnosusPN04were inoculated into modified Lactobacilli MRS broth which containing each of carbon sources separately: glucose, lactose, sucrose, maltose, fructose, in different concentration (0g/L, 5g/L, 10g/L, 15g/L, 20g/L, 30g/L, 40g/L, 50g/L) and incubated at 37ºC for 30h in order to produce differentiated cells which were called minicells. Microscopic cell morphologies were observed under a light microscope with a magnification of 100X.
Differentiated cell isolation
In order to determine the differentiated cells, the isolation techniques described in various combinations to obtain a preparation of desired isolation to homogeneity and free of contaminating parent bacterial cells, cellular debris. Initial differential centrifugation of cell culture was performed at 2000 g for 20 minutes, removes most parent bacterial cells. This step was thus incorporated after the initial 0.45 mm cross-flow filtration stepsto remove small contaminants like bacterial membrane debris. As a result, the finalsterilizedpreparation was checked for the growth on agar plates.The minicells should be not regenerated after incubation at 37ºC.
Drug packaging tests
To determine whetherminicells can be packaged with chemotherapeutic drugs and the kinetics of minicell in response to a concentration of drug in varying times of incubation. Preparations of purified minicells derived from L. rhamnosusPN04 will be separately incubated with the chemotherapeutic drug, paclitaxel (Sigma, U.S.A) (10µg/mL) and cephalosporin(Sigma, U.S.A)within 10 hours at 37°C with rotation. The minicells were harvested by centrifugation at 10 000 g for 5 min and resuspended in sterilebuffered saline gelatin (BSG).Minicellswere then washed 10 times with 10mL of BSG per wash solution.
Drugs were extracted from packaged minicells to prepare for antimicrobial activity. The extract was centrifuged for 5 minutes at 10 000 g to pellet debrisand the clear supernatant was sterilized by filtration (0.45 μm), thus yielding cell-free filtrates.
Antimicrobial activity tests
To examine whether the drugspackaged in the minicellsmaintained the activities, antimicrobial activities were tested on Staphylococcusaureus ATCC 25923, Escherichia coli ATCC 9637, Salmonella typhiATCC 19430, Candida albicans ATCC 14053, Pseudomonasaeruginosa ATCC 27853 by the agar diffusion method. The tested microorganisms were propagated twice and then grown for 18 to 24 h in 10 mL of appropriate growth media. Turbidity of the culture broth was compared with McFarland tubes to give an estimate of bacterial population (1 × 106 CFU/mL). Steriled paper discs (5 mm of diameter) were then prepared and dropped on using 20μL of cell-free filtrate. The inoculated plates were incubated for 18 to 24 h at appropriate temperatures. The measurements recorded were from the edge of the zone to the edge of the wall.
Scanning electron microscope
Morphology of the isolated minicells was observed by scanning electron microscopy (SEM, Jeol JSM-7401F) at 10-15kV. The differentiated cells were mounted on metallic studs with double-sided conductive tape. Finally, the sample preparationswere requiredthe ion coating with platinum by a sputter coater (JFC-1300, Jeol, Tokyo) for 40 seconds in a vacuum at a current intensity of 40 mA (Win, 2006).
Results and Discussion
Cell differentiation in Lactobacillus rhamnosus
Lactobacillus rhamnosus PN04 showed the biochemical tests on the sugars (Table 1, Figure 1). According to the results of biochemical tests of Lactobacillus rhamnosus (Table 1, Figure 1), glucose, lactose, sucrose, maltose, and fructose showed the positive effects based on the changing color from purple to yellow. The study also informed the data of Lactobacillusrhamnosus PN04 isolated in HottuyniacordataThunb. Then, five kinds of sugars including in glucose, lactose, sucrose, maltose, and fructose were used for testing the effect on cell differentiation oL. rhamnosus (Figure 2). The differentiated cells were formed together with the normal rod cells of L. rhamnosus (Figure 2). The differentiated cells were isolated for qualification and quantification.
Table 1: The API 50CHL biochemical testing of Lactobacillus Rhamnosus
| Strip | Substrate | Isolate |
| 0 | Control | negative |
| 1 | Glycerol | negative |
| 2 | Erythritol | negative |
| 3 | D-Arabinose | negative |
| 4 | L- Arabinose | negative |
| 5 | Ribose | positive |
| 6 | D-Xylose | negative |
| 7 | L-Xylose | negative |
| 8 | Adonitol | negative |
| 9 | Β Methyl-D-Xyloside | negative |
| 10 | Galactose | positive |
| 11 | Glucose | positive |
| 12 | Fructose | positive |
| 13 | Mannose | positive |
| 14 | Sorbose | positive |
| 15 | Rhamnose | positive |
| 16 | Dulcitol | negative |
| 17 | Inositol | negative |
| 18 | Mannitol | positive |
| 19 | Sorbitol | positive |
| 20 | α-Methyl-D-Mannoside | negative |
| 21 | α-Methyl-D-Glucoside | negative |
| 22 | N-Acetyl-Glucosamine | positive |
| 23 | Amygdalin | positive |
| 24 | Arbutin | positive |
| 25 | Esculin | positive |
| 26 | Salicin | positive |
| 27 | Cellobiose | positive |
| 28 | Maltose | positive |
| 29 | Lactose | positive |
| 30 | Melibiose | negative |
| 31 | Sucrose | positive |
| 32 | Trehalose | positive |
| 33 | Inulin | negative |
| 34 | Melezitose | positive |
| 35 | Raffinose | negative |
| 36 | Starch | negative |
| 37 | Glycogen | negative |
| 38 | Xylitol | negative |
| 39 | Gentiobiose | positive |
| 40 | D-Turanose | negative |
| 41 | D-Lyxose | positive |
| 42 | D-Tagatose | positive |
| 43 | D-Fucose | negative |
| 44 | L-Fucose | negative |
| 45 | D-Arabitol | negative |
| 46 | L- Arabitol | negative |
| 47 | Gluconate | positive |
| 48 | 2-Keto-Gluconate | negative |
| 49 | 5-Keto-Gluconate | negative |
 |
Figure 1: The API 50CHL biochemical testing of Lactobacillus rhamnosus; Positive tests corresponded to acidification revealed by the purple indicator contained in the medium changing to yellow; For the Esculin test (tube no. 25), a change in color from purple to black is observed.
|
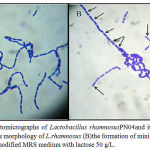 |
Figure 2: Photomicrographs of Lactobacillus rhamnosusPN04and its minicells (100X). (A) the morphology of L.rhamnosus (B)the formation of minicells (black arrow) in the modified MRS medium with lactose 50 g/L.
|
Differentiated cell quantitation
The differentiated cells were isolated and quantified as shown in Table 2 and Figure 3. The statistical analysis identified that carbon sources influenced significantly on the differentiation and the cells were generated depending on the sugar concentrations (p<0.05). As presented in Table 2, the lowest level of producing differentiated cells was found about 34,000 particles when L. rhamnosus was grown in the modified MRS media without sugar. The data presented that the number of obtained cells increased gradually since raising the concentration of sugars from 5 g/L to 50g/L. On the other hand, in the fructose and saccharose conditions, cell differentiation increased at low concentration of these sugars from 5 g/L to 10 g/Land then reduced at high concentration from 15 g/L to 50 g/L).Saccharose effect on the cell phenotype of the bacteria was the least. Actually, during minD expression, saccharose led to the filamentation in cells (Nguyen et al., 2013a). The morphology changed clearly while the wild type of L. rhamnosus was absolutely rod (Figure 1A).According to Table 2, most of the cells weredifferentiatedfrom 146 000 to 787 000 particles when using lactose as carbon source. In the maltose medium, the quantity of differentiated cells was lower in comparison with lactose condition (from 98 700 to 351 000 particles). Through the usage of glucose, the differentiated cells wereonly one half of those numbers in maltose condition. Fructose and saccharose had similar effects on cell differentiation such as between 48 200 to 151000 particles each. At the concentration of 50 g/L, the differentiated cellsproduced in modified MRS media with glucose were 164 000 particles while there were about 351 000 and 787 000 particles in maltose and lactose media, respectively. Meanwhile, the differentiated cells obtained from L. rhamnosus in fructose and saccharosemedia with the highest values at concentration of 10 g/Lin the optimal concentrations (Table 2). In summary, lactose was carbon source affecting on L. rhamnosuscell differentiation and showed the highest number in the concentration of 50 g/L (Table 2).Consequently, theL. rhamnosuscell differentiation occurred highly in MRS modified with lactose. The L. rhamnosus probiotic in the combination of lactose should be studied well. The differentiated cells were achieved by using a procedure to homogenize and eliminate contaminants such as parent bacterial cells, cellular debris. By filtering through the filter (0.45mm), the generated cells had the size less than 400 nm (Figure 4). Therefore, this study could produce the differentiated cells which were called minicellsand used as nano-cells. The study tried to test the storage in sterilebuffered saline gelatin (BSG) and check whether the shape recovered into the rod. Remarkably, the minicells still keep their own shape. The final minicell preparation was suggested to demonstrate the absence of bacterial colonies by plating on MRS agar plates and incubating overnight at 37ºC. The results meant that the buffered saline gelatin could be used as the best medium for storage the minicell for drug delivery.
Table 2: The number ofminicells produced from Lactobacillus Rhamnosus
| g/l | 0a, b | 5 a, b | 10 a, b | 15 a, b | 20 a, b | 30 a, b | 40 a, b | 50 a, b |
| Glucose | 3.39±0.72 | 3.46±0.75 | 6.87±1.59 | 7.48±2.15 | 8.13±2.03 | 9.38±1.97 | 11.7±1.85 | 16.4±1.97 |
| Lactose | 3.46±0.39 | 14.6±2.15 | 25.8±2.72 | 32.3±2.69 | 36.7±2.11 | 52.9±3.31 | 63.5±2.86 | 78.7±2.55 |
| Fructose | 3.41±0.36 | 9.61±2.77 | 15.1±1.40 | 13.9±1.76 | 12.6±2.11 | 11.9±1.93 | 10.5±1.57 | 9.68±2.54 |
| Maltose | 3.43±0.66 | 9.87±1.36 | 18.6±2.16 | 19.2±2.28 | 20.4±2.43 | 23.9±2.35 | 30.7±2.92 | 35.1±2.75 |
| Saccharose | 3.35±0.55 | 4.82±0.30 | 12.9±2.86 | 8.86±0.75 | 7.85±0.76 | 6.24±0.34 | 7.39±1.07 | 5.91±0.38 |
a Data are means ± standard deviations of triplicates for all treatments
b Expressed ×104
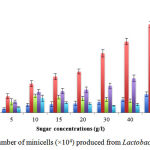 |
Figure 3: The number of minicells (×104) produced from Lactobacillus rhamnosus
|
Antimicrobial activity tests
To test the ability and potency of drug deliver of our minicells, the minicells were packaged with two different drugs incubated with different time. The extracted solutionwas centrifuged and used to test the activities on the above mentioned pathogens. The results showed the activities on both of gram negative and positive bacteria(Table 3).Presented in Table 3 was the inhibition of the growth of S. aureus ATCC 25923, E. coli ATCC 9637, S.typhiATCC 19430, C. albicans ATCC 14053, P . aeruginosa ATCC 27853. The inhibition zone diameters were 17 and19 mm for cephalosporin and paclitaxel, respectively and showed that there were significantly different at incubation time 10 hours at 37°C with rotation(P > 0.05). Therefore, the generated minicells could package many drugs. To test how the mincells could package the drugs, more studies should be done so far.
Table 3: Antibacterial activity of extracted paclitaxel and cephalosporin from drug-packaged minicells of Lactobacillus rhamnosus against bacterial species
|
Indicator Bacteria |
Zone of inhibition (mm) |
|
| Paclitaxel | Cephalosporin | |
| S. typhi | 13.6 ± 0.5 | 11.0 ± 1.0 |
| E. coli | 14.6 ± 0.5 | 12.0 ± 1.0 |
| C. albicans | 12.0 ± 1.0 | 8.3 ± 0.5 |
| P. aeruginosa | 19.3 ± 1.0 | 16.6 ± 0.5 |
| S. aureus | 18.0 ± 1.0 | 14.6 ± 0.5 |
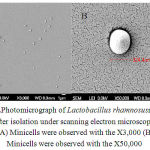 |
Figure4:Photomicrograp of Lactobacillusrhamnosusminicells after isolation under scanning electron microscope. (A) Minicells were observed with the X3,000 (B) Minicells were observed with the X50,000
|
Scanning electron microscopic analysis
The differentiated cells or minicellswere checked for the size and the structure of the minicells(Figure 4). Remarkably, the cells had the sizes from 200 – 400 nm which could be used as nanocells for drug delivery.
Conclusion
The study was the first report that L. rhamnosusPN04in the modified MRS broth with lactose (50g/L) gave the minicell quantity in significantly high number. Because of the cell differentiation, the formulation of L. rhamnosusin probiotic products, yogurts should be well considered. The study also detected the ability of paclitaxel and cephalosporin deliveringminicells by testing the antimicrobial activities on pathogens.The results revealed that carbon source actually affected on the minicell production or cell division of L. rhamnosusPN04 strain. Fructose and saccharose did not affect well on the L. rhamnosusPN04 strain. In the probiotics and yogurts,fructose or saccharose might be used. However, lactose can be used to produce the minicells for drug delivery. More studies should be done so far for the mechanisms and how minicells could package the different drugs.
Refrences
- Adler, H.I., Fisher, C.A, Hardigree, A.A. Miniature Escherichia coli cells deficient in DNA. Proc.Natl.Acad.Sci. USA., 1967; 57: 321-6.
- Brannon P.L. and Blanchette J.O. Nanoparticle and targeted systems for cancer therapy.Adv. Drug Deliv.Rev., 2004; 56: 1649–1659.
- Choi, S.S.,Kim, Y., Han, K.S., You, S., Oh, S., Kim, S.H. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett.Appl.Microbiol., 2006; 42(5): 452.
- Cummings,J. Method for determination of 40-deoxydoxorubicin, 40-deoxydoxorubicinol and their 7-deoxyaglycones in human serum by HPLC. J.Chromatogr.,1985; 341: 401–409.
- Duncan,R. The dawning era of polymer therapeutics.Nat. Rev.Drug Discov.,2003; 2: 347–360.
- Ferrari,M. Cancer nanotechnology: Opportunities and challenges. Nature. Rev. Cancer.,2005; 5: 161–171.
- Jenny, H.Z.Time and Concentration-Dependent Penetration of Doxorubicin in Prostate Tumors. AAPS Pharmsci.,2001; 3 (2): 1-9.
- Langer, R. Drug delivery and targeting.Nature.,1998; 392: 5-10.
- MacDiarmid, J.A., Madrid-Weiss, J., Amaro-Mugridge, N.B., Phillips, L., Brahmbhatt, H.Bacterially-derived nanocells for tumor-targeted delivery of chemotherapeutics and cell cycle inhibitors. Cell Cycle.,2007; 6: 2099-2105.
- MacDiarmid, J.A., Mugridge, N.B., Weiss, J.C., Phillips, L., Burn, A.L., Paulin, R.P., Haasdyk, J.E., Dickson, K.A., Brahmbhatt, V.N., Pattison, S.T., James, A.C., Al Bakri, G., Straw, R.C., Stillman, B., Grahman B., Graham, R.H., Brahmbhatt, H.Bacterially-derived 400 nm particles for encapsulation and cancer cell targeting of chemotherapeutics. Cancer Cell.,2007; 11: 431-445.
- MacDiarmid, J.A.,Amaro-Mugridge, N.B.,Madrid-Weiss, J., Sedliarou, I., Wetzel, S., Kochar, K., Brahmbhatt, V.N., Phillips L., Pattison, S.T., Petti, C., Stillman, B., Graham, R.H., Brahmbhatt, H. Sequential treatment of drug-resistant tumors with targeted minicells containing siRNA or a cytotoxic drug. Nat Biotechnology.,2009; 1-12.
- MacDiarmid, J.A., Brahmbhatt H. Minicells: Versatile vectors for targeted drug or si/shRNA cancer therapy. Current opinion in biotechnology.,2011; 22 (6): 909-916.
- Medina, O.P., Zhu, Y., and Kairemo, K. Targeted liposomal drug delivery in cancer.Curr. Pharm.Des.,2004; 10: 2981–2989.
- Nguyen, T.H.K., Doan, T.T.V., Ha, D.L., Nguyen, N.H. Cell division inhibitor minC from Lactobacillus acidophilus VTCC-B-871 and detection of antimicrobial activities in functional study. Afr. J. Pharm.Pharmacol.,2012; 8(48): 3293 – 3298.
- Nguyen, T.H.K., Doan, T.T.V., Ha, D.L.,Nguyen, N.H. Molecular cloning, expression of minD gene from Lactobacillus acidophilus VTCC-B-871 and analyses to identify Lactobacillus rhamosus PN04 from Vietnam HottuyniacordataThunb. Indian J.Microbiol.,2013a; 53: 385-390.
- Nguyen, T.H.K., Doan, T.T.V., Ha, D.L., Nguyen, N.H. Studies of the paclitaxel delivery and antimicrobial activities generated by MinD from Lactobacillus acidophilus VTCC-B-871. J. pure. appl. microbio., 2013b; 7(1): 379 – 384.
- Philippe, M. Safety aspects of probiotic products.Scand. J.Food.Nutr./Naringsforskning.,2001; 45: 22-24.
- Sanders, M.E. Summary of conclusions from a consensus panel of experts on health attributes of lactic cultures: significance of fluid milk products containing cultures. J. Dairy. Sci., (1993); 76:1819-1828.
(Visited 902 times, 1 visits today)







