N. N. Nwobodo¹*, P. O. Okonkwo² and S. A. Igwe¹
1Department of Pharmacology and Therapeutics, College of Medicine, Enugu State University of Science and Technology, Enugu Nigeria.
2Department of Pharmacology and Therapeutics, College of Medicine, University of Nigeria, Enugu Nigeria.
Abstract
Lipid metabolism of the parasite is associated with alterations in fatty acids and cholesterol in the erythrocyte plasma membrane, which in turn are responsible for changes in permeability and fragility. The augmentation of all the membrane systems of the infected erythrocyte causes the lipid content to rise rapidly, but the parasite lipid composition differs from that of the erythrocyte in many respects. Phospholipid metabolism has been identified as an ideal target for novel anti-malarial chemotherapy due to its vital importance to the parasite. This paper attempts to review the underlying lipid metabolic pathways in the malaria parasite and their potential benefit as likely targets for novel anti-malarial chemotherapy.
Keywords
Lipid metabolism; plasmodium; possible target; chemotherapy
Download this article as:| Copy the following to cite this article: Nwobodo N. N, Okonkwo P. O, Igwe S. A. Lipid Metabolism in Plasmodium: Implication as Possible Target for Chemotherapy. Biomed Pharmacol J 2011;4(1) |
| Copy the following to cite this URL: Nwobodo N. N, Okonkwo P. O, Igwe S. A. Lipid Metabolism in Plasmodium: Implication as Possible Target for Chemotherapy. Biomed Pharmacol J 2011;4(1). Available from: http://biomedpharmajournal.org/?p=1699 |
Introduction
The invasion of human erythrocytes by malaria parasite initiates Plasmodium development in a vacuole bound by erythrocyte-derived membrane, whose asymmetrical distribution of lipids is reversed in its orientation with respect to the parasite plasma membrane. The malaria parasite is incapable of synthesizing fatty acids de novo, but utilizes preformed fatty acids and lipids from the host. An enzyme capable of activating fatty acids, which is necessary for incorporation, into lipids has been localized to membranous structures found within the cytoplasm of the infected erythrocyte1. Lipid metabolism of the parasite may be associated with alterations in fatty acids and cholesterol in the erythrocyte plasma membrane, which in turn are responsible for changes in permeability and fragility2. A study evaluated the constitution of phospholipid classes and the content of cholesterol of various strains of Plasmodium falciparum-infected human erythrocytes grown in invitro cultures in conjunction with drug susceptibility3. Study reveals that uninfected erythrocytes in the culture serve as a major source for the increased lipid content of malaria-infected cells. The alterations of the phospholipid composition of infected cells that results from parasite lipid metabolism are also reflected in the constitution of uninfected red cells, implying lipid exchange between infected and uninfected cells.
Glycerolipid and fatty acid biosynthetic pathways
The augmentation of all the membrane systems of the infected erythrocyte causes the lipid content to rise rapidly, but the parasite lipid composition differs from that of the erythrocyte in many respects: it is higher in diacylphosphatidylethanolamine, phosphatidylinositol, diacylglycerols, unesterified fatty acids, triacylglycerols, hexadecanoic and octadecanoic fatty acids; but lower in sphingomyelin, phosphatidylserine, phosphatidylethanolamine, cholesterol and polyunsaturated fatty acids. Active lipid metabolism accompany the membrane proliferation associated with feeding, growth and reproduction of malaria parasite.
Lipid metabolism is absent from normal mature human erythrocytes4 but the phospholipid content increases by as much as 500% in the infected erythrocytes5,6. It has been previously shown that impairment of phospholipid biosynthesis with polar head analogs, which interfere with natural polar head incorporation either by substitution or competition7-9 with fatty acids10 is lethal to the intra-erythrocytic stage of Plasmodium falciparum in vitro.
In a study aimed at thoroughly studying the mechanism of action of compounds showing marked anti-malarial activity, the effect of the most active compounds on PC (phosphatidylcholine) and PE (phosphatidylethanolamine) biosynthesis was evaluated by measuring the incorporation of radioactive choline into PC as well as the incorporation of radioactive ethanolamine into PE using a cell harvester for rapid serial determination11. A study reported close correlation between impairment of phospholipid biosynthesis and inhibition of in vitro malaria parasite growth12. Compounds showing marked anti-malarial activity in the study, are assayed for their effects on phospholipid metabolism. It further revealed that the most active compounds are inhibitors of de novophosphatidylcholine biosynthesis from choline. The report highlighted that specific anti-malarial effects of choline or ethanolamine analogs, are thus likely mediated by their alteration of phospholipid metabolism; and contended that de novophosphatidylcholine biosynthesis from choline is a very realistic target for novel anti-malarial chemotherapy against pharmacoresistant strains.
Phospholipids are absolutely necessary for parasite membrane biogenesis and it has been shown that impairment of phospholipid metabolism is lethal to Plasmodium falciparum in vitro7-10. When considering the correlation between phospholipid metabolism impairment and parasite growth inhibition, molecules appear to segregate into two groups. The most whose IC50 is higher than 50µmol/L appear to be well distributed along the bisecting line (PL50=IC50). This contrasts with the second group that has a long alkyl chain and IC50 of less than 50µmol/L.
Lipid synthesis by malaria parasite is investigated by quantitatively measuring the parasite’s ability to incorporate 14C-labeled glucose carbon into various lipids in vitro12. Glucose is chosen as the substrate for lipid synthesis because glucose carbon serves as a primary source of the acetate units required for the de novo synthesis of fatty acids and sterols, as a source of the a-glycerol phosphate required for the synthesis of glycerides and phospholipids. Thin layer chromatographic procedures are used to separate the extracted lipid into neutral and phospholipids and to fractionate the neutral and phospholipids into various classes13,14. The individual neutral and phospholipid fractions are recovered from the plates and assayed for 14C content with a lipid scintillation counter15. Results reveal that lipid synthesis observed for all three cell synthesis represent primarily a synthesis of phospholipids; in all instances between 90 to 99% of the total 14C lipid activity is recovered from the phospholipids.
It has been shown that the malaria parasite is incapable of synthesizing lipids de novo and restricted to obtaining preformed fatty acids from the host. Several parasite enzymes involved in lipid biosynthesis from glycerides and free fatty acids as well as enzymes involved in the remodeling of lipid polar head groups have been identified16. A study to determine the in vitro incorporation of sodium acetate into the lipid classes of blood cells infected with malaria parasite reported that free fatty acids of both normal and infected plasma contain most of the activity found in their total lipids. The parasitized blood cells demonstrated greater incorporation of 14C into their lipids than did plasma or normal blood cells. The phospholipid fractions of normal and parasitized blood cells possess most of the 14C activity. A study reported a four-to-fivefold increase in the fatty acid content and a two-to-fourfold increase in phospholipid content of Plasmodium infected erythrocytes.
The high concentration of 14C tagged free fatty acids found in the infected plasma is possibly due to the increased metabolism within the infected cells. It has been shown from experimental evidence that sterols are produced to a greater extent in parasitized than in normal cells, since there is a significant amount of incorporation by sterols and sterol esters of the infected cells.
Several enzymes which are associated with the type II fatty acid synthetic pathway have been identified in Plasmodium and appear to be located in the apicoplast. Plasmodium homologues of enzymes involved in type II fatty acid synthesis has apicoplast targeting sequence and are sensitive to known inhibitors of type II fatty acid synthetase. This enzymatic biosynthetic pathway is a particularly attractive drug target for anti-malarial chemotherapy, since the human host synthesized fatty acids via different pathways utilizing different enzymes. A study revealed the discovery of two genes encoding type II fatty acid biosynthesis proteins: ACP (acyl carrier protein) and KAS III (beta-acetoacyl-ACP synthase III)17. The initiating steps of a type II system require a third protein: malonyl-co-enzyme A:ACPtranscyclase (MCAT). The study described the identification of a single gene from Plasmodium falciparum encoding PfMCAT and the functional characterization of this enzyme. Another study presents evidence that two members of the Plasmodium falciparum acyl-CoA synthetase (PfACS) family are responsible for the activation of long-chain fatty acids by thio-esterification with CoA18. It described co-immunoprecipitation of ankyrin and of ACS1/3 indicating that at least a fraction of these proteins are physically associated with the infected erythrocytes and provide evidence for a novel specific interaction which suggests that such a binding brought these enzymes closer to the host erythrocyte membrane where exogenous fatty acids are available.
A study evaluated the constitution of phospholipid classes and the content of cholesterol of various strains of Plasmodium falciparum-infected human erythrocytes grown in invitro cultures in conjuction with drug susceptibility3. Study reveals that uninfected erythrocyes in the culture serve as a major source for the increased lipid content of malaria-infected cells. The alterations of the phospholipid composition of infected cells that results from parasite lipid metabolism are also reflected in the constitution of uninfected red cells, implying lipid exchange between infected and uninfected cells.
The metabolism and dynamics of lipids in malaria infected erythrocytes have been extensively reviewed19. The phospholipid composition of infected is substantially different from that of normal red blood cells. This altered composition is achieved through various processes, of which de novo synthesis could supply all the needs of the parasite lipid anabolism provided adequate concentration of substrates is supplied. The in vitro culture system is obviously different from in vivo conditions where the large systemic increase in plasma fatty acids and triacylglycerols upon infection can serve the parasite’s lipid metabolism.
The bi-product of lipid metabolism are reactive oxygen intermediates such as superoxide, hydroxyl radical and hydrogen peroxide. These reactive oxygen intermediates (ROIs) damage Plasmodium lipids. Glutathione peroxidase is involved in the detoxification of these reactive oxygen intermediates. Oxidized glutathione is recycled and the reducing equivalents of NADPH generated probably by pentose phosphate cycle. However, it has been proposed that glutamate dehydrogenase provides the reduced NADPH needed for glutathione reductase20. Interestingly, the malaria parasite may supply the host erythrocyte with glutathione which could participate in protecting the host cell from oxidative damage21. It should be noted that the parasite lipid metabolism is intertwined with that of the host’s because of the intimate relationship between the host and parasite.
The biosynthesis of sphingolipidsde novo has been described in Plasmodiumfalciparum22. Studies of intra-erythrocytic development of Plasmodium falciparum have established that sphingomyelin is synthesized by a parasite-specific enzyme23-25, and was important for parasite-mediated nutrient uptake26. However, in contrast to other eukaryotic cells, no discernible amounts of steryl esters are produced, and cholesterol is nearly absent in the malaria parasite27.
The plasma membrane of infected erythrocyte contains more phosphatidylcholine and phosphatidylinositol and less sphingomyelin than the plasma membrane of normal uninfected erythrocyte28. Large increases in the levels of palmitic (C16:0) and oleic (C18:1) acids and major decreases in the levels of polyunsaturated fatty acids such as arachidonic (C22:6) acids, occur as a result of malaria infection. This makes the phospholipid composition very similar to that of acids, such as arachidonic (C20:4) and docosahexanoic (C22:6) acids as a result of infection. Thus, the phospholipid composition is very similar to that of the parasite, indicating that there is intense dynamic phospholipid traffic between the erythrocyte membrane and the membrane of the intracellular parasite. These modifications must be as a result of parasite metabolism of erythrocyte lipids, since mature erythrocytes have negligible lipid synthesis and metabolism. Several studies have shown that the biosynthetic machinery of the parasite can provide all of the new phospholipid molecules.
The intra-erythrocytic stages of Plasmodium falciparum accummulate triacylglycerol, produced using oleate and diacylglycerol as substrates27,29. In contrast to other eukaryotic cells, neither steryl esters nor cholesterol esters, the second neutral lipid species reported to be important for a related apicomplexan, Toxoplasma gondii are detected in Plasmodium falciparum.
The genome of Plasmodium falciparum contains the genes for fatty acid synthase II (FAS II) pathway30. Previous studies have identified and located the FAS II enzymes in the apicoplast31,32. In the FAS II pathway each reaction is catalysed by a discrete enzyme33. Malonyl-ACP is the starting point and is produced from malonyl CoA and ACP catalyzed by the enzyme Malonyl CoA: ACP transacetylase (fabD).
The enzymes of the fatty acid biosynthetic pathway, in the apicomplexan parasites including the Plasmodium species, are predicted to be localized in the apicoplast; based on the N-terminal extensions suggesting such a localization34. Malonyl-CoA itself formed from acetyl-CoA and a single acetyl-CoA carboxylase (ACCase) is predicted to be localized to the apicoplast in the Plasmodium genome. Among the enzymes of the FASII pathway in Plasmodium falciparum, fabI or enoyl-ACP reductase, which catalyses the final step in the chain elongation cycle, has been investigated in great detail from the viewpoint of identifying potent inhibitors. The enzyme catalyses the conversion of trans-2-acyl-ACP to acyl-ACP and requires NADH as the cofactor. The creation of transgenic Plasmodium berghei parasites with the Pfenoyl-ACP reductase replacing the endogenous counterpart, is another interesting development to serve as an in vivo mice model35 for studying drug efficacy. Three condensing enzymes b-ketoacyl-ACP synthases (PfKAS I, II, III) essentially involved in chain initiation and fabB/F (I,II) involved in chain elongation are also being investigated as possible drug targets.
Isoprenoid biosynthetic pathway
The malaria parasite genome provides evidence for the presence of the non-mevalonate pathway for isopentenyl pyrophosphate (IPP) biosynthesis. The presence of 1-deoxy-D-xylose-5-phosphate (DOXP) synthase in the parasite genome has been reported36. Isoprenoids, consisting of isopentenyl pyrophosphate (IPP) repeat units form prosthetic groups of some enzymes and are involved in the synthesis of ubiquinone and dolichol. The DOXP pathway has been fully characterized37. The mechanism of export of IPP is not clear, but the close association of the apicoplast with the mitochondrion is visualized to facilitate its import and ubiquinone biosynthesis in the mitochondrion. Thus inhibitors of DOXP reducto-isomerase may have anti-malarial activity36. The formation of isopentenyldiphosphate and dimethylallyldiphosphate, both central intermediates in the biosynthesis of isoprenoids in Plasmodium falciparum, occurs via the methylerythritol phosphate (MEP) pathway.
Interestingly, metabolic profiles show that DOXP and CDP-ME (4-[cytidine-5-diphospho]-2C-methyl erythritol) are highly accumulated when compared to the other intermediates, mainly in the trophozoite and schizont stages. It is considered that both DOXP and CDP-CE could act as a metabolite reserve, which might be used during schizogony to sustain high demand of isoprenoids, and both intermediates might be key metabolites of the MEP pathway in Plasmodium falciparum. The metabolic results are correlated with the transcript profiles of genes involved in the MEP pathway. Results indicate that MEP pathway metabolite peak preceded maximum transcript abundance during the intra-erythrocytic cycle. The MEP pathway associated transcripts are mostly altered by the drug, indicating that parasite is not strongly responsive at the transcript level. A combined analysis of metabolic and transcription profiles may be a useful procedure for the identification of candidate enzymes as novel drug targets38.
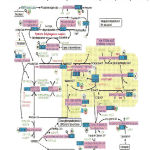 |
Figure 1: Pathway of Lipid Biosynthesis in the Apicoplast of Malaria Parasite. (Retrieved on-line from PubMed http://sites.buji.ac.il/malaria/maps/facidsynthesispath.html) |
Conclusion
A better understanding of the parasite’s lipid metabolism may lead to the development of novel therapeutic strategies which exploit the uniqueness of the malaria parasite.
This lends credence to the existence of novel mechanisms and pathways to malaria infection, thus describing a new intervention strategy in the fight against malaria.
References
- Matesanz F., Duran-Chica I. and Alcina A. The cloning and expression of PfACS1, a Plasmodium falciparum fatty acyl co-enzyme Asynthetase targeted at host erythrocyte cytoplasm. J. Mol. Biol. 291: 59-70 (1999).
- Holz G.G. Lipids and the malaria parasite.Bull. W.H.O. 55(2-3):237-248 (1977).
- Shalmiev G. and Ginsburg H. The susceptibility of the malaria parasite Plasmodiumfalciparum to quinoline-containing drugs is correlated to the lipid composition of the infected erythrocyte membranes. Biochemical Pharmacology.46(3): 365-374 (1993).
- Van Deenen L.L.M. and De Gier J. Lipids of the red cell membrane, in Surgenor G. (ed): In the Red Blood Cell. New York, NY, Academic.p.147 (1975).
- Sherman L. Biochemistry of Plasmodium. Microbiol. Rev. 43: 453 (1979).
- Vial H.J., Ancelin M.L, Philippot J.R. and Thuet M.J. Biosynthesis and dynamics of lipids in Plasmodium infected mature mammalian erythrocytes.Blood Cells.16: 551-555 (1990).
- Vial H.J., Thuet M.J., Ancelin M.L., Philippot J.R. and Chavis C. Phospholipid metabolism as a new target for malaria chemotherapy. Mechanism of action of D-2-amino-1-butanol.Biochem. Phamacol.13: 2761 (1984).
- Ancelin M.L, Vial H.J. and Philippot J.R. Inhibitors of choline transport into Plasmodium-infected erythrocyte are effective anti-plasmodial compounds in vitro. Biochem. Phamacol.34: 4068 (1985).
- Ancelin M.L. and Vial H.J. Quaternary aminonium compounds efficiently inhibit Plasmodium falciparum growth in vivo by impairment of choline transporters. Antimicrobial Agents Chemother.29: 814 (1986).
- Beaumelle B.D. and Vial H.J. Correlation of the efficiency of fatty derivatives in suppressing Plasmodium falciparum growth in culture with their inhibitory effect on acyl-CoA synthetase activity. Mol. Biochem. Parasitol.28: 39 (1988).
- Ancelin M.L, Vialettes F. and Vial H.J. An original method for rapid serial determination of phospholipid biosynthesis.Applications to mammalian lymphocytic cells and a lower eukaryote, Plasmodium falciparum. Anal. Biochem.199: 203 (1991).
- Cenedella R.J. Lipid synthesis from glucose carbon by Plasmodium berghei in vitro. Am. J. Trop. Med. Hyg. 17(5): 680-684 (1968).
- Skipski V.P., Peterson R.F. and Barclay M. Separation of phosphatidyl ethanolamine, phosphatidylserine and other phospholipids by thin layer chromatography. J. Lipid Res.3: 467-470 (1962).
- Krell K. and Hashim S.A. Measurements of serum triglycerides by thin-layer chromatography and infrared spectrophotometry. J. lipid Res. 4: 407-412 (1963).
- Synder F. and Stephens N. Quantitative carbon 14 and tritium assay of thin layer chromatography plates. Anal.Biochem.4: 128-131 (1962).
- Mitamura T. and Palacpac N.M.G. Lipid metabolism in Plasmodium falciparum infected erythrocytes: possible new targets for chemotherapy. Microbes and Infection.5: 545-552 (2003).
- Prigge S.T., He X., Gerena L., Waters N.C. and Reynolds K.A. The initiating steps of a type II fatty acid synthetase in Plasmodium falciparum are catalyzed by PfACP, PfMCAT and PfKAS III. Biochemistry.42(4): 160-169 (2003).
- Tellez M., Matesanz F. and Alena A. The C-terminal domain of the Plasmodium falciparum Acyl-CoA synthetase, PfACS1 and PfACS3 functions as ligand for ankyrin. Mol. Biochem. Parasitol.129(2): 191-198 (2003).
- Vial H.J. and Ancelin M.L. Malaria Lipids, an overview. In: Subcellular Biochemistry. Intracellular Parasites (Eds. Avila J.L. and Harris J.R.). Plenum Press, New York. 18: 259-306 (1992).
- Krauth-Siegel R.L., Muller J.G., Lottspeich F. and Schinner R.H. Glutathione reductase and glutamate dehydrogenase of Plasmodium falciparum, the causative agent of tropical malaria. Eur. J. Biochem. 235: 345-355 (1996).
- Atamna H. and Ginsburg H. The malaria parasite supplies glutathione to its host cell: investigation of glutathione transport and metabolism in human erythrocytes infected with Plasmodium falciparum. Eur. J. Biochem. 250: 670-679 (1997).
- Gerold P. and Schwarz R.T. Biosynthesis of glycosphingolipidsde novo by the human malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol.112: 29-37 (2001).
- Ansorge I., Jeckel D., Wieland F. and Lingelbach K. Plasmodium falciparum-infected erythrocytes utilize a synthetic truncated ceramide precursor for synthesis and secretion of truncated sphingomyelin. Biochem. J. 308: 335-341 (1995).
- Elmendorf H.G. and Haldar K. Plasmodium falciparum exports the Golgi marker sphingomyelin synthase into a tubovesicular network in the cytoplasm of mature erythrocytes. J. Cell Biol. 124: 449-462 (1994).
- Haldar K., Uyetake L., Ghori N., Elmendorf H.G. and Li W.L. The accumulation and metabolism of a fluorescent ceramide derivative in Plasmodium falciparum-infected erythrocytes.Mol. Biochem. Parasitol.49: 143-156 (1991).
- Lauer S.A., Ghori N. and Haldar K. Sphingolipid synthesis as a target for chemotherapy against malaria parasites. Proc. Natl. Acad. Sci. USA92: 9181-9185 (1995).
- Vielmeyer O., Mclntosh M.T., Joiner K.A. and Coppens J. Neutral lipid synthesis and storage in the intra-erythrocytic stages of Plasmodium falciparum. Mol.Biochem. Parasitol.135: 197-209 (2004).
- Hsiao L.L., Howard R.J., Aikawa M. and Taraschi T.F. Modification of host cell membrane lipid composition by the intra-erythrocytic human malaria parasite, Plasmodium falciparum-infected erythrocytes.Mol. Biochem. Parasitol.49: 143-156 (1991).
- Nawabi P.L., Lykidis A., Ji D. and Haldar K. Neutal lipid analysis reveals elevation of acylglycerols and lack of cholesterol esters in Plasmodium falciparum-infected erythrocytes. Eukaryot.Cell.2: 1128-1131 (2003).
- Gardner M.J., Hall N., Fung E., White O. and Berriman N. Genome sequence of the human malaria parasite Plasmodiumfalciparum. Nature.419: 498-511 (2002).
- Waller R.F., Reed M.B., Cowman A.F. and McFadden G.I. Protein trafficking to the plastid of Plasmodium falciparum is via the secretory pathway. EMBO J.19: 1794-1802 (2000).
- Gardner M.J., Tettelin H., Carucci D.J., Cummings L.M., Aravind L., Koonin E.V., Shallom S., Mason T., Yu K., Fujii C., Pederson J., Shen K., Jing J., Aston C., Lai Z., Schwartz D.C., Pertea M., Salzberg S., Zhou L., Sulton G., Clayton R., White O., Smith H.O., Fraser C.M., Adams M.D., Venter J.C. and Hoffman S.L. Chromosome 2 sequence of the human malaria parasite Plasmodium falciparum. Science.282: 1126-1132 (1998).
- Harwood J.L. Recent advances in the biosynthesis of plant fatty acids. Biochem.Biophys.Acta.1301: 7-56 (1996).
- Foth B.J. and Mcfadden G.I. The apicoplast: a plastid in Plasmodium falciparum and other apicomplexan parasites. Int. Rev. Cytol. 224: 57-110 (2003).
- Warna-Mukhi P.L., Sharma S.K., Kapoor M., Suroita N., Suroila A. and Suguna K. Crystallization and preliminary crystallographic analysis of a-hydroxyacyl ACP dehydratase (Fab Z) from Plasmodium falciparum. ActaCrystallogr.60:120-121 (2004).
- Jomaa H., Wiesner J., Sanderbrand S., Altincicek B., Weidemeyer C., Hintz M., Turbachova I., Eberl M., Zielder J., Lichtenthaler H.K., Soldati D. and Beck E. Inhibitors of the non-mevalonate pathway of isoprenoid biosynthesis as anti-malarial drugs. Science.285: 1573-1576 (1999).
- Wilson R.J.M. Parasitic plastids: approaching the end game. Biol. Rev. 80: 129-153 (2005).
- Urbanczyk-Wochniak E., Luedemann A., Kopka J., Selbig J., Roessner-Tunali U., Willmitzer L. and Fernie A.R. Parallel analysis of transcript and metabolic profiles: a new approach in systems biology. EMBO Rep.4: 989-993 (2003).







