Manuscript accepted on :June 28, 2011
Published online on: 27-11-2015
Plagiarism Check: Yes
R. Mensudar
Department of Conservative Dentistry and Endodontics, Sree Balaji Dental College and Hospitals, Chennai (India)
Abstract
Objective Today with the advent of dental bioinformatics, a scientific revolution has evolved in the field of cell and molecular biology, which have given an important contribution by showing the phenomena that occur at the cellular level and elucidating the behaviour of various signals and messengers. The purpose of this study was to develop the insilico model of vascular endothelial growth factor and to determine its interaction with an inhibitor protein, Terrein. Material and Methods The structure of VEGF from Homo sapiens was modelled using Modeler9v3 software. Validation of the modelled structure was done through Procheck. The Q-Site Finder recommends the active site of VEGF and the detailed docking analysis of the complex structure and its interaction energies with Terrein was derived by the docking study using Auto Dock. Results The analysis of the results strongly suggests the potential role of terrain as an anti-inflammatory modulator in pulpal inflammation. Conclusion Within the limitations of the present study, it was concluded that Terrein is a potent anti-inflammatory modulator and play a vital role in the pathogenesis of pulpal inflammation by inhibiting the key regulating growth factor (VEGF).
Keywords
Pulpitis, terrein; vascular endothelial growth facto; bioinformatics; dental pulp; angiogenesis
Download this article as:| Copy the following to cite this article: Mensudar R. Insilico Modeling of Vascular Endothelial Growth Factor and its Interaction with Inhibitor Terrein. Biomed Pharmacol J 2011;4(1) |
| Copy the following to cite this URL: Mensudar R. Insilico Modeling of Vascular Endothelial Growth Factor and its Interaction with Inhibitor Terrein. Biomed Pharmacol J 2011;4(1). Available from: http://biomedpharmajournal.org/?p=1866 |
Introduction
Human dental pulp tissue is a loose connective tissue, which is surrounded by the low compliance of dentin and enamel, which is susceptible to tissue damage when ever there is an increased inflammation. Understanding of pulpal inflammation, pain and healing processes is of great importance in the practice of dentistry. Pulpal pain has been widely researched, yet there are number of unravelled clinical conditions, which are still not well understood.[1, 2] The most challenging common clinical conditions which are encountered in our day to day practices is of symptomatic pulpitis, post-extirpation pain, individual variability of dental pain, or an acutely inflamed pulp. Recent neurophysiological studies have shown that sensitized nerve fibres release various neuropeptides (NP), which in turn modulate the inflammatory process.[3]
During pulpal inflammation, the peripheral inflammatory mediators stimulate and sensitize the sensory nerve fibers (nociceptors) to release the stored NPs. Vasoactive inflammatory substances such as histamine, bradykinin, serotonin, prostaglandins (PGs), and leukotrienes are known mediators that increase the vascular permeability in pulp tissues.[4] Recently, vascular endothelial growth factor (VEGF), which is a glycoprotein that has attracted attention as a potent inducer of vascular permeability (angiogenesis) and is involved in the occurrence and progression of inflammation. However, it was recently demonstrated that VEGF is also produced by human pulp cells (HPC) and that VEGF is mitogenic not only on vascular endothelial cells but also on HPC, in an autocrine manner.[ 5,6] Thus VEGF play a critical role in increasing the vascular permeability of pulp tissue and its activity is 50,000 times stronger than that of histamine.
With the advent of scientific technology, a major advancement has been made in the research field. The word bioinformatics has become a very popular buzz word in the recent scenario. Many scientists find bioinformatics exciting because it holds the potential to divide into a whole new world of uncharted territory. Bioinformatics is a new science and a new way of thinking that could potentially lead to many relevant biological discoveries. It mainly involves the integration of computers, software tools and databases in an effort to address the biological questions. The primary goal of bioinformatics is to increase our understanding and unravel the various mysteries of biological processes. The major research field include
Sequence alignment
Gene finding
Genome assembly
Protein structure alignment
Protein structure prediction
Prediction of gene expression and
Protein-protein interactions
Taken together the existing research based evidence that demonstrates that VEGF is expressed by dental pulp cells and it plays a vital role in the pathogenesis of pulpal inflammation. The purpose of this study was to generate a 3 dimensional model for vascular endothelial growth factor and to find its interaction with a potent inhibitor, namely Terrein.[7]
Materials and Methods
Modeling VEGF
Template Selection
The critical first step in homology modeling is the identification of the best template structure, if indeed any are available. The simplest method of template identification relies on serial pairwise sequence alignments aided by database search techniques such as FASTA and BLAST. When performing a BLAST search, a reliable first approach is to identify hits with a sufficiently low E-value, which are considered sufficiently close in evolution to make a reliable homology model. However, a template with a poor E-value should generally not be chosen, even if it is the only one available, since it may well have a wrong structure, leading to the production of a misguided model.
In this study VEGF protein from H.sapiens which had a percentage identity of 51%, with the template was selected. The template sequence was also submitted to Pfam, PROSITE Data Bases for the pattern, profiles and domains analysis obtained were compared with the target sequence. The vascular endothelial growth factor protein was modelled using 1Wq9, as a template. The amino acid sequence of 1wq9 is got from the PDB database.
The homology modeling procedure can be broken down into four sequential steps:
Template selection
Target-template alignment
Model construction (Modeller9v3)
Model assessment. (Ramachandran plot)
Target Template Alignment
In this study the target template alignment was done using MODELLER 9v3. The MODELLER runs in the command line using the language python. The steering file used for running the MODELLER for the alignment of the target and the template is as follows:
The steering files for the alignment between the target protein and the template protein
# Directories with input atom files:
env.io.atom_files_directory = ‘./:../atom_files’
aln = align_strs_seq(env,
# Name of the input file specifying templates and the
# target sequence:
segfile=’alignment.seg’,
# Output alignment filename (‘PIR’ format)
alnfile=’alignment.ali’,
# Structures’ PDB codes:
knowns=(‘1wq9′),
# Target sequence code:
sequence=’pulpitis’,
# Identity matrix filename:
matrix_file=’fer2.id.mat’,
overhang=4,
# Write out the superposed structures:
write_fit=True)
# Write out the alignment in the ‘PAP’ format, too:
aln.write(file=’alignment.pap’, alignment_format=’PAP’)
Model Building using MODELLER 9v3
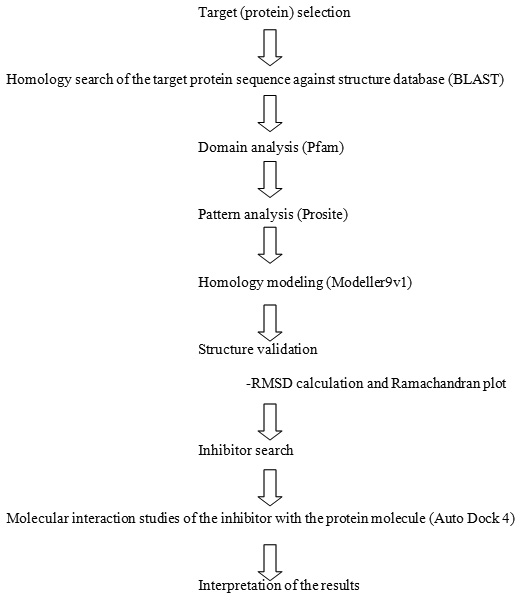
The refined sequence-structure alignment as obtained by MODELLER 9v3was used to construct 3D models of the target with the help of the known structures of the template using the same tool MODELLER 9v3 (FIG 1).
The steering file to generate the model:
# directories for input atom files
env.io.atom_files_directory = ‘./:../atom_files’
a = automodel(env,
alnfile = ‘alignment.ali’, # alignment filename
knowns = ‘1wq9’, # codes of the templates
sequence = ‘pulpitis’) # code of the target
a.starting_model= 1 # index of the first model
a.ending_model = 1 # index of the last model
# (determines how many models to calculate)
a.make() # do the actual homology modeling
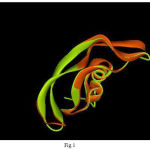 |
Figure 1: Superimposed 3D model (VEGF and 1Wq9) |
Docking
In the field of molecular modeling, docking is a method which predicts the preferred orientation of one molecule to a second when bound to each other to form a stable complex. Knowledge of the preferred orientation in turn may be used to predict the strength of association or binding affinity between two molecules using for example scoring functions. The docking was done using Autodock. AutoDock is a suite of automated docking tools. It is designed to predict how small molecules, such as substrates or drug candidates, bind to a receptor of known 3D structure. AutoDock actually consists of two main programs: AutoDock performs the docking of the ligand to a set of grids describing the target protein; AutoGrid pre-calculates these grids (FIG 2).
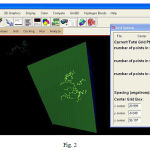 |
Figure 2: Grid Box |
Results
Model Evaluation
PROCHECK checks the stereochemical quality of a protein structure, producing a number of Postscripts plots analyzing its overall and residue-by-residue geometry. The stereo chemical quality of the model was checked by plotting the Ramachandran plot in the RAMPAGE (Structural validation by assessment of the Ramachandran plot). In the present study, the stereo-chemical quality of the VEGF model was checked using RAMPAGE. The modelled protein was submitted to the RAMPAGE server and was assessed by plotting the Ramachandran plot. The Ramachandran plot for the modelled structure had 95.0% in the most favoured region, 8.4% in the additional allowed region, 1.4% in the generally allowed region and 0% in the unfavourable region. The active site of the VEGF protein where ligand binds is got from Q-site finder (TYR21, CYS22, HIS23, PRO24, CYS53, GLY 55, LEU62, GLU63, CYS64, VAL65, and PRO66).
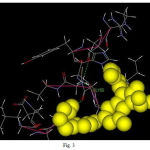 |
Figure 3: Protein ligand interaction |
The analysis of the results showed that VEGF, a potent mitogen for endothelial cells, which promotes angiogenesis shows a strong interaction with the ligand protein, Terrein (FIG3). Therefore it was strongly suggested that Terrein can be a potent anti-inflammatory modulator in the pathogenesis of pulpal inflammation.
Discussion
Pain represents a major stimulus for patients who seek emergency dental treatment. On the other hand, fear of pain represents a significant barrier that discourages patients from seeking routine dental care. Regulation of peripheral NPs release may provide a new therapeutic approach for managing pain and inflammation that accompanies injuries to the pulp and periapex. The understanding of the molecular mechanisms underlying the regulation of neovascularisation and vascular permeability observed in teeth with pulpitis may contribute to the development of therapeutic strategies designed to control these responses and prevent pulp necrosis. It was recently demonstrated by immunohistochemistry that the expression of VEGF is strongly positive in cells constituting the inflammatory infiltrate of teeth with irreversible pulpitis (Artese et al., 2002). Hence experiment to evaluate the effect of blockade of VEGF signalling pathways on the survival of pulp cells on teeth was undertaken.
The dental pulp is a specialized loose connective tissue, containing cells, fibers, ground substance, blood vessels, and nerve endings. It is a low compliance tissue enclosed within the rigid cell walls of dentin. In some pathological clinical situations there is an increase in vascular density. However, excessive blood vessel development can be deleterious and can lead to irreversible pulp pathology because the pulp tissue has limitations for release of internal pressure. The most common cause of pulpitis is the presence of cariogenic bacteria and their byproducts.[3] Vascular endothelial growth factor that is produced by human dental pulp cells is also know as the vascular permeability factor, is a glycoprotein that has the ability to increase the permeability of blood vessels, an important vascular change observed during the inflammatory process.[6] Thus it plays a critical role in angiogenesis and neovascularization, which actually can increase and extend the severity of inflammatory process. VEGF growth factor has a significant homology with platelet derived growth factor which another homology of VRGF family member.
Role of VEGF in Pulpitis
An increase of vascular permeability is involved in the acute phase of pulpitis as well as in acute inflammation elsewhere in the body. However, in the case of pulpitis, an excessive increase of vascular permeability easily results in oedema and necrosis, due to the specific anatomic characteristics of the pulp tissue. Since, the pulp tissue is enclosed in a rigid structure, and blood is supplied only through a small apical foramen, this foramen is used for both blood supply and drainage.[8,9] Furthermore, pulp tissue has no collateral blood supply, so it is difficult to rapidly eliminate filtrated fluid. Thus, pulp tissue, having such a limited system of discharge, is susceptible to irreversible pulpitis. Thus angiogenesis and vasodilatation are well established mechanisms in pulpitis and their key regulator is vascular endothelial growth factor (VEGF).[10,11]
Terrein
In this project an inhibitor, Terrein is used as the ligand binding with the VEGF. Terrein is a bioactive fungal metabolite whose anti-inflammatory properties are virtually unknown.[12] Prediction of protein function using computational support becomes an important tool as the gap between the increasing amount of sequences and the experimental characterization of the respective protein widens. Knowledge of the three dimensional (3D) structure of a protein is a basic requisite for better understanding of its function.[13] It is essential in almost every area of protein research such as enzyme kinetics, ligand – protein binding studies gene characterization and construction, structure based designing of drugs and rational designing of proteins.
In the present study the structure of VEGF from Homo sapiens was modelled using Modeler9v3. Validation of the modelled structure of VEGF from Homo sapiens suggests that the modelled structure is a good model. Q-Site Finder recommends the active site of VEGF. The detailed docking analysis of the complex structure and its interaction energies with Terrein derived by the docking study suggests Terrein to be a potent inhibitor for VEGF. Hence VEGF and its signalling molecules could be used as a therapeutic target to prevent the progression of pulpitis. Hence in future, we can anticipate that this “informatics revolution” in both bioinformatics and dental bioinformatics can help further our understanding of the mechanisms underlying the biological challenges in dentistry. In this approach we can eventually change the current practice of dentistry including diagnostics, therapeutics, and prognosis of common oral diseases.
Conclusion
Within the limitations of the present study, it might be concluded that Terrein is potent anti-inflammatory modulator and its role in the pathogenesis of pulpal inflammation by inhibiting the key regulating growth factor (VEGF). However relatively a very little is known about Terrein and its biological effects are almost unknown. In future bioinformatics can be an emerging field in the biomedical research community and had been gaining momentum in dental medicine.
References
- Matthews B, Andrew D. Microvascular architecture and exchange in teeth. Microcirculation 1995;2:305-13.
- Kim S, Liu M. Effects of selected inflammatory mediators on blood flow and vascular permeability in dental pulp. Proc Finn Dent Soc 1992;88:387-92.
- Narhin M, Kutta J. Dental Caries Progression Correlates with VEGF up-regulation in Patients. Scand J Dent Res 1979;87:23-25.
- Ribatti D. The crucial role of vascular permeability factor in angiogenesis: A Historical Review. Br J Haematol 2004;128:303-9.
- Ferrear N. Vascular endothelial growth factor, basic science and clinical progress. Endocr Rev 2004;25:581-611.
- Ferrara N, Gerber HP. The biology of VEGF and its receptor. Nat MED 2003; 9:669-76.
- Matsushita K, Motani R, Abeyama K, Maruyama I, Torii M. Terrein a new melanogenesis inhibitor and its mechanism. Cell Mol Life Sci 2004;61:289-90
- Artese L, Rubin, Ferrero G, Fioroni M, Santinelli A, Piattelli A. Vascular Endothelial Growth Factor (VEGF) Expression in Healthy and Inflamed Human Dental Pulps. Journal of Endodontics 2002; 28: 20-23.
- Rickoff B. Induction of vascular endothelial growth factor expression in human pulp fibroblasts stimulated with black-pigmented Bacteroides. Journal of Endodontics 1985;85:22-25.
- Teschein B, Scilnder H. Lipopolysaccharide Enhances the Production of Vascular Endothelial Growth Factor by Human Pulp Cells in Culture. Journal of Endodontics 1999;4:1633-1639.
- Derringer KA, Linden RW. Vascular endothelial growth factor (VEGF) expression healthy and inflamed human dental pulps. Journal of Endodontics 2004; 28:20-23.
- Telles PD, Hanks CT, Machado MA. Terrein, a fungal metabolite, inhibits the epidermal proliferation of skin equivalents. J Nat Prod 2002; 65: 345-50.
- Ivanenkov YA, Balakin KV. New Approaches to the Treatment of Inflammatory Disease: Focus on Small-Molecule Inhibitors of Signal Transduction Pathways. Review Article Drugs in R & D 2008;6:397-434







