Manuscript accepted on :June 07, 2011
Published online on: 27-11-2015
Plagiarism Check: Yes
P. Vijayalakshmi and K. Moorthy*
Department of Microbiology, Vivekanandha College of Arts and Science for Women, Elayampalyam - 637 205, Tiruchengode, Namakkal District,Tamil Nadu India
Corresponding Author E-mail:moormicro@gmail.com
Abstract
Pearl spot (discolourised skin, hemorrhagic patches, bloody blotches around fins and mouth of fish) Etroplus suratensis, Cichlidae family were identified at backwaters of Muttukadu, CIBA and Chennai. Diseased fishes were collected and the causative agent isolated from tissues of kidney. A control set of fishes were inoculated with Vibrio vulnificus and it produces the identical signs of vibriosis. Based on morphological and biochemical characters, the pathogen identified as V. vulnificus. Further, the virulence of V. vulnificus tested by Lethal Dosage (LD50) experiment and sensitivity/resistant patterns also assessed by Bauer–Kirby method.
Keywords
Etroplus suratensis; Vibrio vulnificus; Lethal dosage; AST
Download this article as:| Copy the following to cite this article: Vijayalakshmi P, Moorthy K. Assessment of Virulence and Antimicrobial Susceptibility of Vibrio vulnificus Isolated from Etroplus suratensis (Bloch). Biomed Pharmacol J 2011;4(1) |
| Copy the following to cite this URL: Vijayalakshmi P, Moorthy K. Assessment of Virulence and Antimicrobial Susceptibility of Vibrio vulnificus Isolated from Etroplus suratensis (Bloch). Biomed Pharmacol J 2011;4(1). Available from: http://biomedpharmajournal.org/?p=1863 |
Introduction
Fishes are one of the most primitive man’s main foods in the earlier days as a hunter food gatherer. It is the only sector which offers animal protein to a broad-cross section of the society thereby is in an advantageous position to ensure nutritional value. The fish meal is highly proteinaceous in nature, when compared to other animal foods. Pickering and Steward, 1984 reported that overcrowding acts as an aquaculture related chronic stress or which reduces growth besides affecting the immune system in several species of fish like Atlantic salmon, Carp and Sea bream. Due to over exploitation by human population our natural water resources have also become highly polluted through indiscriminate discharge of industrial, sewage and agricultural wastes. Microbial pathogens are associated with the marine and fresh water aquatic animals.
Vibriosis is an economically important disease of fish, marine invertebrates and large marine mammals and is responsible for the high mortality rates in aquaculture worldwide. The genus Vibrio spp. cause fatal diseases commonly in Epinephelus spp. Auguilla spp. Lates calcarifer, Liza macrolepis, Orechromis spp. Among Vibrio spp., Vibrio vulnificus is one of the fish pathogen in marine and brackish waters (Thampuran et al., 1998). Vibrionaceae members distributed in estuarine, marine environment and heterotrophic bacteria. They are halophilic, gram-negative, comma-shaped bacteria and V. vulnificus cause diseases in human as well as terrestrial and aquatic animals. The common names for Vibrio infections of fishes include “red-pest” of eels and “salt- water furunculosis”, “red-boil” and “pike-pest” Peggy et al. (1996). Oliver (1986) reported the production of extracellular enzymes and cytotoxicity by V. vulnificus. Vibrio vulnificus produce toxin such as cytotoxin, cytolysine also produce lipopolysaccharide (Tison et al., 1986 and Yoshid, 1985).
The present study mainly focused on isolation and identification of V. vulnificus from the diseased fishes. A standard procedure was followed for identification of V. vulnificus (Bergey’s Determinative bacteriology, 1990). Virulence of V. vulnificus was assessed by Lethal Dosage (LD50) method.
Materials and Methods
Isolation of the bacterium
Blood samples and tissue homogenates from the injected fishes were used to isolate the bacterium. The blood samples were removed aseptically and then the clean supernatant of tissue homogenates were plated on to Zobell’s Marine agar. The colonies were stained and then identified as Vibrio spp. based on morphology and motility. The identified colonies were subsequently maintained in Alkaline peptone water (Larsen et al., 1998) or Brain Heart Infusion Broth (BHIB). Furtherly it was plated on a selective medium, such as, Thiosulfate Citrate Bile salt Sucrose Agar (TCBS-Hi-media), Vibrio vulnificus selective medium (VVM), and Sodium dodecyl sulfate – Polymixin B- Sucrose medium, (Arias et al., 1999; Jofre et al., 2000 and Bryant, 1987). Identification of V. vulnificus done by Bergey’s Manual Bacteriology (1990).
Antibiotic Sensitivity Test
Sensitivity / Resistant pattern of isolate of Vibrio vulnificus was done by Kirby – Bauer method (Bauer and Kirby, 1966). All the test cultures were inoculated into tryptone soy broth TSB and incubated at 37ºC for 5 hours. Each strain of V. vulnificus spreaded over the Muller Hinton Agar (MHA) plates, the panel of antibiotics were selected and placed aseptically over the MHA plates. The plates were incubated at 37°C for 18- 24 hours. Then the plates were examined for the presence of zone of inhibitions and results were interpreted according to the standard chart (Hi-media, India).
Determination of Lethal Dosage
Five different dose regimes (10-4 -10–8 CFU / fish) were used to kill fifty percent of the challenged fishes, Etroplus suratensis that had been collected from backwater of Muttukadu and acclimatized at 37°C for 30 days prior to experimentation. Twelve fish per dose, six in each replicate were challenged through intramuscular injection, at the base of the dorsal fin with 0.1 ml suspension of the bacterium (Vibrio vulnificus) were inoculated. Injected fishes were kept under observation for 7-10 days. Cumulative mortalities were used in calculating the LD50 (Reed and Muench, 1938).
Results and Discussion
In the present study, the injected fishes were sluggish, necrotic, hemorrhagic spot, boils and blackening of body surface, large sores and granulating lesions with feeble pale gills were identified. The diseased fishes were collected and the samples from kidney and blood were cultured on Zobell’s Marine agar, the non-swarming colonies with serrated margins were observed. Typical green colour colonies were observed on TCBS agar. In normal fish Etroplus suratensis, the bacterium Vibrio vulnificus, isolated from infected fishes were injected intramuscularly and the lesions were observed.
The various biochemical characters of Vibrio vulnificus isolated from infected fishes were tabulated in table 1. The characters of V. vulnificus it includes, gram negative rod, motile, growth at 0%, 0.5%, 3%, 5%, 6% and 8% NaCl, growth at 4ºC, 30ºC and 35ºC, gelatin and starch hydrolysis positive, oxidase and catalase positive, IMViC – Indole negative, MR negative, VP negative, citrate positive, growth on TCBS Agar, gas and acid from glucose, gas from fructose, maltose, sucrose, galactose, mannose, arabinose and xylose. Stephenie et al. (2010) studied the correlation of D- mannitol fermentation with virulence – associated genotypic character in V. vulnificus, which was isolated from oysters and water samples. Indole-negative V. vulnificus strains isolated from a septicemic patient (Amaro, 1995) and indole-positive V. vulnificus isolated from Danish eel farm (Dalsgaard et al., 1998). Feifei Han et al. (2009) reported the clinical and environmental types of V. vulnificus isolated from Louisiana oysters. A culture – free method for the detection of Vibrio vulnificus from costal seawater based on loop – mediated isothermal amplification targeting vcgC gene (Yongjun Li et al., 2010). Based on the present investigation, it is clearly indicated that the isolates from Etroplus suratensis were V. vulnificus.
Table 1: Biochemical characters of vibrio vulnificus.
| S.No | Reactions for the species | V. vulnificus from
E. suratensis |
| 1 | Gram’s Staining | – |
| 2 | Motility | + |
| 3 | Gas from Glucose | – |
| 4 | Acid from Sucrose | – |
| 5 | Acid from Fructose | + |
| 6 | Acid from Maltose | + |
| 7 | Acid from D-Mannose | + |
| 8 | Acids from D-Galactose | + |
| 9 | Acid from D -Xylose | – |
| 10 | Acid from L-Arabinose | – |
| 11 | Indole Production | – |
| 12 | Methyl Red | – |
| 13 | Voges-Proskauer(VP) | – |
| 14 | Citrate utilization | + |
| 15 | Urease activity | – |
| 16 | Nitrate reduction | – |
| 17 | Gelatin hydrolysis | + |
| 18 | Starch hydrolysis | + |
| 19 | Phospholipids | + |
| 20 | Catalase | + |
| 21 | Oxidase | + |
| 22 | Growth on TCBS agar | + |
| 23 | Growth at 0% Nacl | – |
| 24 | Growth at 0.5% Nacl | – |
| 25 | Growth at 3% Nacl | + |
| 26 | Growth at 5% Nacl | + |
| 27 | Growth at 6% Nacl | + |
| 28 | Growth at 8% Nacl | – |
| 29 | Growth at 4o C | – |
| 30 | Growth at 30o C | + |
| 31 | Growth at 35 o C | + |
This study also showed the optimum temperature and salinity for the growth of organism. Temperature and salinity form a major controlling factor in aquaculture system. The optimum temperature lies between 30°-35°C supports normal growth (Beena, 2000). Fishes were much susceptible to temperature changes, which lead to temperature shock, followed by stress and thus are prone to infections. Salinity also forms important limiting factors in brackish water culture systems. The result of the present study, the sodium chloride concentration lies between 3% – 6% respectively.
Sensitivity and Resistant pattern of Vibrio vulnificus (54 – isolates) were studied and tabulated in table 2. A panel of antibiotics were employed, among these Gentamycin (25.5mm), Norfloxacin (25.0mm), Lomefloxacin (25.0mm), Nalidixic acid (23.5mm), Levofloxacin (22.5mm) and Co-trimoxazole (24.0mm) mean values were found to be sensitive and Tetracycline and Cephotaxime fail to inhibit V. vulnificus isolates. Antibiotics are frequently used to cure diseases but there is always a risk of bacteria developing resistance and residues in the product (Fjalestad et al., 1993). Vibrio vulnificus isolates sensitive with many antibiotics and resistant with Tetracycline and Cephotaxime. Li et al. (1999) reported that Cefriaxone, Nalidixic acid, Chloramphenicol and Sulphamethoxazole were sensitive with isolates of V. vulnificus isolated from moribund silver sea bream.
Table 2: Sensitive and Resistance pattern of Vibrio vulnificus (54 isolates) to Etroplus suratensis.
| S. No | Antibiotics | Symbol | Disc content (mcg) | Zone of inhibition(mm) | Result | ||
| Minimum | Maximum | Mean ± SEM* | |||||
| 1 | Gentamycin | G | 10 | 21 | 30 | 25.5 ± 4.5 | S |
| 2 | Tetracycllin | T | 30 | – | – | – | R |
| 3 | Norflaxacin | NX | 10 | 22 | 28 | 25 ± 3 .0 | S |
| 4 | Nalidixic acid | NA | 30 | 21 | 26 | 23.5 ± 3.5 | S |
| 5 | Cephotaxime | CE | 30 | 18 | 22 | 20.0 ± 2.0 | R |
| 6 | Lomefloxacin | LO | 10 | 22 | 28 | 25.0 ± 3.0 | S |
| 7 | Levofloxacin | LE | 5 | 19 | 26 | 22.5 ± 3.5 | S |
| 8 | Co-trimoxazole | CO | 1.25/23.75 mcg | 20 | 28 | 24.0 ± 4.0 | S |
*- Standard Error of the mean
The occurrence of resistance to OXA by fish pathogen been reported to have increased over the recent years. Development of drug resistance by fish pathogen has frequently been reported. Disease resistance genes have not been identified in fish. However, the production of transgenic fish with enhanced resistance to specific diseases remains as a possibility for the future (Fjalestad et al., 1993). Although the present study recommends the use of sensitive drugs in aquaculture, it is suggested that these antibiotics should be assessed against the set of standards as reported by Austin and Austin (1987) in order to ensure safety.
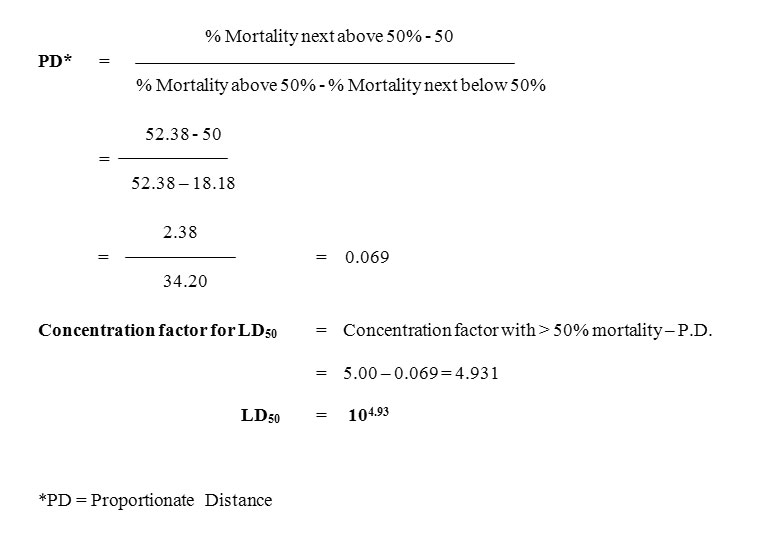
The lethal dose (LD50) values of Vibrio vulnificus were recorded in table 3. The LD50 value for the juvenile Etroplus suratensis was found to be 104.93. Melanisation followed by reddening and swelling at the site of injection were observed as immediate changes, one to three hours post injection with 10-7and 10-8 CFU / fish of V. vulnificus. Mean lethal dose 50% LD50 represent the number of bacteria needed to kill 50% of the inoculated fishes (Reed and Muench, 1938). This test has given a more quantitative assessment of the vaccine potency than the Relative Percent Survival (RPS) method (Ellis, 1988). Higher lethality due to injection challenge was by direct access of the bacterium to the circulatory system.
Tables 3 (a) : Survival pattern of Etroplus suratensis injected with Vibrio vulnificus (Intramuscular Route).
| Dilution Factor | Replicates | Day Post Injection (Dpi) | Total Mortality | Total Survival | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||||
| 10-4 | R1 | 6 | 6 | 6 | 6 | 5 | 5 | 4 | 4 | 8 |
| R2 | 6 | 6 | 6 | 6 | 6 | 5 | 4 | |||
| 10-5 | R1 | 6 | 6 | 5 | 5 | 4 | 4 | 3 | 7 | 5 |
| R2 | 6 | 5 | 5 | 4 | 4 | 3 | 2 | |||
| 10-6 | R1 | 6 | 5 | 5 | 4 | 4 | 3 | 3 | 8 | 4 |
| R2 | 6 | 6 | 5 | 4 | 3 | 2 | 1 | |||
| 10-7 | R1 | 5 | 5 | 4 | 3 | 3 | 2 | 1 | 11 | 1 |
| R2 | 6 | 5 | 4 | 4 | 3 | 2 | 0 | |||
| 10-8 | R1 | 5 | 5 | 5 | 3 | 2 | 1 | 0 | 12 | 0 |
| R2 | 6 | 5 | 4 | 4 | 3 | 2 | 0 | |||
Table 3 (b) : Determination of LD50 values of Vibrio vulnificus isolated from Etroplus suratensis
| CFU 0.1ml/fish | Mortality | Survival | Cumulative Mortality | Cumulative Survival | Mortality rate | Mortality Percentage |
| 108 | 12 | – | 42 | – | 42.42 | 100.00 |
| 107 | 11 | 01 | 30 | 01 | 30.31 | 96.80 |
| 106 | 08 | 04 | 19 | 05 | 19.24 | 79.10 |
| 105 | 07 | 05 | 11 | 10 | 11.21 | 52.38 |
| 104 | 04 | 08 | 04 | 18 | 4.22 | 18.18 |
Biosca et al. (1993) reported the presence of the capsule in Vibrio vulnificus biotype 2 and its relationship to virulence for eels. Capsule expression, siderophore production, ability to use hemin and exotoxin production expresses the virulence factors for human (Amaro et al., 1995). Amaro et al. (1997) reported that the lipopolysaccharide O-side chain of V. vulnificus E is a virulence determinant for eels. Iron acquisition system is involved in the virulence mechanism of V. vulnificus. Sung Young Goo et al. (2006) reported the presence of OmpU, a major outer membrane proteins is an important virulence factor involved in the adherence of V. vulnificus to the host cell. Lee et al. (2010) reported the presence of I1pA protein of V. vulnificus is an important virulence factor involved in the adhesion. The utilization of N-Acetylneuraminic acid and a Sialic acid involved in V. vulnificus pathogenesis (Jeong et al., 2009). Fouz et al. (2002) reported LD50 value of V. vulnificus to be 1.0 X 10-6 CFU / ml and 1.1 X 10-7 CFU / ml for eel and tilapia group.
The virulence of the bacterium depended on the type of species, its susceptibility and also the some extent and environmental conditions. The results of the present study revealed that Vibrio vulnificus can become a potential threat to the culture of brackish water species. The present study fulfilled that this kind of study will certainly promote the identification and prevention of Vibriosis from the aquaculture industries.
Acknowledgement
The author is thankful to Central Institute of Brackish water and Aquaculture (CIBA), Govt. of India, Muttukadu, Chennai, Tamil Nadu for providing Lab facilities and encouragement.
References
- Amaro, C., Fouz, B., Biosca, E.G., Marco-Noales, E., Park, S., and Colloda, R., The lipopolysaccharide O-side chain of Vibrio vulnificus E is a virulence determinant for eels. Infect. Immmunology, 65: 2475-2479 (1997).
- Amaro, C., Biosca, E.G., Fouz, B., Alcaide, E., and Esteve, C., Evidence that water transmits Vibrio vulnificus, biotype 2 infections to eels. Applied Environmental Microbiology, 61: 1133-1137 (1995).
- Arias, C.R., Aznar, R., Garay, E., and Pujalte, M.J., Identification of Vibrio spp. (other than Vibrio vulnificus recovered on CPC agar from marine natural samples. International Microbiology, 3: 51-53 (2000).
- Arias, C.R., Aznar, R., Garay, E. and Pujalte., M.J., Identification of Vibrio spp. (other than Vibrio vulnificus) recovered on CPC agar from marine natural samples. International Microbiology, 3: 51-53 (2000).
- Arias, C.R., Macian, M.C., Aznar, R., Garay, E., and Pujalte., M.J., Low incidence of Vibrio vulnificus, among Vibrio isolates from sea water and shellfish of the western Mediterranean Coast. Journal of Applied Microbiology, 86: 125-134 (1999).
- Austin, B., and Austin, D.A., Bacterial fish pathogens, Diseases in farmed and wild Fish. Ellis Horwood Publishers, Chicester. PP: 364 (1987).
- Beena Tilak, Water quality. Sea Food Export Journal. 31: 598-663 (2000).
- Biosca, E.G., Esteve, C., Garay, E. and Amaro, C., Evaluation of the AP120E system for identification and discrimination of Vibrio vulnificus of biotype 1 and 2. Journal of fish Disease, 16: 79-82 (1993).
- Bryant, R.G., Jarvis, J., and Janda, J.M., Use of Sodium dodecyl sulfate-Polymyxin B sucrose medium for isolation of Vibrio vulnificus from shellfish. Applied Environmental Microbiology, 53: 1556 – 1559 (1987).
- Dalsgaard, I., and Dalsgaard, A., Improved isolation of Vibrio vulnificus from seawater and sediment with Cellobiose-Colistin agar. Applied Environmental Microbiology, 64: 1721 – 1724 (1998).
- Feifei Han, Shuaihua Pu, Aixin Hou and Beilei Ge., Characterization of Clinical and Environmental types of Vibrio vulnificus isolated from Louisaiana oysters. Food borne pathogens and Disease, 1251-1258 (2009).
- Fjalestad, K.T., Gjedram, J. and Gjerda, B., Genetic improvement of disease resistance in fish. Aquaculture, 111: 65-75 (1993).
- Fouz, B., Alcaid, E., Barrera, R., and Amaro, C., Susceptability of Nail tilapia (Oreochromis niloticus) of Vibrio vulnificus biotype 2 (serovar E). Aquaculture, 212: 21-30 (2002).
- Jofre, J., and Blanch, A.R., A selective medium and specific probe for detection of Vibrio vulnificus. Applied Environmental Microbiology, 66: 855-859 (2000).
- Larsen, J.L., Dalsgaard, I., and Dalsgaard, A., Occurrence of Vibrio vulnificus biotype in Danish marine Environment. Applied Environmental Microbiology, 64: 7 (1998).
- Li, J., Yie, J., Fu, W., Foo, R.W., Hu, Y., Woo, N.Y. and Xu, H., Antibiotic resistance and plasmid profiles of Vibrio isolates from cultured Sparus sarba. Wei Sheng Wu Xue Bao , 39: 461- 468 (1999).
- Oliver, J.D., Wear, J.E., Thomas, M.B., Warner, M. and Linder, K., Production of extracellular enzymes and cytotoxicity by Vibrio vulnificus. Diagnostic Microbiology of Infectious Diseases, 5: 99-111(1986).
- Peggy, A.R. and Ruth Francis-Floyd. Vibrio infection of Fish. This document, one of the series of the Fisheries and Aquatic Science Department, Florida Co-operative Extension Service, Institute of Food and agricultural Science (IFAS), University of Florida. Original Publication, 1996.
- Reed, L.J. and Munch, H., A Simple method of estimating fifty percent End point. American Journal of Hygiene, 27: 493 – 497 (1938).
- Stephenie L Drake, Whitney, B., Levien, J.F., Angelo DePaola., and Lee-Ann Jaykus., The correlation of D- mannitol fermentation with virulence – associated genotypic character in Vibrio vulnificus, which was isolated from oysters and water samples in the Gulf of Mexico. Food borne pathogens and Disease, 7: 97-102 (2010).
- Sung Young Goo., Lee, H., kim, W.H., Han, K., Lee, H.J., Kim, S.M., Kim, K., Lee, K., and Park, S., Identi fication of OmpU of Vibrio vulnificus as a fibrionectin- Binding protein and its role in bacterial pathogenesis. Infection and Immunity, 74: 5586-5594 (2006).
- Thampuran, N. and Surendran, P.K., Occurence and distribution of Vibrio vulnificus in tropical fish of shell fish from Cochin (India). Lett. Applied Microbiology, 26: 110-112 (1998).
- Tison, D.L., and Kelly, M.T., Virulence of Vibrio vulnificus strain from marine environments. Applied Environmental Microbiology, 51: 1004 – 1006 (1986).
- Yongjun Li, Zheng, Z., Zhao, Y., Wei, X., and Zhu, L., A culture-free method for the detection of Vibrio vulnificus from costal seawater based on loop-mediated isothermal amplification targeting vcgC gene. Acta Oceanologica Sinica, 29: 93-97 (2010).
- Yoshid, S.L., Ogawa, M. and Mizuguchi, Y., Relation of capsular material and colony opacity to virulence of Vibrio vulnificus. Infect. Immunology, 47: 446 – s451(1985).







