B. Nagaraj, P. Liny*, P. Sreedhar Reddy, N.B. Krishnamurthy, D. Jhansi Rani and V. B. Mazumdar
1Department of Biotechnology, Shridevi Institute of Engineering and Technology, Tumkur, Karnataka - 572 106 India. 2Department of Biotechnology, Nagarjuna College of Engineering and Technology, Bangalore, Karnataka - 572 110 India.
Abstract
Biodiesel are monoalkyl esters of long-chain fatty acids, preferentially methyl and ethyl esters, derived from renewable feedstock, such as vegetable oils or animal fats. Its properties are close to diesel fuels, and therefore biodiesel becomes a strong candidate to replace the diesel fuel. Biodiesel can also be used as a low carbon alternative to heating oil. Rhizopus Oryzae fungus cells have been demonstrated to efficiently catalyze the methanolysis of vegetable oils for biodiesel production in solvent-free systems. To efficiently reuse the whole cell biocatalyst, the cells can be immobilized on a nonwoven fabric. In contrast to extracellular lipase, no enzyme purification and immobilization steps are required when preparing the immobilized whole cell biocatalysts since cell immobilization could be achieved spontaneously during cell growth
Keywords
Biodiesel; Rhizopus oryzae; fungus cells; whole cell biocatalyst; immobilization
Download this article as:| Copy the following to cite this article: Nagaraj B, Liny P, Reddy P. S, Krishnamurthy N. B, Rani D. J, Mazumdar V. B. Biodiesel Production Catalyzed by Fungus Cell Immobilization in Fibrous Support. Biomed Pharmacol J 2010;3(2) |
| Copy the following to cite this URL: Nagaraj B, Liny P, Reddy P. S, Krishnamurthy N. B, Rani D. J, Mazumdar V. B. Biodiesel Production Catalyzed by Fungus Cell Immobilization in Fibrous Support. Biomed Pharmacol J 2010;3(2). Available from: http://biomedpharmajournal.org/?p=1609 |
Introduction
Biodiesel refers to a vegetable oil- or animal fat-based diesel fuel consisting of long-chain alkyl (methyl, propyl or ethyl) esters. Biodiesel is typically made by chemically reacting lipids (e.g., vegetable oil, animal fat (tallow) with an alcohol. Biodiesel burns up to 75% cleaner than conventional fuels from petroleum fuels. It substantially reduces unburned hydrocarbons, carbon monoxide and particulate matter in exhaust fumes. Biodiesel is environmental friendly and renewable. Also ozone harming potential of biodiesel is nearly 50% less than conventional diesel fuels. Biodiesel consists of a mixture of alkyl esters of fatty acids derived from the transesterification of vegetable or animal oils with alcohol1.
Lipase (Triacyl glycerol ester hydrolase) is an enzyme that catalyses the hydrolysis of triacylglycerols to fatty acids, mono and diacylglycerols and glycerol. Lipase also catalyses the transesterification of lipid to biodiesel under mild conditions by reducing process cost in terms of energy consumption and capital equipment requirements2. The extracellular and intracellular microbial lipases could effectively catalyze transesterification (methanolysis) of oils by short-chain alcohols to produce biodiesel.
Rhizopus Oryzae comes under Mucoraceae family. Rhizopus oryzae have been demonstrated to efficiently catalyze the methanolysis of vegetable oils for biodiesel production in solvent-free systems 3, 4.To efficiently reuse the whole cell biocatalyst, the cells were subsequently immobilized within biomass support particles made out of reticulated polyurethane foam 5, 6.
In this study, for industrial biodiesel production, immobilized whole cell biocatalyst was prepared from R. oryzae cells in a conical flask using low cost porous nonwoven fabric as the support matrix for cell immobilization. Rhizopus oryzae whole cell immobilized within biomass support particles was used for the methanolysis of soybean oil to increase the rate of biodiesel production.
Materials and Methods
Collection of Rhizopus Oryzae
Rhizopus oryzae is a common fungal species which can be seen growing on the cooked rice which kept unused for a day or two .For our present study, R.oryzae was considered as it is found to be having more amount of lipase activity. Lyphilized Rhizopus oryzae pure culture obtained from MTCC Chandigarh (MTCC no: 3831). These fungus cells were then sub cultured on the solid media (PDA). Then the liquid media was prepared for culturing the fungus (R.oryzae) and for immobilizing cells.
Media for isolation and culturing
Potato dextrose agar media
150 grams of diced potatoes, 10grams of glucose (dextrose), and 7.5 grams of agar and 500ml of distilled water are mixed and the pH is set to 6.9. The contents are kept for autoclaving at 121˚ C for 30 minutes and at 15 lbs pressure.
Liquid media for immobilization
5 grams of powdered dry black grapes skin ,2 grams of olive oil, 0.2 grams of KH2PO4, 0.05 grams of KCl, 0.05 grams of NaNO3, 0.05 grams of MgSO4, the components mixed in 500 ml of water and the pH is set to 4.6. The contents are kept for autoclaving at 121˚C for 30 minutes and at 15 lbs pressure.
Spread plate method
PDA media plate is prepared, dip bent glass rod in a beaker containing 95% alcohol, and then aseptically transfer loop full of culture is in the centre of PDA media plate. The inoculated Petri plate is covered and it is kept for incubation at 35˚C for Fungus mycelia grown at 30 °C were transferred from the surface of solid culture media with culture loop to a slope solid culture medium. Cells grown on the slope for 96 h were washed with 5 ml distilled water and loosen with culture loop to prepare a spore solution under aseptic condition.
Preparation of immobilizing material
Stainless steel mesh of dimensions length 12.5 cm, width 3.0 cm & thickness 0.5 mm is used for supporting the immobilizing material. Polypropylene cloth sample which is the material selected for immobilizing the fungus species was collected from Sofinco industries Pvt. Ltd, Rajajinagar, Bangalore. The polypropylene cloth is cut into a rectangular shape of the dimensions (length 12.5 cm × width 3.0 cm × thickness 0.5 mm). This piece of polypropylene cloth acts as an immobilizing material. A steel mesh was taken an it was also cut into a rectangular shape of the same dimensions of (length 12.5 cm × width 3.0 cm × thickness 0.5 mm).The polypropylene fabric and steel mesh was layered together to be used as an immobilized matrix and rolled together into a stoppered conical flask.This matrix is weighed and it is noted down. Cell immobilization matrices were sterilized by autoclaving at 120 °C for 30 min and cooled to room temperature before use.
Simultaneous growth and immobilization of fungus (Rhizopus Oryzae)
The fungus cells which were initially cultured on solid media of potato dextrose agar media is taken out. These cultured cells are then are aseptically transferred into the liquid media for the growth. Once the fungus sample is transferred into the liquid media, the matrix which was prepared was introduced into the conical flask containing the inoculum. A temperature of 30˚C is maintained and air of 4 vvm is passed for aeration. The sample is then kept for incubation for 72- 90 hours. During this period the fungus cells grows in liquid media and simultaneously get immobilized on the non- woven fabric material. Once immobilized the matrix was removed from the liquid media it is subjected for drying. Once these immobilized cells are dried these are subjected for permeabilization process, by immersing immobilized cells in acetone for 90 minutes. These permeabilized cells were incubated at 30˚C for 3 days 7 .The cells matrixes were weighed and amount of cell immobilized is noted down.
Production of Bio diesel by Immobilized Whole Cell Biocatalyst
For transesterification of soybean oil with methanol, one piece of immobilization matrix containing dried cells as prepared above were placed in a 250 ml conical flask. This is followed by adding 38.6 grams of soybean oil (substrate), 1.4 grams of methanol, and water based on the experiment condition to reach predetermined water content 8, 9. The reaction mixture was incubated at 150 rpm at 30˚C. 200 microliter of reaction solution was removed every 24 hours, which is then centrifuged at 10,000 rpm and mixed with equal volume of ethyl acetate in hexane. The ME (methyl ester content) in the reaction mixture was determined by gas chromatography mass spectroscopy (GC-MS). GC-MS was carried out in Department of Organic Chemistry, IISC, Bangalore. In the GC-MS method, sample is dissolved in the mobile phase. This mobile phase travels across a stationary phase. It will carry with components of the mixture. The movement of the sample based on retardation factor (RF). Analysis requires a pure gaseous sample. The sample is maintained at inlet temperature of 2500C, pressure 64.5kPa, column flow rate at 1.3mL/min with linear velocity of 45.9cm/sec and sampling at a rate of 0.5 sec.
Results and Discussion
Isolation and characterization
The lyophilized fungal samples obtained from MTCC were plated on potato dextrose media (PDA). The fungal colonies were observed on PDA media with white cottony mass structure after incubation of 72 hours was observed in Fig 1.
Immobilization of fungus on nonwoven matrix
The fungus cells were observed to be immobilized on the matrix support after inoculation of the fungus (Rhizopus Oryzae) after incubation for 90 hours. These immobilized cells were permeabilized in acetone. Pictures of fibrous matrix before cell immobilization (A and B) and after cell immobilization (C and D) are shown in Fig 2 and 3.
 |
Figure 1: Picture showing Rhizopus Oryzae grown on PDA media.
|
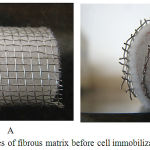 |
Figure 2 Pictures of fibrous matrix before cell immobilization (A and B).
|
 |
Figure 3: Pictures of fibrous matrix after (C and D) cell immobilization.
|
Production of biodiesel
The immobilized cells when incubated along with the substrate i.e soyabean oil and alcohol and water for trans-esterification process, the sample collected was sent for GC-MS analysis. From the GC-MS analysis it was been seen that the three peaks for palmetic acid ME, oleic acid ME and lenoleic acidwas represented in Fig 4. ME were obtained according to molecular weight, these Methyl Esters are the composition of biodiesel produced from soyabean oil. These compounds were identified from their molecular weights such as Lenoleic acid ME (295), Oleic acid ME (296), Palmetic acid ME (271). Typical Soybean Oil Methyl Ester Profile (National Biodiesel Board) is represented in Table 1.
 |
Figure 4:GC MS plot showing the presence of biodiesel for sample after 72 hours of immobilization. |
Table-1 Typical Soybean Oil Methyl Ester Profile (National Biodiesel Board)
| Fatty Acid | Weight percent | Molecular weight | Formula |
| Palmitic | 12.0 | 270.46 | C15H31CO2CH3 |
| Stearic | 5.0 | 298.52 | C17H35CO2CH3 |
| Oleic | 25.0 | 296.50 | C17H33CO2CH3 |
| Linoleic | 52.0 | 294.48 | CH3(CH2)4CH=CHCH2CH=CH(CH2)7 CO2CH3 |
| Linolenic | 6.0 | 292.46 | CH3(CH2CH=CH)3(CH2)7 CO2CH3 |
Effective methanolysis of oils using extra cellular microbial lipase have been studied by several researchers also it is reported that it takes 30-60 hours to reach 80% biodiesel conversion without using an organic solvent 10,11,12. Although lipase-catalyzed methanolysis reaction for biodiesel production could be carried out by using immobilized extracellular lipase with an aim to reuse the enzyme and cut down enzyme cost, extracellular enzymes may require purification procedures that are too complex and costly for industrial applications 13. Furthermore, enzymes recovered through such operations are generally unstable. Biodiesel produced by lipases without any organic solvent, and thus the enzymatic process becomes environmentally benign 4, 14.
Immobilizing cells to biomass support particles allows for easy separation of the whole cell biocatalyst from the reaction mixture and facilitates its reuse during repeated batch reaction. When methanolysis was carried out with the immobilized cells in the presence of water, the level of methyl ester (ME) production is almost the same as that achievable with extracellular lipase but with substantial reduction in the cost of immobilized biocatalyst preparation 8, 15 .
Utilizing whole cell biocatalyst instead of free or immobilized enzyme is a potential way to reduce the cost of catalyst in lipase-catalyzed biodiesel production. These findings suggested that the use of whole cell biocatalyst immobilized within a suitable matrix may offer a promising means of biodiesel production for industrial application with the simplicity of the biocatalyst production process. Nonwoven fabric is a suitable macro porous fibrous matrix for immobilization of fungus cells. In contrast to extracellular lipase, no enzyme purification and immobilization steps are required when preparing the immobilized whole cell biocatalysts since cell immobilization could be achieved spontaneously during cell growth 16.
Conclusion
The study indicated a low cost lab scale set up for immobilization of fungus whole cells on fibrous support. The result indicated that bio diesel was produced from immobilizing the fungus Rhizopus oryzae into non woven polypropylene matrix. Under the experimental conditions, it indicated that fungus whole cells catalyzed lipase can be a cost cutting strategy for extraction and purification of lipase from micro organisms for biodiesel production. The degree of efficiency has also improved by the use of whole cell in transesterification. Further immobilized fungus can be re- used for biodiesel production.
References
- Colucci, Jose A, Ernesto E. Borrero and Fabio Alape. J. American Oil Chemists’ Society, 82, 525-530 (2005).
- Dong Hwan Lee, Jung Mo Kim, Hyun Yong Shin, Seong Woo Kang & Seung Wook
- KimBiotechnology and Bioprocess Engineering,11,522-525 (2006).
- Zeng, J., Du, W., Liu, X. Y., & Liu, D. H. Journal of Molecular Catalysis B Enzymatic, 43, 15–18 (2006).
- Li, W., Du, W., & Liu, D. H. Process Biochemistry, 42, 1481–1485 (2007).
- Fukuda, H. (2002). Journal of Molecular Catalysis B Enzymatic, 17, 157–165.
- Ban, K., Kaieda, M., Matsumoto, T., Kondo, A., & Fukuda, H. Biochemical Engineering Journal, 8, 39–43 (2001).
- Kaieda, M., Samukawa, T., Matsumoto, T., Ban, K., Kondo, A., Shimada, Y., Noda, H., Nomoto, F., Ohtsuka, K., Izumoto, E., Fukuda, H. Journal of Bioscience and Bioengineering, 88, 627–631 (1999).
- Tamalampudi, S., Talukder, M. R., Hama, S., Numata, T., Kondo, A., & Fukuda, H. Biochemical Engineering Journal, 39, 185–189 (2008).
- Watanabe, Y., Shimada, Y., Sugihara, A., Noda, H., Fukuda, H., & Tominaga, Y. Journal of the American Oil Chemists’ Society, 77, 355–360 (2000).
- Al-Zuhair, S., Jayaraman, K. V., Krishnan, S., & Chan, W. H. Biochemical Engineering Journal, 30, 212–217 (2006).
- Wang, L., Du, W., Liu, D., Li, L., & Dai, N. Journal of Molecular Catalysis B Enzymatic, 43, 29–32 (2006).
- Nie, K., Xie, F., Wang, F., & Tan, T. Journal of Molecular Catalysis B Enzymatic, 43, 142–147 (2006).
- Du, W., Li, W., Sun, T., Chen, X., & Liu, D. H. Applied Microbiology and Biotechnology, 79, 331–337 (2008).
- Fukuda, H., Hama, S., Tamalampudi, S., & Noda, H. TIBTECH, 26, 668–673(2008).
- Oda, M., Kaieda, M., Hama, S., Yamaji, H., Kondo, A., Izumoto, E., Fukuda, H. Biochemical Engineering Journal, 23, 45–51 (2005).
- Atkinson, B., Black, G. M., Lewis, P. J. S., & Pinches, A. Biotech. Bioeng. 21, 193–200 (1979).







