Manuscript accepted on :August 12, 2009
Published online on: 20-11-2015
Plagiarism Check: Yes
N. Vijaya Sree, P. Udayasri, V. Suresh and K. R. S. Sambasiva Rao
Centre for Biotechnology, Acharya Nagarjuna University, Guntur - 522 510 India
Abstract
Osteoporosis is a severe disease faced by most people in the world. It has been estimated that a total of 6,43,000 people in Europe are currently suffering from an osteoporosis – related hip fracture & 1.5 million osteoporosis – related fractures, including 7,00,000 vertebral & 2,50,000 hip fractures each year. Although there is no cure for osteoporosis a broad range of therapies have been approved by the Food and Drug Administration for postmenopausal women to prevent or treat osteoporosis by counter medications such as calcium and vitamin D, estrogen therapy, and newer medications such as “Antiresorptive agents”(i.e., Selective Estrogen – receptor modulators) and Anabolic therapy. As drug treatments have benefits as well as possible side effects and risks. In the place of various drug therapies scientists as well as doctors prefer the patients to undergo nanoparticles treatment for osteoporosis. There are several experimental evidences given by researchers for treatment of osteoporosis by nanoparticles. This review summarises about osteoporosis and the use of nanoparticles in treating osteoporosis.
Keywords
Osteoporosis; Post menopause; Drug therapy; Nanoparticles
Download this article as:| Copy the following to cite this article: Sree N. V, Udayasri P, Suresh V, Rao K. R. S. S. Osteoporosis: Use of Nanoparticles for the Treatment of Osteoporosis. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Sree N. V, Udayasri P, Suresh V, Rao K. R. S. S. Osteoporosis: Use of Nanoparticles for the Treatment of Osteoporosis. Biomed Pharmacol J 2009;2(2). Available from: http://biomedpharmajournal.org/?p=1069 |
Introduction
Osteoporosis is a disease in which bones become fragile and more likely to break. If not prevented or if left untreated, osteoporosis can progress painlessly until a bone breaks. These broken bones, also known as fractures, occur typically in the hip, spine, and wrist. Any bone can be affected, but of special concern are fractures of the hip and spine. A hip fracture almost always requires hospitalization and major surgery (Lindsey R. 1990). It can impair a person’s ability to walk unassisted and may cause prolonged or permanent disability or even death. Spinal or vertebral fractures also have serious consequences, including loss of height, severe back pain, and deformity.
Osteoporosis is a disease characterized by low bone mass and structural deterioration of bone tissue, leading to bone fragility and an increased susceptibility to fractures, especially of the hip, spine and wrist, although any bone can be affected. In simpler terms, osteoporosis is a condition in which the bones become weak and can break from a minor fall or, in serious cases, from a simple action such as a sneeze (Ralston SH. 1997). Also Osteoporosis literally means ‘porous bones’. Bones are made up of a thick outer shell and a strong inner honeycomb mesh of tiny struts of bone. Osteoporosis means some of these struts become thin or break. This makes the bone more fragile and prone to break. It often remains undetected until the time of this first broken bone. Broken wrists, hips and spinal bones are the most common fractures in people with osteoporosis (Amin, S. 2004 May).
Causes Of Osteoporosis
Two types of cells are constantly at work in bones:
Construction cells.
Demolition cells.
Demolition cells break down old bone and construction cells build new bone. Some drugs work by slowing down the activity of the demolition cells while others stimulate the construction cells to build more bone. Some drugs work on both sets of cells. The aim is to strengthen bones to prevent them from breaking because it is these broken bones, particularly broken hips and spinal fractures which can cause pain and have other debilitating effects on people’s lives.
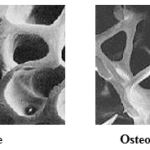 |
Figure 1:
|
Osteoporosis is a severe disease faced by most people in the world. It has been estimated that a total of 6,43,000 people in Europe are currently suffering from an osteoporosis – related hip fracture (I.O.F, 2003) & 1.5 million osteoporosis – related fractures, including 7,00,000 vertebral & 2,50,000 hip fractures, occur in the USA each year (Riggs BL 1995). For adult women, the estimated lifetime risk of sustaining an osteoporotic fracture is one in three. These fractures result in increases disability, excess mortality and reduced quality of life (Pal B 1999). The burden of osteoporosis on quality of life and health care costs can be reduced through offering effective treatment options to postmenopausal women diagnosed with osteoporosis (Smith R 1985; Smith R 1987).
Osteoporosis is a condition characterized by low bone mass and deterioration of bone micro architecture leading to increased susceptibility to fracture and consequent painful morbidity (Pande KC et al., 2000; Pande KC 2002). Osteoporosis poses a major public health treat: In the United States, 10 million individuals alone suffering from this condition which most of women are suffering compared to men. Osteoporosis is responsible for more than 1.5 million fractures annually, pre-dominately of the hip, spine and wrist. The estimated national direct expenditures for osteoporotic and associated fractures were $17 billion in 2001 and the cost is rising (according to N.O.F).
Osteoporosis increases the risk of bone fractures, especially in the hips, spine, and wrists. Although it can affect anyone, the risk of developing osteoporosis increases with age, affects women significantly more often than men, and is most prevalent in Caucasian and Asian women (Teotia SPS et al., 1990). According to the National Osteoporosis Foundation (NOF), 10 million people in the United States have osteoporosis and another 34 million have low bone mass and are at risk of developing the disease. Of those who have osteoporosis, 80 Percent are women (WHO-Study Group 1994).
Most of the people at risk for osteoporosis are not aware of it. It is called a “silent disease” because there are usually no symptoms until a person has a bone fracture. This breakage, frequently in the hip, the vertebrae of the spine, or in the wrist, can occur with very little pressure and can cause the person significant pain and protracted or permanent disability. If the fracture causes severe debility where the patient’s general health is affected, it may be a contributing factor in the death of the patient.
According to estimated figures, osteoporosis was responsible for more than 2 million fractures in 2005, including approximately:
297,000 hip fractures
547,000 vertebral fractures
397,000 wrist fractures
135,000 pelvic fractures
675,000 fractures at other sites
The total number of fractures due to osteoporosis is expected to rise to more than 3 million by 2025 (Fast Facts, October 5, 2005).
To prevent the osteoporosis which is a major problem faced today by the world, there are so many organisation formed which include:
National Osteoporosis Foundation (N.O.F)
International Osteoporosis Foundation (I.O.F)
The World Osteoporosis Day is on 20th October which is conducted every year by these two organisations N.O.F & I.O.F.
Two Types Of Osteoporosis (Osteoporosis Overview NIAMS 2005 June)
Primary or high turnover or age-related osteoporosis
It is the primary bone disease like post-menopausal, senile osteoporosis, immobility and idiopathic juvenile osteoporosis (Savvas M et al., 1988). It occurs in some women aged 50-75 due to the sudden decrease in estrogen as a result of menopause. This causes rapid calcium loss from the bones, making the women susceptible to hip, wrist, forearm, and spinal compression fractures. Age-related osteoporosis occurs in men and women older than 75 years of age and may be due to a decrease in calcium absorption or vitamin D deficiency. Changes in lifestyle, calcium or vitamin D supplements, or other medications decreasing bone loss may slow the progression of this type of osteoporosis. (Ungan M, Tumer M. 2001)
Secondary or low turnover osteoporosis
Occurs as a part of systemic disorder, when bone loss and formation are not equal and more bone is broken down than replaced (Stein E & Shane E, 2003). It affects both men and women and may be due to several different disorders including rheumatoid arthritis, hyperparathyroidism, Cushing’s disease, chronic kidney disease, multiple myeloma, or drugs such as anti-epileptics, glucocorticoids or lithium, thyrotoxicosis, hypogonadism, malabsorption syndrome, scurvy10, rheumatoid arthritis, alcoholism, diabetes mellitus, drugs (like corticosteroids, thyroxin8, heparin, antiepileptics and cytotoxic drugs), COAD, primary biliary cirrhosis, osteogenesis imperfect and after gastrectomy. Treatment of the underlying disease or cause may slow the loss of bone density in secondary osteoporosis (Prakash C 1999).
Bones are primarily a combination of type-I collagen protein and calcium phosphate (Guyton AC 1986). The protein forms a spongy network that is “mineralized” by the addition of the calcium compound to make the bones both strong and flexible. Bone is living tissue that is slowly but continuously replaced. During a process called bone resorption, cells called osteoclasts dissolve bone on a microscopic scale and enzymes break down the collagen network. This is followed by the formation of new bone by cells called osteoblasts, which secrete osteocalcin and precursors to collagen and create a new protein framework. The framework is then mineralized to create new bone. This on-going process is called bone turnover or bone remodeling and it takes place throughout the body, normally replacing about 8-10 percent of the body’s bone each year(Molnar FJ 1999).
During childhood, bone formation proceeds at a faster rate than bone resorption, and bone mass increases to peak at about 30 years of age. After this peak, bone formation slows and resorption begins to outpace it, resulting in a decline in bone mass with age. An inadequate intake of calcium and vitamin D during childhood, the use of medications that contain steroids (such as asthma medications), anorexia, inactivity, smoking, and excess alcohol consumption can all increase the risk of a person developing osteoporosis later in life (Phillipov G 1998).
Some diseases, such as thyroid disease, Cushing’s disease, rheumatoid arthritis, kidney disease, hyperparathyroidism, and vitamin D deficiency can also have an effect on bone health. (Adami S et al., 1993). Those with a strong family history of osteoporosis may also be at an increased risk of developing it themselves.
Women who go through menopause may experience an increased rate of bone mass loss with a decrease in estrogen. Going through menopause early can exacerbate the loss. According to the NOF, some women can lose up to 20 Percent of their bone mass in the first 5 to 8 years following menopause. Men with decreased testosterone levels are also at risk for increased bone loss.
Primary osteoporosis is further divided into:
Idiopathic
which occurs in children and young adults where the cause is not known.
Type I
affecting females between 50-70 years, involves trabecular bone predominantly; leads to fracture of distal forearm and vertebrae and level of PTH is low.
Type II
affecting elders between 70-75 years of both sexes and involves both cortical and trabecular bone causing fractures of pelvis, femoral neck, proximal humerus and proximal tibia. Level of PTH is high. Serum level of 1-25(OH), vitamin D is low in both type I and type II.
Symptoms
People cannot feel their bones getting weaker. They may not know that they have osteoporosis until they break a bone. A person with osteoporosis can break a bone from a minor fall, or in serious cases, from a simple action such as a sneeze. Vertebral (spinal) fractures may initially be felt or seen in the form of severe back pain, loss of height, or spinal deformities such as kyphosis or stooped posture. In many cases, a vertebral fracture can even occur with no pain. This often results in the curvature of the spine at the shoulders in older people sometimes called a “widow’s hump
The appearance of the widow’s hump or a fractured wrist or hip from a fall may be the first actual symptoms of osteoporosis unless the doctor has been measuring the bone density. Men also should watch for a loss of height, change in posture or sudden back pain. There are a number of risk factors that increase a person’s likelihood of having osteoporosis.
Mechanism
Osteoporosis is a disease mainly of post-menopausal ladies and elderly males. It results due either to excessive bone resorption or diminished new bone formation or both. Advances in molecular genetics and cell biology have revolutionalised our understanding of the basic mechanisms that underlie the regulation of bone-remodelling and have shown the importance of genetic factors in osteoporosis. Clinical studies have shown the association between polymorphism of several candidate genes and bone mass. The reduction in vitamin D supply or activity, aggravates osteoporosis and predisposes to fracture. Lack of exposure to sun-light due to restricted mobility and decreased 25-hydroxylase activity in old age leads to reduced formation of 25-hydroxy vitamin D. Age related deficiency of renal enzyme 1-hydroxylase impairs conversion of 25 hydroxy-vitamin D to active 1.25-dihydroxyvitamin D, leading to decreased bone loss.
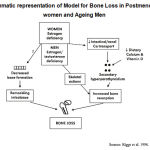 |
Diagram 1:Diagrammatic representation of Model for Bone Loss in Postmenopausal women and Ageing Men.
|
ALP
To test for increased levels that may point to a problem with the bones.
Protein electrophoresis
To identify abnormal proteins produced by a certain of cancer (called multiple myeloma) that can break down bone
Testosterone
To check for deficiency in men
FSH
To check for menopause
Parathyroid hormone
To check for hyperparathyroidism
Thyroid tests
Such as T4 and TSH to screen for thyroid disease
Vitamin D
Deficiencies can lead to decreased calcium absorption
Blood calcium levels
This test is usually normal in osteoporosis but may be elevated with other bone diseases
Blood tests may include
Laboratory Tests
The goals with testing are to determine whether a patient has osteoporosis, has low bone mass and an increased risk of developing the disease, is menopausal and/or hormone-deficient, and/or has an underlying condition that may be causing or exacerbating bone loss. Testing may be done to screen for bone density loss or to evaluate bone status when a person has an unexpected bone fracture and may be used to monitor osteoporosis therapy for effectiveness. Diagnostic imaging, a non-laboratory test, is used in the Bone Mineral Density test, the primary screening and diagnostic test for osteoporosis (Murby Brian & Fogelman I 1988)
TESTS
Lifestyle
Current cigarette smoking, drinking too much alcohol, consuming an inadequate amount of calcium or getting little or no weight-bearing exercise, increases the chances of developing osteoporosis.
Menopause/Menstrual History
Normal or early menopause (brought about naturally or because of surgery) increases the risk of developing osteoporosis. In addition, women who stop menstruating before menopause because of conditions such as anorexia or bulimia, or because of excessive physical exercise, may also lose bone tissue and develop osteoporosis.
Bone Structure and Body Weight
Small-boned and thin women (under 127 pounds) are at greater risk.
Race: Caucasian and Asian women are more likely to develop osteoporosis. However, African American and Hispanic women are at significant risk for developing the disease.
Family History and Personal History of Fractures as an Adult
Susceptibility to fracture may be, in part, hereditary. Young women whose mothers have a history of vertebral fractures also seem to have reduced bone mass. A personal history of a fracture as an adult also increases the fracture risk.
Gender
There are more chances of developing osteoporosis greater in case of a woman. Women have less bone tissue and lose bone more rapidly than men because of the changes involved in menopause.
Age
The older the age, the greater the risk of osteoporosis. Bones become weaker and less dense as the age.
Taking one of the following medications can increase one’s risk as well
Seizure medication
Immunosuppressive drugs
Steroids (prednisone, hydrocortisone, dexamethasone)
Heparin
Lithium
Excess Thyroxine, thyroid replacement (Franklyn JA & Sheppard MC 1990).
In addition, having a history of one of the following diseases can increase both a woman and man’s risk of developing osteoporosis (Satterfield T et al., 2003):
Hyperparathyroidism, having an overactive parathyroid gland
Hyperthyroidism, having an overactive thyroid gland
Severe liver disease
Kidney failure
Pituitary tumor
Adrenal disease
Malabsorption
Multiple sclerosis
Rheumatoid arthritis
Multiple myeloma
Lymphoma
Leukemia
Diabetes
Risk Factors for Men
Heredity
Race — White men appear to be at the greatest risk for developing osteoporosis, although the condition can affect people of all ethnic groups
Undiagnosed low levels of testosterone
Falling
Inadequate physical activity
Age — Bone loss increases with age
Chronic disease that alters hormone levels and affects the kidneys, lungs, stomach and intestines
Smoking tobacco
Alcoholism
Lifelong low calcium intake
Low body weight
Risk Factors for Women
European or American ethnic background
Personal history of fracture as an adult
Poor general health
Smoking tobacco
Low body weight, less than 127 pounds
Estrogen deficiency
Early menopause, before age 45
Surgical removal of the ovaries before age 45
Prior to menopause, having a time in life when the individual went more than a year without a menstrual period
Taking medical therapy that lowers estrogen levels, such as for breast cancer or endometriosis
Lifelong low calcium intake
Alcoholism
Poor vision despite correction, like wearing glasses
Falling
Inadequate physical activity
Risk Factors
Some drugs seem to protect one site in the body, such as the spine, but have not been proven to protect others, like the hip, which is why you may be prescribed one drug rather than another. Drug treatments are taken to reduce the risk of broken bones. They are increasingly being prescribed for people whose risk of fracture is considerably increased. Factors such as age, in case of smoking, have low bone density, have already broken a bone very easily or take corticosteroid tablets will be used to determine whether there is need to start a drug treatment.
Although there is no cure for osteoporosis a broad range of therapies have been approved by the Food and Drug Administration for postmenopausal women to prevent or treat osteoporosis: from over-the-counter medications such as Calcium and vitamin D, to estrogen therapy, and newer medications such as “Antiresorptive agents”(i.e., Selective Estrogen – receptor modulators) and Anabolic therapy (Eastell R 1998). All drug treatments have benefits as well as possible side effects and risks. No treatment is right for everyone. Remember that medicines affect people indifferent ways so a drug may cause side effects for one person yet not another (Beaglehole R 1988).
Diagrammatic representation of Model for Bone Loss in Postmenopausal women and Ageing Men
Bone Markers, blood and urine tests that may sometimes be ordered to help evaluate and monitor the rate of bone resorption and formation include:
Bone Resorption tests
C-telopeptide (C-terminal telopeptide of type 1 collagen (CTx)) – a fragment of the protein matrix
N-telopeptide (N-terminal telopeptide of type 1 collagen (NTx)) – another fragment of the protein matrix
Deoxypyridinoline (DPD) – a collagen breakdown product with a ring structure
Pyridinium Crosslinks – a group of collagen breakdown products that includes DPD
Bone formation tests
Bone-specific alkaline phosphatase (ALP) – one of the isoenzymes (types) of ALP; associated with osteoblasts and thought to have a role in bone mineralization
Osteocalcin (bone gla protein) – a protein created by osteoblasts; part of the non-collagen portion of the new bone structure; some of it also enters the bloodstream
Non-Laboratory Tests
The bone mineral density (BMD) test is the primary test used to identify osteoporosis and low bone mass. One of the preferred and most accurate ways to measure BMD is Dexa-Scan (dual-energy x-ray absorptiometry or DXA). It uses a low energy x-ray to evaluate bone density in the hip and/or spine. BMD is often reported in terms of peak bone mass in young adults. A BMD value that is less than 1 standard deviation below the young adult mean is considered normal. BMD in osteopenia has a value between -1 and -2.5 standard deviations below the young adult mean while osteoporosis BMD values are even lower and are at least -2.5 standard deviations below the mean.
Portable BMD screening devices, utilized at some pharmacies, health fairs, etc., are used to scan people’s heels or fingers. These scans are not as accurate as the DXA but may be used as an initial scan. When these tests are positive for decreased bone density, a DXA scan may be performed for confirmation.
Other diagnostic imaging tests that may be done to measure BMD and to detect osteoporosis include CT scans (computerized tomography), X-rays and ultrasounds (Osteoporosis: The Diagnosis, NIAMS 2005 November).
One other diagnostic imaging test used to evaluate the condition of the bones is a bone scan, which is not to be confused with the bone density scan or BMD. While the BMD test is used to identify low bone density that is indicative of osteoporosis and is non-invasive, a bone scan is a nuclear medicine test used to rule out other serious conditions of the bones. To perform this test, a radioactive tracer is injected into a vein in the arm. The tracer then travels through the blood and is absorbed by the bones. The level of radioactivity detected in the bone is evaluated and can point to conditions or diseases such as metastatic cancer, infection, causes of unexplained bone pain, or Paget’s disease. This type of scan can discov
discover problems with the bones much earlier than a regular X-ray and may be ordered when patients have a high frequency of bone fractures.
Diagnosis
Specialized tests called bone mineral density (BMD) tests can measure bone density in various sites of the body. Experts recommend a type of BMD test using a central DXA (Kanis J et al., 1994)
A BMD test performed by a central DXA can:
Tell if a person has low bone density before a fracture occurs
Tell if a person’s bones are losing bone density or staying the same when the test is repeated at intervals of one year or more
Predict the chances that a person will have a fracture in the future
Help a person and their healthcare provider decide if treatment is needed
Medicare reimburses for BMD testing every two years
An increase in BMD testing and osteoporosis treatment was associated with a decrease in hip fracture incidence. BMD is an important determinant of fracture risk even in nursing home patients. There has been a five-fold increase in office visits for osteoporosis (from 1.3 to 6.3 million) in the past 10 years.
It is important to understand that bone is not a hard and lifeless structure; it is, in fact, complex, living tissue. Bones provide structural support for muscles, protect vital organs, and store the calcium essential for bone density strength. Because bones are constantly changing, they can heal and may be affected by diet and exercise. Until the age of about 30, the individual build and store bone efficiently. Then, as part of the natural aging process, these bones begin to break down faster than new bone can be formed. In women, bone loss accelerates after menopause, when her ovaries stop producing estrogen – the hormone that protects against bone loss (Jacqui Wise 1988)
Think of the bones as a savings account. There is only as much bone mass in the account as deposited. The critical years for building bone mass are from prior to adolescence to about age 30. Some experts believe that young women can increase their bone mass by as much as 20 Percent – a critical factor in protecting against osteoporosis.
A bone mass measurement is the only way to tell whether there is osteoporosis or not. Specialized tests called bone density tests can measure bone density in various sites of the body (MayoClinic.com).
A bone density test can
Detect osteoporosis before a fracture occurs
Predict the chances of fracturing in the future
Determine the rate of bone loss and/or monitor the effects of treatment if the test is conducted at intervals of a year or more.
Normal Spine
B. Moderately Osteoporotic Spine
C. Severely Osteoporotic Spine
The only sure way to determine bone density and fracture risk for osteoporosis
is to have a bone mass measurement also called BMD test.
National osteoporosis foundation (NOF Feburary 25, 2005) Guidelines indicate, BMD testing should be performed on:
All women aged 65 and older regardless of risk factors
Younger postmenopausal women with one or more risk factors (other than being white, postmenopausal and female).
Postmenopausal women who present with fractures (to confirm the diagnosis and determine disease severity).
Medicare covers BMD testing for the following individuals aged 65 and older:
Estrogen deficient women at clinical risk for osteoporosis
Individuals with vertebral abnormalities
Individuals receiving, or planning to receive, long-term glucocorticoid (steroid) therapy
Individuals with primary hyperparathyroidism
Individuals being monitored to assess the response or efficacy of an approved osteoporosis drug therapy.
Medicare permits individuals to repeat BMD testing every two years.
There are several ways to measure bone mineral density; all are painless, noninvasive and safe and are becoming more readily available. In many testing centers we don’t even have to change into an examination robe.
The tests measure bone density in the spine, hip and/or wrist, the most common sites of fractures due to osteoporosis. Recently, bone density tests have been approved by the FDA that measure bone density in the middle finger and the heel or shinbone. Bone density is compared to two standards, or norms, known as “age matched” and “young normal.” The age-matched reading compares this bone density to what is expected in someone of the same age, sex and size. The young normal reading compares the density to the optimal peak bone density of a healthy young adult of the same sex.
Types of BMD Tests
There are several different machines that measure bone density. Central machines measure density in the hip, spine and total body. Peripheral machines measure density in the finger, wrist, kneecap, shin bone and heel.
DXA (Dual Energy X-ray Absorptiometry) measures the spine, hip or total body.
pDXA (Peripheral Dual Energy X-ray Absorptiometry) measures the wrist, heel or finger.
SXA (single Energy X-ray Absorptiometry) measures the wrist or heel.
QUS (Quantitative Ultrasound) uses sound waves to measure density at the heel, shin bone and kneecap.
QCT (Quantitative Computed Tomography) most commonly used to measure the spine, but can be used at other sites.
pQCT (Peripheral Quantitative Computed Tomography) measures the wrist.
RA (Radiographic Absorptiometry) uses an X-ray of the hand and a small metal wedge to calculate bone density.
DPA (Dual Photon Absorptiometry) measures the spine, hip or total body (used infrequently).
SPA (Single Photon Absorptiometry) measures the wrist (used infrequently).
Treatment
The bone density test will tell the doctor if it is normal, osteopenic (low bone mass) or osteoporotic. Based on these results and the risk factors for fracture, the individual and the doctor may select among the following treatment options. (Juby AG & Davis P, 2001)
Treatment for Women & Men
Prevention
No matter what the bone density is, all women should optimize their lifestyle to help prevent bone loss (Magnus JH et al., 1996). This includes:
Adopting a regular exercise regimen of weight-bearing exercises, such as walking or jogging, dancing, weight lifting, racquet sports and using resistance machines.
In addition, it is important to get enough vitamin D. A daily intake of 400 IU, but no more than 800 IU, each day is recommended. Obtaining adequate amounts of vitamin D from the food may be difficult. The main sources of dietary vitamin D are fortified milk (100 IU/cup), egg yolks (25 IU/yolk) and oily fish (vitamin D content varies). Sunlight exposure causes vitamin D production in the skin, but this effect is blocked by sunscreen. Many people need vitamin D supplements to achieve an adequate intake. Most multi-vitamins contain 400 IU of vitamin D.
Ensuring a daily calcium intake of 1,000 mg per day to age 50, and 1,200 to 1,500 mg per day for those over age 65 also is recommended. Calcium Counter offers a basic guideline for maintaining good bone health through adequate calcium consumption.
Medication
If the bone mass and the risk factors put the individual at high risk for fracture, then the doctor also may want him to take medication either to treat or prevent osteoporosis. There are many medications available. All have risks and benefits. Only the individual and his doctor can select which medication is right for him.
Bisphosphonates
These medications are very effective in increasing bone mass at all ages and reduce fractures by about 5 Percent. Current bisphosphonates approved for osteoporosis include alendronate (Fosomax) and risidronate (Actonel). Doctor’s may recommend taking bisphosphonates with supplemental calcium and vitamin D (Black D et al., 1996). These medications can be hard to absorb and they must be taken on an empty stomach first thing in the morning with water only. The individual then must remain upright for at least 30 minutes before eating or drinking anything else. Rarely, these medications can cause oesophageal irritation and ulceration. There are daily and weekly regimens of bisphosphonates; both appear equally effective at increasing bone density (Rosen CJ & Kessenich CR 1996).
Calcitionin
This medication is a nasal spray and some evidence suggests it may reduce vertebral fractures although the studies are small. Unlike other medications, it appears to help reduce the pain associated with fractures. While calcitionin is currently only FDA approved for the treatment of osteoporosis in postmenopausal women, evidence suggests that it may have similar effects of men.
Testosterone Replacement Therapy
This medication may be prescribed to men with low testosterone levels and has been shown to increase muscle mass and strength, libido, bone mass and hair growth. (Ferguson KJ et al., 1989)
Parathyroid Hormone (PTH)
Teriparatide, a form of parathyroid hormone, has been shown to stimulate bone formation and increases bone mineral density. An 11-month study conducted by Orwoll and the Oregon Health Science at the University of Portland, found that men with osteoporosis who took PTH had a spine bone mineral density (BMD) increase of 6 Percent and a hip BMD increase of 1.5 Percent. Teriparatide is self-administered as a daily injection for up to two years.
Kyphoplasty
A new treatment for osteoporosis spine fractures is called kyphoplasty. Kyphoplasty is a minimally invasive procedure, which means only tiny incisions are used. Through an incision, a small balloon is inserted into the collapsed bone to restore its shape. It is then filled with a substance that hardens and helps the bone expand. Long-term trials of this procedure are ongoing. Although osteoporosis has been studied for a number of years, no current effective prevention and treatment methods exist for this disease. There are several major barriers that exist for the use of any pharmaceutical agents to stimulate new bone formation (Kasper MJ et al., 1994, Kasper MJ et al., 2001).
Nanoparticles For The Treatment Of Osteoporosis
On the other hand, based on their unique mesoscopic physical, chemical, thermal, and mechanical properties, nanoparticles offer a high potential for several biomedical applications, including bioanalysis and bioseparation, tissue specific drug therapeutic applications, gene and radionuclide delivery . In order to be used effectively in fighting diseases, specific surface chemistry of the nanoparticles need to be tailored for their desired biomedical applications. Magnetic nanoparticles are also of interest. Specifically, the main interest for the use of magnetic nanoparticles in biomedical applications is that an in homogeneous external magnetic field exerts a force on them, and thus they can be manipulated or transported to a specific diseased tissue by a magnetic field gradient.
They also have controllable sizes, so that their dimensions can match either that of a virus (20–500 nm), of a protein (5–50 nm) or of a gene (2nm wide and 10–100 nm long). In addition, magnetic particles are of interest because they do not retain any magnetism after removal of the magnetic field.
Specifically, inorganic biodegradable nanoparticles (including ceramics like hydroxyapatite) will be functionalized with bioactive chemicals such as bone morphogenetic protein-2 (BMP-2) that bond to bone of low mass. Such bioactive groups will be placed on the outer surface of the magnetic nanoparticle systems using various techniques (such as covalent chemical attachment). After bonding specifically to osteoporotic bone and not healthy bone, magnetic nanoparticle systems will deliver bioactive compounds to locally increase bone mass.
Lastly, the outer coating of the embedded nanoparticle systems will be created to have different biodegradation rates for the release of bone-building agents over various time spans; this will allow for not only quick bone formation but also long-term sustained bone regeneration. One potential advantage of formulating HA magnetic nanoparticles are that as the magnetic particles accumulate, e.g., in bone tissue, they can play an important role in detection through MRI to locate, monitor and control drug activities. They are several trends to cure osteoporosis using the nanoparticles (Arya SN,1999).
Nowadays scientists are using Functionalized Calcium Phosphate-based Nanoparticles for the Treatment of Osteoporosis.
Functionalization of Aminophase Molecules on Nanomaterials
For the attachment of bioactive compounds, they use silane chemistry. Alkoxysilanes were chosen in this study because of their ability to graft to hydroxyl terminated surfaces of HA. It is expected that the surface energy of biomaterials can be controlled using the attachment site (e.g., silane), spacer (e.g., alkane), and end group (e.g., amine). It is important to note that although aminophase chemistry has been used for glass, little to no reports are in the literature for its use on HA.
Applications of Magnetic Nanoparticles for the Treatment of Osteoporosis
Some of these novel drug carrying systems will then distribute pharmaceutical agents locally to quickly increase bone mass. Specifically, the main interest for the use of magnetic nanoparticles in biomedical applications is that an inhomogeneous external magnetic field exerts a force on them, and thus they can be manipulated or transported to a specific diseased tissue by a magnetic field gradient. In addition, magnetic particles are of interest because they do not retain any magnetism after removal of the magnetic field.
Specifically, inorganic biodegradable nanoparticles (including ceramics like hydroxyapatite) will be functionalized with bioactive compounds that bond to bone of low mass. Such bioactive groups will be placed on the outer surface of the magnetic nanoparticle systems using various techniques (such as covalent chemical attachment). After bonding specifically to osteoporotic bone and not healthy bone, magnetic nanoparticle systems will deliver bioactive compounds to locally increase bone mass. Lastly, the outer coating of the embedded nanoparticle systems will be created to have different biodegradation rates for the release of bone-building agents over various time spans; this will allow for not only quick bone formation but also long-term sustained bone regeneration.
Future Directions
In osteoporosis research, there are several major barriers that exist for the use of any pharmaceutical agents to stimulate new bone formation. Because of these limitations that even the best strategies to sufficiently increase bone mass (although, to date, still unproven) require at least one year to see any change; a time period not acceptable especially for the elderly. For these reasons, in this study we used nanotechnology (or the design of materials with 10/sup -9/ m dimensions) to develop novel drug-carrying systems that will specifically attach to osteoporotic (not healthy) bone. Moreover, some of these novel drug carrying systems will then distribute pharmaceutical agents locally to quickly increase bone mass.
Efforts will focus on the prolonged release of bioactive agents (bone morphogenetic protein-2 (BMP-2)) to efficiently regenerate enough bone for the patient to return to a normal active lifestyle. Particularly, inorganic biodegradable nanomaterials (including ceramics like hydroxyapatite or HA) will be functionalized with bioactive chemicals. Such bioactive groups will be placed on the outer surface of the nanoparticle systems using various techniques. The outer coating of the embedded nanoparticle systems will also be created to have different biodegradation rates for the controlled release of embedded bioactive agents to the target site.
References
- Adami S, Passeri M, Ortolani S, Broggini M et al. Effects of Oral Alendronate and Intranasal Salmon Calcitonin on Bone mass and Biochemical markers of Bone – Turnover in Post-menopausal women with osteoporosis. Bone 1995; 17 (4): 383-90. 18.
- Amin, S. (2004 May, Updated). Osteoporosis American College of Rheumatology (© 2006)
- Arya SN. Recent Trends in the treatment of Osteoporosis, Sinha KK, Thakur D, (ed) New Trends in Medicine, Ranchi 9th BAPICON (Dr KK Sinha, Booty Road, Ranchi) 1999; 135-9.178 Journal of Indian Academy of Clinical Medicine _ Vol. 5 _ No. 2
- Beaglehole R. Post-menopausal oestrogens seem to reduce Coronary Heart Disease. Br Med J (Indian Edition) 1988; 4: 667-8
- Black D, Cummings SR, Karpf DB et al. Randomised trial of effect of alendronate on risk of fracture. Lancet 1996; 348: 1535-41. 20
- Black DM, Reiss TF, Nevitt MC et al. Design of the Fracture Intervention Trial. Osteoporosis International 1993; 3:529-39
- British National Formulary, Tavistock Square, London WC1H 9JP, England and P.O. Box 151, Wallingford Oxon OX 10 8 QU, England. British Medical Association and The Pharmaceutical Press respectively 1998; 35: 334-6
- British National Formulary-38, Tavistock Square, London WC1H 9JP, UK and Lambeth High Street, London SE1 7JN, UK, British Medical Association and Royal Pharmaceutical Society of Great Britain respectively, Sept 1999; 38:342-3
- Eastell R. Treatment of postmenopausal osteoporosis, N Engl J med 1998; 338: 736-46
- Fast Facts [47]. National Osteoporosis Foundation (2005 October 5)
- Ferguson KJ, Hoegh C, Johnson S. Estrogen replacement therapy: A survey of women’s knowledge and attitudes. Arch Int Med 1989;149:133-6
- Franklyn JA, Sheppard MC. Thyroxin replacement treatment and osteoporosis. Br Med J (Indian Ed.) 1990; 6: 353-4
- Guyton AC. Parathyroid Hormone, Calcitonin, Calcium and Phosphate metabolism, Vit D, bone and teeth, Dana Dreibelbis, (ed) Guyton-Text Book of Medical Physiology. 7th Edition : HARCOURT Brace JOVANOVICH Inc., The CURTIS Centre, Independence Square West, Philadelphia PA 19106, W.B. Saunders Company 1986; 936-53
- Harrison’s Principles of Internal Medicine, 14th Ed., New Delhi, McGraw Hill, Health Professions Division 1998; 2: 2247-53
- I.O.F, osteoporosis in the European community: action plan. I.O.F 2003
- Jacqui Wise. Hormone replacement therapy increases risk of breast cancer. Br Med J (Indian Edition) 1988; 13: 1116
- Juby AG, Davis P. A prospective evaluation of the awareness, knowledge, risk factors and current treatment of osteoporosis in a cohort of elderly subjects. Osteoporos Int 2001;12:617
- Kanis J, Melton LJ, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis, J Bone Miner Res 1994; 9:11 37-41
- Kasper MJ, Peterson MG, Allegrante JP. The need for comprehensive educational osteoporosis prevention programs for young women : results from a second osteoporosis prevention survey. Arthritis Rheum 2001;45:28-34
- Kasper MJ, Peterson MG, Allergrante JP, et al. Knowledge, beliefs, and behaviour among women concerning the prevention of osteoporosis. Arch Fam Med 1994;3:696-702
- Lindsey R. Post-menopausal osteoporosis, W. Sircus, Current Medicine-2, Royal College of Physicians of Edinburgh, Churchill Livingstone 1990; 2: 65-83
- Magnus JH, Joakimsen RM, Berntsen GK, et al. What do Norwegian women and men know about osteoporosis? Osteoporosis Int 1996;6:31-6
- MayoClinic.com. Bone density test: Measure your risk of osteoporosis
- Molnar FJ, Man-Son-Hing M, Dalziel WB, et al. Assessing the quality of newspaper medical advice columns for elderly readers. CMAJ 1999;161:393
- Murby Brian, Fogelman I. Bone mineral measurements in clinical practice. Journal of Applied Medicine 1988; 14: 115-24
- N.O.F. (National osteoporosis foundation). Disease statistic Feburary 25,2005[www.nof.org]
- Osteoporosis Overview [52]. NIAMS(2005 June, Revised)
- Osteoporosis: The Diagnosis [14]. NIAMS (2005 November, Revised)
- Pal B. Questionnaire survey of advice given to patients with fracture. BMJ 1999;318(7182):500-1
- Pande KC, de Takats D, Kanis J, et al. Development of a questionnaire (OPQ) to assess patient’s knowledge about osteoporosis. Maturitas 2000;237:75-81
- Pande KC. Prevalence of low bone mass in healthy Indian population. JIMA 2002;1000:598-600
- Phillipov G, Phillips PJ, Leach G, et al. Public perceptions and self-reported prevalence of osteoporosis in South Australia. Osteoporos Int 1998;8:52-6
- Prakash C. Involutional Osteoporosis. API-Medicineupdate (Part I) 1999; 9: 387-95
- Ralston SH. Osteoporosis. Br Med J (Indian Edition) 1997; 13: 956-9
- Riggs BL, Melton III LJ. The worldwide problem of osteoporosis; Bone 1995; 17 (suppl 5): 505s-11s
- Rosen CJ, Kessenich CR. Comparative Clinical Pharmacology and Therapeutic use of Biphosphonates in Metabolic Bone Diseases. Drugs 1996; 51 (4): 537-51
- Satterfield T, Johnson SM, Slovic P, et al. Perceived risks and reported behaviours associated with osteoporosis and its treatment. Women Health 2003;31:21-40
- Savvas M, Brincat M, Studd JWW. Postmenopausal osteoporosis. Journal of Applied Medicine 1988; 14: 157-60
- Smith R. Disorders of Skeleton, Weatherall DJ, Ledingham JCG, Warell DA (ed). Oxford Text Book of Medicine International Ed. ELBS, Great Clarendon Street, Oxford OX2 6DP, UK 1985; 17.10-17.15
- Smith R. Osteoporosis: Cause and management. Br Med J (Indian Edition) 1987; 3: 95-101
- Stein E, Shane E. Secondary osteoporosis. Endrocrinol Metab clin N Am 2003; 32:115-34
- Teotia SPS, Teotia M. Osteoporosis (Osteopenia) Shah SJ, Anand MP, Metha AB, Sainani GS, Vishwanathan M (ed). API Text Book of Medicine, Bombay, Association of Physicians of India 1990; 4: 65-83
- Ungan M, Tumer M. Turkish women’s knowledge of osteoporosis. Fam Pract 2001;18:199-203
- WebMD A to Z Health Guide from WebMD: Medical Tests, Bone Mineral Density
- WebMD A to Z Health Guide from WebMD: Medical Tests, Bone Scan
- WHO-Study Group. Assessment of Fracture risk and its application to screening for post-menopausal osteoporosis-WHO Technical Report series, 843. World Health Organisation Geneva 1994.







