A. Hasmah 1*, Azimahtol Hawariah L. P.2 and Judith Hohmann3
1School of Health Sciences, Health Campus, Universiti Sains Malaysia, 16150 Kota Bharu, Kelantan, Malaysia. ²School of Biosciences and Biotechnology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, Bangi 43600, Selangor, Malaysia ³Institute of Pharmacognosy, University of Szeged, Hungary. Corresponding Author E-mail: hasmah@kck.usm.my
Abstract
Cytochrome c is a central protein that plays a role in triggering apoptosis in cell induced by any agent either drug or plant extracts. Here, we present data of an active antiproliferative agent 7,3’,5’-trihydroxyflavanone (3HFD) that is elucidated from the plant Hydnophytum formicarium showed to trigger cytochrome c release in treated MCF-7 cell. By performing Western Blot, cytochrome c was seen to be elevated throughout the experiments. The increase level of cytochrome c was confirmed by ELISA and remarked as the beginning of the caspase cascade without altering caspase-8 level. In conclusion, the 3HFD seems to significantly induced apoptosis via mitochondria pathway as reflected by up regulation of cytochrome c.
Keywords
Cytochrome c; MCF-7 cels; Hydnophytum formicarium
Download this article as:| Copy the following to cite this article: Hasmah A, Hawariah L. P. A, Hohmann J. Release of Cytochrome c in MCF-7 Cells Treated With 7, 3’, 5’-Trihydroxyflavanone of Hydnophytum Formicarium. Biomed. Pharmacol. J.2009;2(1) |
| Copy the following to cite this URL: Hasmah A, Hawariah L. P. A, Hohmann J. Release of Cytochrome c in MCF-7 Cells Treated With 7, 3’, 5’-Trihydroxyflavanone of Hydnophytum Formicarium. Biomed. Pharmacol. J.2009;2(1). Available from: http://biomedpharmajournal.org/?p=603 |
Introduction
Cytochrome c is an ancientprotein, developed early in the evolution of life. The familiar function of cytochrome c, is its role as a carrier of electrons (Reed, 1997). It is a small,mobile molecule that shuttles electrons through the last stepof aerobic energy production. These electrons are obtained fromthe dATP, which are shuttledthrough a series of proton pumping proteins (Yang et al., 1997). Cytochrome c shuttles these electrons in the narrow spacebetween the two mitochondrial membranes. It diffuses from proteinto protein, picking up electrons from one huge membrane-boundcomplex and placing them at their final destination on another (Liu et al., 1996).
Cytochrome c and the mitochondria play a central role in apoptosis, signaling the cell to begin the process of programmed cell death (Goldstein et al., 2005a; Goa et al., 2001). Apoptosis is triggered when something is amiss with the cell: DNA damage, detachment from neighbors, growth factor deprivations, infection, or a host of other signs. The cell then initiates one or more cascades of signaling proteins that spread the message through the cell and ultimately orchestrate a controlled self-destruction. Apoptosis is essential in many natural processes, such as the coordinated growth and selective pruning that shapes a growing embryo. If the system is corrupted, however, the consequences are dire, leading to degenerative diseases if overactive and allowing the growth of cancers if blocked (Goldstein et al., 2005b). Although studies of plant-derived anti-cancer agents are fast-progressing, but the precise mechanism of plant-derived agents on the inhibition of cancer cell growth is still not completely understood. Previous studies reported that cytochrome c play as central role in apoptosis regulation, and contribute significantly to the pathogenesis of cancer.
In this study, we tested the 7, 3’, 5’-trihydroxyflavanone (3HFD) that is elucidated from the plant Hydnophytum formicarium (Hasmah et al. 2008) of the rubiaceae family which is native to Malaysia and Indonesia (Huxley, 1992) for cytochrome c signaling on human breast cancer cell line, MCF-7. This compound was reported to exert potent antiproliferative activity towards MCF-7 cell without affecting normal cell line, MDBK (Hasmah et al. 2008). Previously chloroform extract of this plant was reported to have anti multidrug resistant towards mouse lymphoma cell lines transfected with human mdr1 gene with moderate effect (Hasmah et al. 2004). Here in this study, we treated MCF-7 cells with the concentration of 3HFD as reported by Hasmah et al. (2008) to observe the expression of cytochrome c with regard to the involvement of procaspase-8. We also performed the ELISA analysis to figure out the concentration of cytochrome c release in treated and untreated control cells.
Materials and Method
Cell culture
MCF-7 human mammary carcinoma cells were obtained from the American Type Culture Collection (ATCC) and maintained in DMEM supplemented with 10% fetal bovine serum and 2 mM glutamine (Lee et al., 2003).
Isolation of cytosolic fractions
Cytosolic extracts were prepared as previously described by Yang et al. (1997). Briefly, treated cells were harvested by centrifugation and washed with ice-cold phosphate-buffered saline and re-suspended in 5 volumes of extraction buffer containing 250 mM sucrose. Cells were homogenized and the homogenates were centrifuged twice at 750 × g for 10 min at 4°C. The supernatant was then centrifuged at 10,000 × g for 15 min at 4°C, and the resulting mitochondrial pellets were discarded. The supernatant was then dissolved in electrophoresis sample buffer and used for Western blotting.
Western Blotting
After electrophoresis, proteins were blotted onto polivynyl-difluoride membranes (PolyScreen, Nen Life Science). Membranes were dried, pre-blocked with 5% non-fat milk in phosphate-buffered saline and 0.1% Tween-20, then incubated with a primary antibody for caspase-8 and cytochrome c (Clone 7H8.2C12) (all from Pharmingen), and detected with horseradish peroxidase-labeled antibodies to rabbit or mouse IgG. Following exposure on a Kodak BIOMAX x-ray film, densitometry analysis was done with a GS 670 Imaging Densitometer with the software Molecular Analyst (Bio Rad). Blots were stripped with Re-Blot Plus (Chemicon) before reprobing with β-actin antibody to determine equal loading.
ELISA analysis of cytochrome c.
Quantification of cytochrome c concentration was assayed by means of ELISA analysis according to User Protocol QIA74 provided in the test kit by Qiagen. The assay was done according to the manufacturer’s instructions. Briefly, MCF-7 cells were treated with 3HFD at 9 µg/ml 3HFD for 0, 3, 6, 12 and 24 in 5% CO2 at 37 °C. Control were treated with 1% DMSO. After the treatment period, cells were counted and then pelleted at 1,500 rpm for 10 min. Cells were then re-suspended in chilled Cell Lysis Buffer and incubated at room temperature before centrifugation at 1000 × g for 15 min. The supernatant (cytosolic extract) was then diluted for 5 times and assayed immediately. Alternatively, supernatant could be aliquot and stored at -80 °C. The protein concentration for each sample set was then assayed using standard protocols. Assay mixture was prepared in a 96-well plate and mixed with calibrator diluents, sample and standard and shielded with provided plastic cover and left for 2 hours at room temperature. After 2 hours, 96 well plates were washed with wash buffer and dried. Lastly, cytochrome c conjugate solution were added and incubated for 2 hours, washed and mixed with substrate solution for 30 minutes followed by adding stop solution and mixed well. The mixture was read with a Dynex MRX microtiter plate reader at 450 nm and 540 nm. The concentrations were determined from regression equation of standard curve times dilution factor.
Result
During apoptosis, initiator caspases are activated in response to proapoptotic signals (Diaz et al., 2003). By SDS-PAGE and subsequent Western Blot analysis with a caspase-8 specific antibody, it was found that 3HFD treatment did not lead to the activation of the initiator caspase-8. Procaspase-8, expressed in two functionally active isoforms, caspase-8a and caspase-8b (Diaz et al., 2003) was not processed. From immunoblotting, the two bands observed were the 55/50-kDa procaspase-8 isoforms (Figure 1) similarly as reported by Sun et al. (1999) and Dirsch et al. (2001) and the active p18 subunit could not be detected. As processing of this caspase did not occur, it is possible that the other initiator caspase, caspase-9 may be involved in 3HFD-induced apoptosis.
Cytochrome c seems to be a major trigger for the assembly of this complex, and various studies have found that cytochrome c is released from the mitochondria into the cytosol during cell death (Li et al. 1997). When cytochrome c levels in the cytosol were examined, we detected increasing levels in the 3HFD-treated MCF-7 cells (Figure 1). Untreated control cells did not exhibit similar high levels of cytochrome c, indicating that the release of cytochrome c from the mitochondria into the cytosol was an effect of 3HFD treatment.
 |
Figure 1:
|
We further examined the concentration of cytochrome c by means of enzymatic assay through the same experimental period. The level of cytochrome c concentration increased in time dependence manner. This result supported the Western Blot analysis and high peaks were observed at 24 hours treatments (Figure 2).
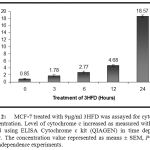 |
Figure 2:
|
Discussion
Western Blott and ELISA analysis has evidenced the importance of cytochrome c in MCF-7 treated 3HFD apoptosis. These data provided basis for 3HFD mechanism of action through mitochondria pathway. Release of cytochrome c will triggered a formation of activation of caspase cascade to produce apoptotic characteristic such as nuclear condensation, substrate degradation and fragmentation DNA as suggested by Slee et al. (1999). Slee et al. (1999) also reported that cytochrome c involves in activation of downstream caspases (kaspase-2, -8 -9 dan -10), and upstream caspases (kaspase-3, -6 dan -7). Liu et al. (1996) concluded that cytochrome c will trigger the processing of casopase 3. Reed (1997) suggested that cytochrome c is a central role of apoptosis that released from mitochondria. and function as central controller of apoptosis. The exact mechanism involved in cytochrome c release is still undiscovered (Renz et al., 2001).
Determination of cytochrome c placement is also important to support this apoptosis precursor through Western blot analysis. In this study, we evaluated the concentration of cytochrome c in treated cells using ELISA assay to support the elevated expression in Western Blot analysis. Han et al. (2003) reported the evidenced of cytochrome c in cell cytosol through immunoflourescene libeling with FITC cytochrome c specific antibody. Luetjens et al. (2001) reported the multiple kinetic release of cytochrome c in MCF-7 with caspase-3 drug induced apoptosis (staurosporin and valiomysin) and tumour necrosis factor TNF-alfa. Bax or Bid from Bcl-2 family was evidenced to influence the cytochrome c release from mitochondria through the phosphorilation process (Jürgensmeier et al., 1998; Li et al., 1998; Luo et al., 1998). Smac/Diablo also reported to influence the cytochrome c release (Carson et al., 2002; Kandasamy et al., 2003) in prostate cancer cell (Ln CaP). This is due to the needs of Smac to combat epidermal growth inhibitor.
Caspase-8 was reported to influence cytochrome c release through Bid processing and triggered the activation of caspase 9 (Granville et al., 1999). However, in this study, caspase-8 was not processed. This finding supported study by Scaffidi et al. (1999) which stated that caspase-8 was not activated in early stage of caspase activation in CD95 signaling. This is because the existing of two types of different cell which were: type I involve caspase-8 activation at early stage and type II occurred at late stage of aggregation involved mitochondria. Dirsch et al. (2001) and Tang et al. (2000) also reported the inactivation of procaspase 8 in MCF-7 treated with helenalin and staurosporin respectively.
Previous reports have found that MCF-7 cells are relatively insensitive to many chemotherapeutic agents due to the absence of caspase-3 (Yang et al., 2001). Our studies here have shown that the mechanism for apoptosis is functional in MCF-7 and 3HFD is able to trigger cytochrome c release through mitochondria pathway. Therefore, finding new therapeutic agents that induce tumor cells apoptosis in a manner independent of caspase-3 with promoting cytochrome c release may have important clinical implications. By releasing cytochrome c without requiring caspase-3, 3HFD may evoke an apoptotic pathway different from clinical oncology drugs such as doxorubucin and etoposide (Yang et al., 2001) thus making it a promising agent for combination chemotherapy that merits further study.
Acknowledgement
This project was supported by Universiti Sains Malaysia (RLKA), Universiti Kebangsaan Malaysia, and University of Szeged, Hungary.
References
- Carson, J.P., Behnam, M., Sutton, J.N., Du, C., Wang, X., Hunt, D.F., Weber, M.J., & Kulik, G., Smac is required for cytochrome c-induced apoptosis in prostate cancer LNCaP cells. Cancer Research 62: 18-23 (2002).
- Diaz, G.D., Li, Q., & Dashwood, R.H., Caspase-8 and apoptosis-inducing factor mediate a cytochrome c-independent pathway of apoptosis in human colon cancer cells induced by the dietary phytochemical chlorophyllin. Cancer Research 63: 1254-1261 (2003).
- Dirsch, V.M., Stuppner, H., & Vollmar, A., Helenalin triggers a CD95 death-receptor-independent apoptosis that is not affected by overexpression of Bcl-XL or Bcl-2. Cancer Res. 61: 5817–5823 (2001).
- Goa, W., Pu, Y., Lou, K.Q., & Chang, D.C., Temporal relationship between cytochrome c release and mitochondrial swelling during UV-induced apoptosis in living HeLa cells. Journal of Cell Science 114: 2855 – 2862 (2001).
- Goldstein, J.C., Muňoz-Pinedo, C., Ricci, J.E., Adams, S.R., Kelekar, A., Schuler, M., Tsien, R.Y., & Green, D.R., Cytochrome c is released in a single step during apoptosis. Cell Death and Differentiation 12: 453-462 (2005a).
- Goldstein, J.C., Rodier, F., Garbe, J.C., Stampfer, M.R., & Campsi, J., Caspase-independent cytochrome c release is sensitive measure of low-level apoptosis in culture models. Aging Cell 4: 217 – 222 (2005b).
- Granville, D.J., Shaw, J.R., Leong, S., Carthy, C.M., Margaron, P., Hunt., D.W., & McManus, B.M., Release of cytochrome c, Bax migration, Bid cleavage and activation of caspase 2, 3, 6, 7, 8, and 9 during endothelial cell apoptosis. American Journal of Pathology 155(4): 1021-1025 (1999).
- Han, B.S., Hong, H.S., Choi, W.S., Markelonis, G.J., Oh, T.H., & Oh, Y.J., Caspase-dependent and independent cell death pathway in primary cultures of mesencephalic dopamignergic neurons after neurotoxin treatment. The Journal of Neoroscience 23(12): 5069-5078 (2003).
- Hasmah,A., Hohmann, J., Molnar, J., & Azimahtol Hawariah, L.P., Multidrug resistance reversing activity of methanol extract and fractions of Hydnophytum formicarium Jack (Rubiaceae). Journal of Tropical Medicinal Plants 5(2): 173-177 (2004b).
- Hasmah,A., Hohmann, J., Azimahtol Hawariah, L.P., Molnar, J., & Forgo, P. Antiproliferative compounds from Hydnophytum formicarium. Journal of Tropical Medicinal Plants 9(2): 366-371 (2008).
- Huxley, A.J. (1992) New Royal Horticultural Society dictionary of gardening. Jld. 4. London: Macmillan Press.
- Jürgensmeier, J.M., Xie, Z., Deveraux, Q., Ellerby, L., Bredesen, D., & Reed, J.C., Bax directly induces release of cytochrome c from isolated mitochondria. Proceeding National Academic Science USA 95: 4997-5002 (1998).
- Kandasamy, K., Srinivasula, S.M., Alnemri, E.S., Thompson, C.B., Korsmeyer, S.J., Bryant, J.L., & Srivastava, R.K., Involvement of proapoptotic Bax and Bak in tumor related necrosis factor-related apoptosis-inducing ligan (TRAIL)-induced mitochondrial disruption and apoptosis: Differential regulation of cytochrome c and Smac/DIABLO release. Cancer Research 63: 1712-1721 (2003).
- Lee, A.T.C., Azimahtol Hawariah, L.P., & Tan, A.N., Styrylpyrone Derivative (SPD) induces apoptosis in a caspase-7-dependent manner in the human breast cancer cell line. Cancer Cell International 3: 16-23 (2003).
- Li, P., Nijhawan, D., Budihardjo, I., Srinivasula, S.M., Ahmad, M., Alnemri, E.S., & Wang, X., Cytochrome c and dATP-dependent formation of Apaf-1/Caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489 (1997).
- Li, H., Zhu, H., Xu, C., & Yuan, J., Cleavage of BID by caspase-8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491-501 (1998).
- Liu, X., Kim, C.N., Yang, J., Jemmerson, R., & Wang, X., Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86: 147-157 (1996).
- Luetjans, C.M., Kögel, D., Reimertz, C., Düßmann, H., Renz, A., Schulze-Osthoff, K., Nieminen, A.L., Poppe, M., & Prehn, J.H.M., Multiple kinetics of mitochondrial cytochrome c release in drug-induced apoptosis. Molecular Pharmacology 60: 1008-1019 (2001).
- Luo, X., Budihardjo, I., Zou, H., Slaughter, C., & Wang, X., BID, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94: 481-499 (1998).
- Reed, J.C., Cytochrome c: can’t live with it-can’t live without it. Cell 91: 559-562 (1997).
- Renz, A., Berdel, W.E., Kreuter, M., Belka, C., Schulze-Oshtoff, K., & Los, M., Rapid extracellular release of cytochrome c is specific for apoptosis and marks cell death in vivo. Blood 98: 1542-1548 (2001).
- Scaffidi, C., Schmitz, I., Krammer, P.H., & Peter, M.E., The role of c-FLIP in modulation of CD95-induced apoptosis. The Journal of Biological Chemistry 274: 1541-1548 (1999).
- Slee, E.A., Harte, M.T., & Kluck, R.M., Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, 3, 6, 7, 8, and 10 in a caspase-9 dependent manner. Journal Cell Biology 144: 281-292 (1999).
- Sun, X.M., MacFarlane, M., Zhuang, J., Wolf, B.B., Green, D.R., & Cohen, G.M., Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem. 274: 5053–5060 (1999).
- Tang, D., Lahti, J.M., & Kidd, V.J., Caspase-8 activation and Bid cleavage contribute to MCF-7 cellular execution in a caspase-3-dependent manner during staurosporine-mediated apoptosis. The Journal of Biological Chemistry 275: 9303-9307 (2000).
- Yang, J., Liu, X., Bhalla, K., Kim, C.N., Ibrado, A.M., Cai, J., Peng, T.I., Jones, D.P., & Wang, X., Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275: 1129–1132 (1997).
- Yang, X.H., Sladek, T.L., Liu, X., Butler, B.R., Froelich, C.J., & Thor, A.D., Reconstitution of Caspase-3 sensitizes MCF-7 breast cancer cells to Doxorubicin- and Etoposide-induced apoptosis. Cancer Res. 61: 348–354 (2001).







