Manuscript accepted on :May 10, 2009
Published online on: 14-11-2015
Plagiarism Check: Yes
S. M. More1, A. V. Girde1 and M. M. V. Baig2*
1Department of Microbiology, Yeshwant Mahavidyalaya, Nanded - 431 602 India.May 10, 2009.
2Department of Botany and Department of Biotechnology, Yeshwant Mahavidyalaya, Nanded - 431 602 India.May 10, 2009
Correspondung Author E-mail:mmvb@indiatimes.com
Abstract
The synthesis ability of the plant pathogenic fungus Alternaria alternata (Fr.) Keissl. in production of protease in submerged cultures was studied using different substrates. The fungus was able to produce protease in the medium in different quantity. The most suitable carbon source was soybean seed powder containing medium followed by casein hydrolysate. The protease was active in a range of pH 4.0 to 7.5. The enzyme was maximally active at 45°C and was stable for several hours at temperature up to 50°C.
Keywords
protease; Alternaria alternate; carbon source; properties
Download this article as:| Copy the following to cite this article: More S. M, Girde A. V, Baig M. M. V. In Vitro Protease Synthesis by the Seed Borne Alternaria Alternata (FR.) Keissl. Biomed. Pharmacol. J.2009;2(1) |
| Copy the following to cite this URL: More S. M, Girde A. V, Baig M. M. V. In Vitro Protease Synthesis by the Seed Borne Alternaria Alternata (FR.) Keissl. Biomed. Pharmacol. J.2009;2(1). Available from: http://biomedpharmajournal.org/?p=654 |
Introduction
Seed borne plant pathogens produce a wide range of enzymes in response to the stored food material in the seeds 1. Seed-borne pathogen fungi cause losses in terms of seed quality and quantity in most of the grain crops causing loss in germination and storability of the seed.
Seeds of many pulses crop are known to harbour large amount of seed borne fungi that affects the germination and seedling emergence during the course of growth. Thus seed deterioration due to these seed borne fungi is attributed to ability of production of hydrolytic enzyme. In pulses, the seed borne fungi produces protease which hydolyses the stored protein in the seed 2.
This aspect of seed borne fungi was investigated in the earlier study3. The seed borne fungi associated with soybean was isolated and the dominant fungi were screened for the production of proteases. The deterioration of soybean seeds rich in protein was correlated with extracellular production of protease by seed borne fungi. A comparative account of five fungi viz. Alternaria alternata, Aspergillus flavus, A. niger, Fusarium oxysporum, Penicillium digitatum were studied for protease synthesis. Here a detailed study of protease produced by the A. alternate is presented.
Materials and Methods
Isolation of fungi
Untreated seeds were obtained from various sources – breeders, retailer, farmers etc. these seeds were assessed for presence of fungi using standard blotter method as recommended by International Seed Testing Association 4,5. A. aternata was isolated from the soybean seeds.
Enzyme production
A. aternata was maintained on Czapek medium supplemented with different carbon source instead of sucrose. The effect of different carbon source as substrates in the enzyme production was also studied by using different carbon substrate instead of sucrose. Czapek medium broth with 1% casein hydrolysate or 1% soybean seed powder were used as enzyme production medium in further study. The procedure for enzyme production was carried out as reported earlier3.
Partial Purification of Enzyme
The culture filtrate obtained after 8 days of incubation was used as crude enzyme preparation and was subjected to partial purification. One hundred ml of culture filtrate was processed for precipitation using 60-90 % ammonium sulphate. The precipitate was redissolved in 0.02 M Phosphate buffer at pH 7.0 and was dialyzed overnight against same buffer.
Enzyme determinations
Protease was determined using casein as substrate as described. The amino acid released was estimated by Lowry’s method 6. The enzyme activity was determined as the amount of amino acids released/unit time/g of protein. One unit of enzyme was defined as the amino acid released / unit time / gm of protein.
Effect of pH on the enzyme activity
The effects of pH on the enzyme activity was determined using buffers with pH values from 3.5 to 10.5.7
Effect of temperature on enzyme activity
The effects of temperature on the activity of enzymes were carried out at temperature ranging from 20 to 65 ºC. The thermal stability was determined by incubating the enzyme at 30, 40, 50 and 60ºC for 1 hour then the enzymes preparation was incubation in an ice bath. The enzyme activity was determined under standard conditions.
Polyacrylamide gel electrophoresis
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis (PAGE) was performed with 10 % acrylamide gel. The gel was loaded with 100 μg of protein and with a constant current of 6 mA. Staining was performed with Coomasie brilliant blue. Destaining was done by 7% acetic acid with frequent changes and gels were stored in 2 % acetic acid.8
Result
Species of Alternaria are known to synthesize a variety of enzyme depending upon availability of substrate. The isolated fungus was maintained on Czapek agar and was further used for evaluation of protease production. A series of experiments were undertaken to assess the ability of the fungus utilize various carbon source for synthesis and secretion of protease.
alternata used in the present study was isolated form the seeds. Several strain of A. alternata were isolated from different seeds, the most dominant strain based on the radial growth on the agar medium plate was selected for further studies.
alternata was grown on Czapek medium supplemented with different carbon source. The eight days old culture filtrate was used as crude enzyme source as recorded in the earlier study. A. alternata synthesizes proteases in both the media. The synthesis increased with increase in time of incubation the media; however the amount of enzymes varied. Maximum enzymes were secreted in soybean powder medium (Table no. 1) followed by Czapek medium with casein hydrolysate.
Table 1: Production of protease by A. alternata in submerged cultures.
| Carbon source | Biomass | Protease |
| (1 %) | (mg) | (U/mL) |
| Xylose | 27±7 | 0.05±0.6 |
| Glucose | 32±5 | 0.19±0.5 |
| Maltose | 43±4 | 0.18±0.3 |
| Lactose | 15±6 | 0.07±0.5 |
| Cellobiose | 27±4 | 0.08±0.3 |
| Sucrose | 35±3 | 0.11±0.4 |
| Xylan | 26±3 | 0.07±0.2 |
| CMcellulose | 19±3 | 0.10±0.2 |
| pectin | 29±4 | 0.12±0.5 |
| Starch | 32±3 | 0.14±0.4 |
| Casein hydrolysate | 57±5 | 0.20±0.4 |
| Ovoalbumin | 51±4 | 0.18±0.3 |
| Soybean seed powder | 63±4 | 0.23±0.4 |
The biomass of A. alternata produced on various carbon source varied with the carbon source. The suitable carbon source seemed to be soybean seed powder followed by casein hydrolysate. But from the data it is also clear that despite of the carbon source A. alternata produced protease constitutively.
The degree of enzyme production was found to be related with their adaptation potential which might be different in these fungi. Some properties of the extracellular protease from A. alternata was studied. The synthesized protease was more active in an acidic of pH (4.5- 7.5), while the best pH for protease activity was between pH 4.0 and 6.0 (Fig. 1). It also suggests the existence of only one group of protease, one with optimum pH between 4.0-6.0. The enzyme was optimally active at 40ºC (Fig. 2) and they retained more than 95% of initial activity after 60 min at 50ºC (data not shown). The partially purified culture filtrate containg protease was subjected to SDS PAGE which exihibited a molecular weight of 29 kD.
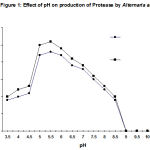 |
Figure 1:
|
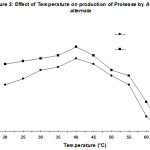 |
Figure 2:
|
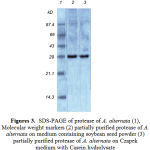 |
Figure 3:
|
In the present work it is shown that A. alternata produces protease necessary to degrade protein stored in the seeds. The secretion of protease provides this phytopathogenic fungus with the ability to attack hosts.
The results showed the capability of A. alternata to produce proteases. The enzymes may be involved in the capability of the fungus to invade plant tissues. A. alternata protease was identified as an acidic protease (Fig. 1). However, It has been suggested that the proteases may facilitate located penetration of the plant cell wall by breaking down the fibrous glycoproteins that contribute to cell wall stability9. Plant pathogenic fungi like Fusarium, Alternaria, Rhizoctonia etc. produced serine alkaline proteases, which are responsible for nutrient- mobilizing and primarily function in the support of fungal growth after host cell death 10, 11.
Acknowledgements
This work was financially supported by University Grants Commission New Delhi.
References
- Cherry, J.P. Phytopath., 73, 317-321 (1982).
- Bilgrami, K.S., Sinha, R.K., Prasad, T., Jamaluddin, Roy, A.K. Indian Phyopath., 29, 374-377 . (1976).
- More, S.M., Girde A.V., Baig M.M.V. J. Pure and Appl. Microbiol. 2(2),447-450, (2008).
- ISTA. Proc. Int. Seed Asso., 32, 565-589(1966).
- de Tempe, J. Proc. Int. Seed Test. Asso., 28, 133-151(1953).
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.L. J. Biol. Chem., 193, 265-274 (1951).
- Gomori, G. Methods in Enzymology, Vol. I. Academic Press, New York (1955).
- Bryan, J S. Basic Protein and Peptide Protocols, Vol 32 Humana Press Inc., Totowa, NJ, 23-34 (1994).
- Carpita, N.C., Gibeaut, D.M. Plant J., 3, 1-10 (1993).
- North, M.J. Microbiol. Rev., 46, 308-340 (1982)
- Ries, S.M., and Albersheim, P. Phytopath., 63, 625-629(1973).







