Vandana Singh*1, R. C. Saxena2 and A. K. Singh¹
1Pest Control and Ayurvedic Drug Research Laboratory, Vidisha (India).
2Department of Chemistry, R.G.P.V. University, Bhopal (India).
Corresponding Author E-mail: vandanatomar@yahoo.com
Abstract
Flavonoids are present widely in Angiospermic plants and are associated with several pharmacological activities including antibacterial, antidiabetic, antifungal, anti-allergic and anti-oxidant activities. Flavonoids because of their low toxicity compared to other active plant compounds can be ingested in significant quantities by human beings in their diet. Flavonoid can initially prevent the ageing, cancer and inflammation effectively rather than cure because they inhibit the occurrence of oxidative stress in the cell. Tolerance to ultraviolet and altered behavior of T, B, NH and mast cells and neutraphills in their presence assure the activation of immune system. Body’s action against them as foreign compound induces phase II enzyme which are mutagens and carcinogens eliminating. The present article shows the significance of flavonoids as potential drug, emphasizing their extraction out of Indian ethnomedicinal plant Tephrosia purpurea because of its easy availability and study of MIC value of particular flavonoid found effective on very much prevailed microbe born diseases.
Keywords
Immunomodulatory; ethnomedicinal; minimum inhibitory concentration; Antimicrobial
Download this article as:| Copy the following to cite this article: Singh V, Saxena R. C, Singh A. K.A flavonoid out of Tephrosia purpurea extract and its antimicrobial effect.Biomed. Pharmacol. J.2008;1(2) |
| Copy the following to cite this URL: Singh V, Saxena R. C, Singh A. K.A flavonoid out of Tephrosia purpurea extract and its antimicrobial effect.Biomed. Pharmacol. J.2008;1(2). Available from: http://biomedpharmajournal.org/?p=516 |
Introduction
The production of free radicals (reactive oxygen containing molecule) in all in energy producing mechanism result in oxidative stress linked with cancer, ageing, atherosclerosis, inflammation and neurodegenerative diseases. Flavonoids are actively involved in the detoxification of highly reactive species.
Detoxification of hydrogen peroxide in vacuole has been proposed by Yamaski H in 1997.
2 FlaOH + H2O2 = 2 Flavo* + 2 H2O
Flavonoid Peroxidase Flavonoid phenoxyl radical
The resulting flavonoid Phenoxyl radical can react with ascorbic acid (ASA) to generate the monodehydroascorbic acid radical (MDA*).
2 Flavo* + 2 ASA = 2 FlavOH + 2 MDA*
MDA* can in turn nonenzymatically generate ASA and DHA (Dehydorascorbate reductase).
Tephrosia purpurea of family Leguminosae are very rich in flavonoid. It is a small annual shrub used in the treatment of asthma. Roots are useful to cure cuts and wounds. Whole plant is used in spleen enlargement and stem used as tooth brush cure many dental disorder. It cures colic pain when used with sendha salt.
Flavonoids are synthesized by the phenylpropanoid metabolic path way in which the amino acid phenylalanine is used to produce 4-coumaroyl-CoA.
Material and Method
 |
Figure 1:
|
Plant Material
The plant material (near about 2 Kg) collected from local surroundings after weighing, washed thoroughly and dried in air at room temperature for more than one month. The dried plant material was grinded to powder to about 40-60 mesh size. Then it was weighted and stored carefully in bottles for extraction and chemical testing.
Extraction and Isolation
Extraction was carried out in Soxhlet apparatus in the laboratory with 90% ethanol as solvent. The extract was concentrated in vaccum evaporator to a good yield of 6.3%.
The biologically active compound was separated from the crude extract by column chromatography using EtoAc: ccl4 solvent system as mobile phase and silica gel as stationary phase.
The compound was than isolated followed by TLC using same solvent combination for that fraction in column.
Table 1: showing the weight of plant material after drying and percentage loss in weight of plant material of Tephrosia purpuria.
| S. No. | Description | Weight in
gms. |
| 1. | Wt. Of plant material in wet fresh condition | 240 gms. |
| 2. | Wt. Of plant material after drying condition | 160 gms. |
| 3. | Loss in weight on drying | 240–160 = 80 gms. |
| 4. | Percentage loss after drying | 40% |
Table: 2 Percentage yield of Tephrosia purpuria using different solvents in Soxhlet apparatus.
| S.No. | Solvent
used |
Wt. of Of Powdered
Material |
Volume
of Solvent |
Wt. of
extract |
Character
of extract |
Percentage
of yield |
| 1. | 90% Ethanol | 160 gms. | 750 ml. | 7.20 ml. | Green in
semisolid state |
6.3% |
| 2. | Water | 160 gms. | 500 ml. | 8.00 ml. | Greenish
yellow semisolid state |
7.5% |
Table: 3 TLC of 90% alcoholic extract of the plant Tephrosia purpuria.
| S.No. | Solvent System | Ratio | Visual
light |
Iodine
Chamber |
Rf Value | Fraction
Code |
| 1. | EtoAc: CCl4
|
1:1 | Blackish
green |
Dark
Green |
0.92 | FR – 1 |
| 2. | CHCl3: MeOH
|
2:1 | Olive
green |
Dark
Green |
0.86 | FR – 2 |
Observation and Results
The tentative structure of compound was elucidated as Mf. C21H20O11.
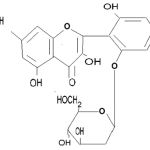 |
Formula 1:
Click here to View figure |
Molecular Formula -3, 7, 2’ ́ trihydroxy 6’ ́ – O – β – D glucopyranosyl flavone
Molecular Formula – C21H21O11
Molecular Weight – 449
M.P. – 210 – 212 0C
It was light greenish amorphous powder separated as fraction-1 from column. It was blackish green colored compound with Rf value 0.92 on TLC was followed by acid hydrolysis, methylation and glycosidal test.
IR
3524 (OH), 1650 (C = O – γ – pyrone), 1590, 1565, 1515 (C = C aromatic)
PMR
(Py) -8.35 (3H, d, J = 9.6, H – 5, 2’, 6’), 7.15 (1 H, br. s, 3 – OH), 7.44 (4H, m, aromatic protons) 10.30 (1H, br. s, 7 – OH), 9.80 (1H, br. s, 4’ – OH), 13.62 (1H, s, 3 – OH).
On analysing under different analytical procedure showed maximum peak in ultraviolet spectrum at 270 mm.
Table 4: Chemical Properties of Tephrosia purpurea
| S.No. | Properties | Percentage |
| 1.
2. 3. 4. 5. |
Ash content
Water soluble ash content Acid soluble ash content Fiber value Saponification value |
30
70 4.2 Not more than 30 68 |
Discussion
The known compound 3,7,2’ – trihydroxy 6’- O – β – D glucopyranosyl flavone was identified by CoTLC with authentic sample and structure was established by comparison of measured UV, NMR and mass spectral data with spectroscopic data available from literature. 3,7,2’ – Trihydroxy 2’- O – β – D glucopyranosyl flavone showed 1H and 13CNMR data in full agreement with those given by Malikov and Yuldashev (2002).
The chemical structure of flavanonols includes 3 – Hydroxyflavanones or Dihydroflavonols. They contain two asymmetric carbon atoms. Flavanols differed from flavanones by the presence of strong peaks in their mass spectra. It is demonstrated that flavanonols exhibit a base peak for [M –57] + ion whereas a peak for the [M – 43] + ion was typical of flavones. It should be noted that an alcoholic solution of flavanonols at room temperature can undergo cis-trans epimerization to form a new isomeric compound.
Several such compounds have been isolated from plants of different families.Genistein and Daidzein soy flavonoid appear to reduce the risk of prostrate and breast cancer, Hesperidins raises the blood levels of the good high-density lipoprotein and lowers the bad low-density lipoprotein and triglycerides. It also possesses significant anti-inflammatory and analgesic effect.
Quercetin can inhibit the growth of head and neck cancers and can stop reverse transcriptase, the method HIV uses to replicate itself. It has demonstrated significant anti-inflammatory because of direct inhibition of several initial process of inflammation. As it inhibits both the production and release of histamine and other allergic inflammatory mediators. Tangeretin induces apoptosis programmed cell death in leukemia cells but does not harm normal cells Prenylnaringenin are very potential antioxidant. Epicatechin improves blood flows and thus seems good for cardiac cells.
The flavon compound is isolated from Tephrosia Purpurea was found to be anti-microbial when tested in experimental rats. A detail study of this compound is needed to be established.
Biological activity
The flavone isolated from alcoholic extract of stems and leaves of Tephrosia purpurea showed antimicrobial activity against Staphylococcus aureus. The bacterial strain used for the present study was procured from M.T.C.C. Chandigarh.
Table 5: Minimum inhibitory concentration (MIC) value of different fraction of flavonoids compound of Tephrosia purpurea against Staphylococcus aureus MTCC 96.
| S.No. | Fraction | MIC |
| 1.
2. |
FR- 1
FR-2 |
0.72 0.34 |
For antimicrobial activity disc diffusion was followed. The agar medium was used. The plates were incubated at 37 0C over night and examined for zones of growth inhibition.
The minimum inhibitory concentration of the extract was determined for Staphylococcus aureus using the two fold serial micro dilution method with saline at a final concentration ranging from 10,000 mg / ml. to 0.0024 mg / ml. The MIC values were taken as the lowest concentration of the extracts in the wells of the microtiter plate. These plates were interpreted as visible growth of microorganisms. Thus growth of bacteria is noticed inhibited with moderate zone of inhibition.
References
- Chaudhary Rashmi (2005), have reported ant asthmatic activity in alcoholic extract of Tephrosia purpurea. PhD thesis B.U., Bhopal – 221.
- Malikov and Yuldashev (Chemistry of Natural compound vol. – 38 No. – 5)
- Guido flamini, Maria pardini, Ivano Morelli, Kuddisi Ertugrul (2002) Phytochemistry 61, pp. – 433 – 437.
- Koli M.C., R. Chaudhary, Sourabh Kumar, Deepti Shukla and R.C. Saxena (2002). An isoflavonol glycoside from the stem of Euphorbia hirta (Asian Journal of Chemistry vol. – 14 No. – 3, 1668-1673)
- Kamgang R, Vidal, Poukam Kamgne E, I. Fonkoua Penlap N Beng V, Bivole Sida M: Activities of aqueous extract of Mallotus oppositi folium on Shigella dysenteriae. An induced diarrhea in rat clin Exp Pharmocol Physiol 2006; 33; 89-94.
- Sashidharan, L. yoga, Latha Z. Zuraini, S. Suryani, S. Sangetha, L. Shirbi: Indian Journal of Pharmacology, vol. – 39, October 2007, issue 5.







