Kishu Tripathi¹ and Shobha Kulshreshtha2
¹Surya College of Pharmacy, Lucknow.
²Head,Department of Pharmacology, SN Medical College, Agra.
Abstract
Metal ions after forming complexes with an antibiotics alters the antimicrorial activity of an antibiotics alone.
Keywords
Metal ions; antibiotics
Download this article as:| Copy the following to cite this article: Tripathi K , Kulshreshtha S. Metalloantibiotics-III. Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Tripathi K , Kulshreshtha S. Metalloantibiotics-III Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=372 |
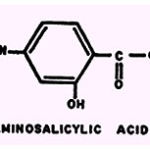
p-Aminosalicylic acid (PAS)
The antitubercular activity of such drugs as INH, PAS, and thiosemicarbazone is affected by the ability of the compounds to form complexes with Cu2+. The Cu2+ complex of PAS was later found to be as active as PAS, but the effectiveness of both substances is enhanced by excess Cub (89). the Cu2+ as well as the Fe2+, Co2+, Mn2+, and Cr2+ complexes of PAS are less tuberculostatic than the pure corm- pound Other investigators reported that Co2+ enhances (103), and that the majority of metallic ions tested suppress (17), the antitubercular activity of PAS. The toxicity of Cu2+ or Hg++ for frog heart tissue is suppressed by PAS (28) but the Fe2+ complex is more toxic towards mice than is PAS itself (39).
 |
Scheme 1
|
 |
Scheme 2
|
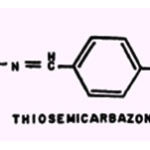
Thiosemicarbazone.
This substance is derived from a well-known metal reagent, thiosemicarbazide. The effects of metallic ions and thiosemicarbazone were found to possess greater antitubercular activity than the unchelated compounds (62). In common with INH and PAS, thiosemicarbazone suppresses the toxicity of Cu2+and Hg2+ towards isolated frog heart tissue (28).
 |
Scheme 3
|
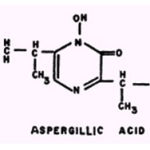
Aspergillic acid.
The antimicrobial activity of aspergillic acid is suppressed by Fe++ and enhanced by Bi3+. The potency of the compound is also enhanced by Co2+, Ni2+, Zn2+, and As3+,
 |
Scheme 4
|
but to a lesser extent than by Bi3+ (46, 47). Goth (45) has postulated that aspergillic acid is antitubercular because of its ability to chelate Fe++ which he observed to be essential for growth of mycobacteria.
 |
Scheme 5
|
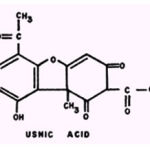
Usnic acid.
Johnson et al. (57) have suggested that usnic acid may owe its antitubercular action to inhibition of phosphorylation reactions, because antimicrobial concentrations of the compound suppress oxidative phosphorylation of rat liver and kidney homogenates.
 |
Scheme 6
|
Miscellaneous Antimicrobial Compounds
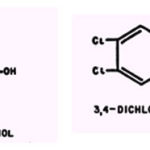
Compounds whose Antimicrobial Activity is Influenced by Metallic Cations.The inhibitory effect of penicillin and bacitracin on the growth of Micrococcus pyogeneis enhanced by Co2+,(104); and the inhibition by 2 ,3-dimercaptopropanol (British anti-Lewisite) of the binding of penicillin by whole cells is counteracted by Co2+ and Mn2+ (30). Bacitracin has been observed to be precipitated by salts of heavy metals and to be partially inactivated by British anti-Lewisite (11).Mg2+, Mn++, Ca++, and Fe,++(+) suppress the ability of polymyxin to inhibit Pseudomonas aeruginosa; a Mg2+&:drug ratio of 400:1 is required for maximum activity of Mg2+ (73).The ability of novobiocin to inhibit gram negative bacteria is suppressed by Mg2+&, and, to a lesser extent by Ca2+, Sr4+, and Ba3+ (19, 117).Co2+ slightly enhances the ability of chloramphenicol to inhibit growth of B. subtilis. An analogue of chloramphenicol, 3,4-dichlorophenylserine, is inert against strains of Enterobacteriaceae but prevents sporulation of Aspergilus niger by the irreversible binding of essential Cu2+ (108).The ability of 1-nitroso-2-naphthol to inhibit growth of gram positive bacteria is suppressed by Fe2+ and slightly enhanced by Cu2+ and Co2+;Ni2+, Zn2+, and Mn2+are inactive.
 |
Scheme 7
|
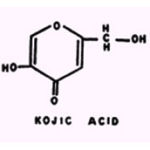
Kojic acid inhibits P. fluorescens (but not B. subtilis) more strongly in the presence of certain concentrations of Al+++,
Cu++, or Fe2+; similarly,Stove (100) noted that metal derivatives of kojic acid are betterfungicides than is the nonchelated substance.
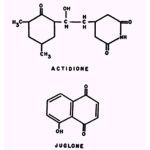
The ability of actidione to inhibit growth ofSaccharomyces cerevisiae is suppressed by oxalate;and the growth inhibitory action of juglone towards B. subtilis is strongly enhanced by Hg2+and Co2+, moderately enhanced by Al+++, Ni++, Cu2+, Zn2+, and Mn2+, and suppressed by citrate, tartrate, and oxalate.
 |
Scheme 8
|
 |
Scheme 9
|
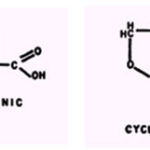
a-Picolinic acid, when tested with B. subtilis, is suppressed strongly by Co2+ or Ni2+ and moderately by Cu2+ or Zn2+. With P. fluorescens, the ions are inactive except for Cu++, which possesses slight reversing ability. However, the toxicity of high concentrations of Co2+ towards cell of each species is suppressed by a-picolinic acid (118). Previous studies demonstrated that Co2+,but not Cu2+, suppresses the antitubercular activity of a-picolinic acid hydrazide (34) and that Cu2+, Fe2+, Mn2+, and Mg2+ suppress the inhibition of metallo-enzymes of rice plants by a-picolinic and fusarinic (5-butylpicolinic) acids (102).
 |
Scheme 10
|
The ability of cycloserine (oxamycin) to inhibit growth of B. subtilis is suppressed by citrate, tartrate, oxalate, phosphate, or EDTA,whereas the antagonism of the substance towards growth of P. fluorescen is suppressed only by Cu2+(118).
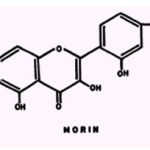
Antagonism of morin towards B. subtilis is, like that of cycloserine, suppressed by citrate, tartrate, or oxalate; the inhibition by morin of P. fluorescens is suppressed strongly by Co++, and to a lesser extent by Fe++(+), oxalate, or phosphate (118).
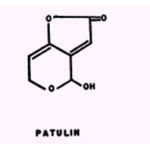
The activity of patulin is suppressed by Mn2+, Ca2+, and Ba3+, and slightly enhanced by Sb+ , Co2+, Cd2+’, Ni2+, and Zn2+.
References
- B6nicke, R., And Reif, W. 1952 Untersuchungen Tiber Die Tuberkulostatische Wirksamkeit Und Die Schwermetallkom- Plexbildung Der P-Aminosalicylsiure Und Ihrer Derivate. Beitr. Klin. Tuberk., 107, 379-386.
- Brock, T. D. 1956 Studies On The Mode Of Action Of Novobiocin. J. Bacteriol., 72, 320-323.
- Colizzi, C., And Garattini, S. 1953 The Inhibition Of The Action Of Copper By Iso-Nicotinoyl Hydrazide. Atti. Soc. Lombarda Sci. Med. E Biol., 8, 45-50.
- Erlenmeyer, H., Bxumler, J., And Roth,W. 1953 Metallkomplexe Und Tuberkulostatische Aktivitat. Helv. Chim. Acta,36, 941-949.
- Foye, W. 0. 1955 Metal Chelates And Anti- Tubercular Activity. J. Am. Pharm. Assoc., 44, 415-418.
- Goth, A. 1945 The Antibacterial Activity Of Aspergillic Acid And Its Probable Mode Of Action. J. Lab. Clin. Med., 30, 899-902.
- Goth, A. 1946 Potentiation Of The Antibiotic Activity Of Aspergillic Acid By Bis- Muth. Science, 104, 330.
- Goth, A. 1946 The Effect Of Cobalt On The Antitubercular Activity Of Aspergillic Acid. Federation Proc., 5, 180.
- Johnson, R. B., Feldott, G., And Lardy, M. A. 1950 The Mode Of Action Of The Antibiotic Usnic Acid. Arch. Biochem., 28, 317-323.
- Liebermeister, K. 1950 Zum Wirkungs- Prinzip Schwefelhaltiger Tuberkulosta- Tischer Chemotherapeutica. Z. Naturforsch. 6b 79-86
- Newton, B. A. 1953 Reversal Of The Anti- Bacterial Activity Of Polymyxin By Divalent Cations. Nature, 172, 160-161.
- Roth, W., Zuber, P., Sorkin, E., And Erlenmeyer, H. 1951 Uber Die Eigen- Shaften Einiger Metallkomplexe Der P-Amino-Salicylsdure. Helv. Chim. Acta, 34, 430-433.
- Stoves, J. L. 1954 Chemotherapy And Selective Toxicity. 3. Chelation Of Trace Metals. Mfg. Chemist, 25, 148-150.
- Tamari, K., And Kaji, J. 1954 The Mechanism Of The Growth Inhibitory Action Of Activity Of Fusarinic Acid On Plants. J. Biochem. (Japan), 41, 143-165.
- Tesi, A., And Pisani, 1). 1952 Antibiosi E Chemioterapia Della Tubercolosi Speri- Mentale. Sperimentale, 102, 298-313.
- Trace, J. C., And Edds, G. T. 1955 The Influence Of Cobalt On The Action Of Antibiotics. Am. J. Veterinarv Research, 15, 639-642.
- Volkmann, C. M., And Beerstecher, E., Jr. 1955 Microbiological Effects Of Ring- Substituted Chloro Analogues Of Phenyl- Serine. J. Bacteriol., 70, 476-480.
- Weinberg, E. D. 1957 Suppression Of The Antibacterial Activity In Vitro Of Novobiocin By Chelating Agents And By Divalent Cations. Antibiotics Annual 1956-1957, Medical Encyclopedia, Inc., New York, N. Y., In Press.
- Weinberg, E. D., Cook, E. A., And Wisner, C. A. 1956 The Mutual Effects Of Co+ And A-Picolinic Acid Hc1 On Bacterial Bacteriol. Proc., P 42-43.







