Manuscript accepted on :10-12-2024
Published online on: 27-01-2025
Plagiarism Check: Yes
Reviewed by: Dr. Chunyan Ren and Dr. Manju Jakhar
Second Review by: Dr. Pranjal Sachan
Final Approval by: Dr. Jihan Seid Hussein
Santosh Nelogi1* , Kiran Kaur2
, Kiran Kaur2 , Prashant Karni1
, Prashant Karni1 and Amit Porwal3
and Amit Porwal3
1Department of Prosthodontics, KLEVK Institute Of Dental Science, KLE Academy Of Higher Education And Research, Belgavi, Karnataka, India.
2 Malata Crescent, Success, Perth, Western Australia.
3College of Dentistry, Jazan University, Saudi Arabia.
Corresponding Author E-mail: drsantoshnelogi@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/3119
Abstract
Background: The enhancement of implant surfaces to promote better osseointegration and reduce infection risks is critical in biomedical applications. Gold nanoparticles (GNP) have shown promise in improving the biological performance of implant materials. This study explores the effects of GNP-coated titanium surfaces on the proliferation, differentiation, and mineralization of MG63 osteoblast-like cells. Methods: Titanium discs were coated with gold nanoparticles synthesized using a green, environmentally friendly method. The coated surfaces were characterized using SEM, EDAX, and SPR techniques to confirm the presence and stability of the GNP. MG63 cells were cultured on these GNP-coated titanium discs, and their growth, proliferation, and differentiation were assessed using fluorescence microscopy, Von Kossa staining, vinculin focal adhesion proteins, and alkaline phosphatase (ALP) activity measurements. The antimicrobial efficacy of the GNP-coated surfaces was also evaluated against Staphylococcus aureus and Escherichia coli. Results: Titanium surfaces coated with (GNP) at 5 ppm significantly enhanced the growth and differentiation of MG63 cells, with no evidence of acute cytotoxicity. The GNP-coated surfaces facilitated improved cell attachment, proliferation, and mineralization, indicating strong osteogenic potential. Additionally, the GNP-coated surfaces exhibited notable antimicrobial efficacy, MICrecorded at 0.3135 mg/cm³ for Staphylococcus aureus and 0.2915 mg/cm³ for Escherichia coli. Conclusion: Gold nanoparticle coatings at a concentration of 5 ppm significantly improve the osteogenic potential of titanium surfaces while also providing antimicrobial protection. These findings suggest that GNP-coated titanium implants could offer a dual benefit of enhanced osseointegration and infection prevention, making them a promising option for future clinical applications. Further in vivo studies are necessary to validate these results and understand the broader implications of GNP in biomedical implants.
Keywords
Antimicrobial nanoparticles; Gold nanoparticles; Implant surface treatment; Osteogenic activity; Osseointegration
Download this article as:| Copy the following to cite this article: Nelogi S, Kaur K, Karni P, Porwal A. Surface-functionalized Titanium Implant with Gold Nanoparticles on Osteogenic and Antimicrobial Behavior. A Novel Preliminary Research on mg 63 Cell Line. Biomed Pharmacol J 2025;18(1). |
| Copy the following to cite this URL: Nelogi S, Kaur K, Karni P, Porwal A. Surface-functionalized Titanium Implant with Gold Nanoparticles on Osteogenic and Antimicrobial Behavior. A Novel Preliminary Research on mg 63 Cell Line. Biomed Pharmacol J 2025;18(1). Available from: https://bit.ly/3Qcs6b3 |
Introduction
Implant dentistry has emerged as a widely accepted fixed prosthetic solution for replacing missing teeth, where achieving direct contact between the implant surface and bone is crucial for long-term success 1. Studies have shown that the topographical features of implant surfaces significantly influence bone cell behaviour. Consequently, various modification technologies, have been developed to enhance the rate of bone-to-implant integration 2, 3.
A major challenge in implant dentistry Peri-implantitis is a primary factor contributing to implant failure. Roughly 30.7%. of implants are affected by peri-implant mucositis, while 9.6% are impacted by peri-implantitis 4. To address this issue, bioactive antimicrobial molecules are being reintroduced into implant biomaterials to influence cell behaviour and reduce the risk of infection (5). Additionally, nanoparticle coatings on implant surfaces have shown the potential to lower implant failure rates by providing antimicrobial and osteoconductive properties 5.
GNP have garnered significant attention due to their diverse applications in drug delivery, biological imaging, and therapeutic interventions. Evidence suggests that GNP exhibit both osteogenic and antibacterial properties, making them promising candidates for enhancing implant surfaces6-25. This research assesses the osteogenic differentiation and antimicrobial effects of titanium surfaces modified with GNP, using the human osteosarcoma (MG-63) cell line as a model. The GNP in this study were synthesized using green chemistry methods, which involve plant extracts as reducing agents and stabilizers. This approach is both cost-effective and environmentally friendly, producing biocompatible nanoparticles without harmful byproducts 26-30.
The functionalization of titanium surfaces with GNP is intended to improve osseointegration, leading to faster and more predictable bone formation, enhanced implant stability, and bactericidal properties 5.
As far as we are aware, this work represents the initial exploration into the impact of GNP-functionalized implant surfaces prepared through green synthesis. Utilising botanical extracts for nanoparticle, minimizes the use of hazardous chemicals and waste, offering a sustainable alternative within green chemistry 31-37.
Materials and Methods
GNP Preparation and Analysis
Genuine Pterocarpus marsupium wood(PM) (sample NO: RMRC922 – ICMR) and Sigma-Aldrich, India gold(III) chloride were bought to synthesize (GNPs), 1 mL of an aqueous extract of PM heartwood was introduced into a 10 mL gold solution (III) chloride (0.5 mM) at 40ºC. A noticeable colour shift suggested GNP production.
The GNPs were then centrifuged at 17,000 RPM and the sample was centrifuged at a temperature of 4ºC for 20 minutes using a Kubota 6500 centrifuge.
The resultant GNP were cleaned and suspended. The GNP size, polydispersity index, and zeta potential were determined using a Malvern Instruments Zeta Seizer. Additionally, the samples were analysed using Transmission Electron Microscopy (TEM) with a Hitachi H-7500 microscope. The GNPs seemed uniform, and colloidal and had a diameter of approximately 20 nm. They showed polydispersity but did not experience any sedimentation .38
Titanium disc prep
Titanium alloy sample preparation for experimentation
Titanium alloy (Type IV Ti) was obtained from Munani Metals. Standardized discs were made that are 10 mm across and 3 mm thick. These discs were divided into two subgroups (n=20): Group A, consisting of plain titanium discs, and Group B, consisting of titanium discs treated with a 5 ppm concentration of gold nanoparticles. Every aspect of the sample and technique was assessed by an impartial statistician
Methodology for Assessing Surface Roughness
First, a steam cleaner with 0.3 MPa pressure was used to clean the representatives from every group. Next, they were cleaned with ultrasonic waves to remove any residual particles on the surface. After drying, The texture of the outer layer of all specimens was assessed both quantitatively and qualitatively. To do quantitative analysis, we utilised (Surtronic S-128 – Taylor Hobson) to assess the average roughness character (Ra) for every sample.
Green-synthesized GNP was applied layer by layer to Group B (Test). Group A (Control) and Group B (Test) The discs were subsequently analysed using scanning electron microscopy (SEM) 39.
Cell culture
The osteosarcoma cell strain (CRL-1427 – ATCC), sourced from NCCS Pune, India, was cultured in DMEM, and bought from Sigma-Aldrich Co.
The medium was enriched with essential growth factors and nutrients to promote cells with Dulbecco’s Modified Eagle Medium (DMEM) (Sigma-Aldrich Co.) with 5% CO2, 95% air, 100% relative humidity, 10% foetal bovine serum (FBS Gibco), and 2 mN glutamine.
For the experimental methods in both Group B (test) and Group A (control), a precise concentration of 1 × 10⁵ cells per well of titanium discs containing MG63 cells was meticulously planted. The injected cells were nurtured for 72 hours at a constant temperature of 37°C, with 5% CO2 and .
MTT test assay
The MTT assay was employed to assess the growth of MG63 cells on titanium surfaces in
group A and group B MG63 cells, which were cultured in three separate samples and kept in an incubator for 72 hours. Each well was filled with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) for evaluating cellular biochemical assignment with 50 μL of reagent and cultured for another four hours following the incubation time. The obtained formazan crystals were dissolved in and kept for 30 minutes. The OD was measured by using spectrophotometry at a wavelength of 492 nm40.
The ALP Assay
The ALP Assay was conducted using MG63 cells growing in triplicate. In the culture process, the activity of ALP was evaluated by introducing p-nitrophenyl phosphate. The concentration of resultant p-nitrophenol was then quantified using Bio-Tek equipment at a wavelength of 405 nm by spectrophotometry.
Antimicrobial Activity
The antimicrobial efficacy of green-synthesized GNP was assessed using the MIC method. Serial dilutions of the GNP were prepared to determine the MIC against (Staphylococcus aureus,) and (Escherichia coli) bacteria. The bacterial cultures were maintained at 10⁵-10⁶ CFU/ml and incubated. The MIC of GNP against (S. aureus) and (E. coli) was measured using a spectrophotometer at different GNP concentrations.
Evaluation of MG63 cellular proliferation
The proliferation and activity of MG63 cells on both uncoated titanium discs (GROUP A) and titanium discs functionalised with gold nanoparticles (GROUP B) were assessed using acridine orange (AO) staining. An acridine orange treatment was performed after the cells were immobilised. The stained samples were examined and analysed using a FluoView Confocal Microscope (FV1000; Olympus) to evaluate the shape and arrangement of cells on the titanium surfaces.
Von Kossa staining
Von Kossa staining was conducted on MG63 cells after they were cultured for fourteen days. The cells were initially fixed for ten minutes, followed by dehydration through a graded series of ethanol concentrations, ranging from 70% to 100%.
After dehydration, the samples were rehydrated and treated with 2% silver nitrate. They were then exposed to direct sunlight for 20 minutes to allow for the precipitation of calcium salts. Following this, the samples were treated with 5% sodium thiosulfate for 3 minutes to remove unreacted silver, and an acid fuchsine counterstain was applied for 5 minutes to enhance contrast. The samples were then dried and prepared for image analysis.
Vinculin immunofluorescence staining
Vinculin immunofluorescence staining, enhanced by GNP, is employed to investigate cell adhesion and cytoskeletal organization. Vinculin plays a critical role in cell adhesion, securing cells to the cellular scaffold To assess the influence of GNP on attachment factors., MG-63 cells were subjected to an overnight incubation. Subsequently, The cells were treated with GNP and incubated for five days.
Post-treatment, the cells were fixed, cells were fixed and then cleaned with PBS and made permeable. Following permeabilization, the cells were incubated for 30 minutes with 1% bovine serum albumin (BSA) in PBS. The cells were then exposed overnight at 4–8°C to primary antibodies against diluted vinculin. The cells were treated with fluorescently marked antibodies for 1 hour upon cleaning with PBS containing 0.05% Tween. Vinculin expression was then assessed using fluorescence microscopy.
Analysis biostatistics
An impartial statistician examined the data and provided the M ± SD. The assessment was carried out with SPSS 22.0. ANOVA was used to find significant differences in all measured indices, and the Dunnett test (for multiple comparisons) compared GROUP A and GROUP B. A p-value less than 0.001 indicates the significance of the statistic.
Results and Discussion
Due to their biocompatibility, corrosion resistance and desirable mechanical properties, titanium and its alloys have been the commonly used material for orthopaedic and dental implants over several decades5. However, these surface characteristics making titanium implants biocompatible also lead to bacterial colonisation and biofilm formation, increasing the risk of implant-associated infections. These injuries represent a significant source of infection, which most commonly ultimately leads to implant failure and costly patient procedures6. Conventional surface treatments for titanium implants emphasise passivation or re-passivation of the material to facilitate biocompatibility through the induction of a stable, adhering titanium oxide layer. Whilst this layer is inadequate in its own right to prevent bacterial adhesion and subsequent biofilm formation that could lead to a loss of long-term implant efficacy, this protective layer is used, in conjunction with other treatments that reduce reactive oxygen species and enhance fluid overload protection 7-9,37.
The surface attributes of implants can be enhanced by nanotechnology, especially the use of nanoparticles37, GNP are distinguished because of their biological compatibility. Gold nanoparticles (GNP) were synthesized using a green synthesis method, which is recognized for its simplicity, cost-effectiveness, and environmentally friendly nature as it avoids producing harmful by-products commonly associated with synthetic approaches 33. Phytochemical analysis of the plant extract indicated the successful synthesis of GNP, with characteristic peaks observed at 2.10 and 9.50, confirming the presence of pure gold.
The Surface Plasmon Resonance (SPR) observed at 538 nm signalled the formation of primarily spherical GNP. These particles exhibited a negative charge with a zeta potential of -27.8 ± 1.23 mV, which suggests good stability due to electrostatic repulsion, thereby preventing aggregation 37.
Scanning Electron Microscopy (SEM) of the uncoated titanium discs revealed the existence of several concentric ridges with smooth and randomly distributed edges (Fig-1).
 |
Figure 1: SEM image of the uncoated commercially pure titanium disc.Click here to view Figure |
In contrast, the SEM analysis of GNP-coated titanium discs displayed a uniform distribution of nanoparticle clusters across the surface, signifying successful GNP deposition (Fig 2 and 3).
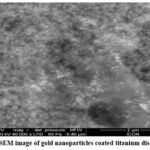 |
Figure 2: SEM image of gold nanoparticles coated titanium disc surfaces.Click here to view Figure |
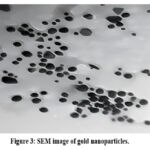 |
Figure 3: SEM image of gold nanoparticles.Click here to view Figure |
The elemental analysis further confirmed the integration of GNPs into the titanium surface (Figure 4). The GNP were spherical with an approximate diameter of 20 nm. Prior studies by C.J. Murphy have shown that spherical GNPs of this size are non-cytotoxic to human cells 41.
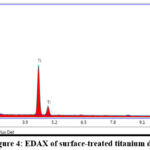 |
Figure 4: EDAX of surface-treated titanium disc.Click here to view Figure |
In this investigation, we employed GNP with a diameter of 20 nm, characterized by a spherical shape, negative surface charge, and a concentration of 5 ppm. This choice was guided by prior studies evaluating the impact of particle size, surface properties, and modifications on GNP 41, 42. The primary objectives were to examine how titanium discs coated with these GNP affect the proliferation of MG63 cells and to evaluate the antimicrobial effectiveness of the GNP.
Assessment of Gold Nanoparticle-Enhanced Titanium Discs on Bone Cell
The MG63 cell line was employed to evaluate the osteogenic activity of GNP. Using acridine orange staining, we observed that MG63 cells on GNP-coated titanium discs GROUP B (5 ppm concentration) exhibited increased attachment, proliferation, and density, alongside improved spreading and enhanced cell-to-substrate contact (Fig-5 ). Conversely, the control group GROUP A showed more spherical cells on the uncoated titanium discs (Fig-6).
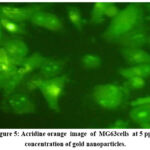 |
Figure 5 : Acridine orange image of MG63cells at 5 ppm concentration of gold nanoparticles.Click here to view Figure |
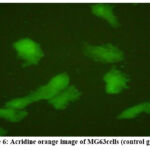 |
Figure 6 : Acridine orange image of MG63cells (control group).Click here to view Figure |
A comparative study of MG63 cell proliferation revealed a significant increase in cell viability in the GNP-treated group, reaching 104.4% at 24 hours, compared to 41% viability in the GROUP A control group (Table 1). This trend persisted, with the GROUP B GNP-treated group maintaining a viability of 107.4% after 72 hours (Table 2 and Graph Fig-7). These results highlight the osteogenic potential of GNP, enabling sustained cell viability and proliferation over extended periods. The specific cellular response to this concentration of GNP has not been widely documented, making it a notable finding in this research.
Table 1: Percentages of proliferation rates in different time periods at different concentrations
| CONTROL | 24hrs | 48hrs | 72hrs |
| 46% | 54% | 57% | |
| 5PPM GNPs | |||
| 104.4% | 109.7% | 107.4% |
Table 2: Comparison of groups at different time points ANOVA
| Time points | Groups | Mean | SD | Mean rank | H-value | P-value |
| 24hrs | Control | 41.00 | 7.00 | 2.00 | 7.2000 | 0.0270* |
| 5ppm GNPs | 100.33 | 3.72 | 8.00 | |||
| 48hrs | Control | 53.03 | 6.50 | 2.00 | 7.2000 | 0.0270* |
| 5ppm GNPs | 102.27 | 6.48 | 8.00 | |||
| 72hrs | Control | 49.03 | 12.11 | 2.00 | 7.2610 | 0.0270* |
| 5ppm GNPs | 101.63 | 5.20 | 8.00 |
ALP activity and Von Kossa staining were used to detect early markers of osteoblastic activity, bone remodelling, and turnover. After 14 days of treatment with 5 ppm GNP, Von Kossa staining confirmed the presence of mineral deposits in MG63 cells (Fig- 8,9).
 |
Figure 7: Graph showing MG 63 cell proliferation comparison at different time intervals.Click here to view Figure |
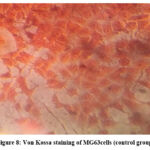 |
Figure 8: Von Kossa staining of MG63cells (control group).Click here to view Figure |
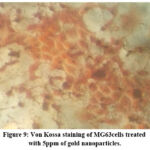 |
Figure 9: Von Kossa staining of MG63cells treated with 5ppm of gold nanoparticles.Click here to view Figure |
ALP activity in the GROUP B GNP-treated cells proliferation was higher (0.23 ± 0.005) compared to the GROUP A control group (0.22 ± 0.007), with a P-value of < 0.001 (Table 3). These results corroborate previous studies on osteoblast differentiation and mineralization, reaffirming the osteogenic potential of GNPs 31-35.
Table 3: Comparison of groups at different time points ANOVA
| Group (alkaline phosphatase) | Mean & standard division |
| Loaded 5ppm | 0.23+/-0.005 |
| Control | 0.22+/-0.005 |
Further analysis focused on the role of GNPs in altering the composition and organisation of focal attachment proteins, particularly vinculin. Vinculin is crucial for stabilizing focal adhesion complexes by binding to talin, which links integrins to actin filaments. The study demonstrated that cells treated with GNPs showed significantly enhanced vinculin expression and organisation compared to the control group, indicating that GNP may improve cell adhesion by stabilizing these complexes (Fig 10 and 11).
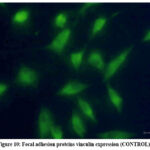 |
Figure 10: Focal adhesion proteins vinculin expression (CONTROL).Click here to view Figure |
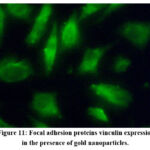 |
Figure 11: Focal adhesion proteins vinculin expression in the presence of gold nanoparticles.Click here to view Figure |
This enhancement in focal adhesion is closely linked to cellular differentiation, which can contribute to improved implant stability and integration. The results suggest that GNP not only elevate vinculin levels but also alters its arrangement, potentially affecting focal adhesion dynamics in MG-63 cells 43-46.
Minimum Inhibitory Concentration (MIC)
This research explored the antimicrobial efficacy of GNP-coated titanium discs against Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria while also examining their impact on osteoblast proliferation, differentiation, and mineralization. MIC testing demonstrated that 20 nm GNP possess significant antimicrobial properties, with MIC values determined at 0.3135 mg/cm³ for S. aureus and 0.2915 mg/cm³ for E. coli. These findings are consistent with previous research that has highlighted the bactericidal nature of GNP 17-19. Furthermore, the study supports the osteogenic potential of GNP, as documented in earlier research 26-35. This investigation confirms the biological compatibility, bone formation potential, and antibacterial capabilities of GNP-coated titanium discs.
Study Constraints
The data could provide for further alternative hypotheses and interpretations of the research. Here are some potential areas to consider.
Particle size influence: The study was carried out with 20 nm GNPs, but other particle sizes may result in other observations. The effect of these small or large particles on cell behaviour and antimicrobial properties may be distinct.
Potential systemic effects: The study is limited to local effects, but as GNPs can enter the bloodstream, systemic effects must also be considered. It could be a potential interaction with the immune system or getting stuck in other organs.
Investigate alternative coating methods:
A layer-by-layer specific method to coat titanium discs with GNPs was used in the current study for further research on how the coating might affect the distribution, stability, and efficacy of the GNP layer.
Address long-term stability and effectiveness
Consider hypothesizing about the long-term stability of the GNP coating and its potential impact on implant performance over extended periods.
Addressing these alternative hypotheses and adequate interpretations would constitute a more complete analysis as the implant tissue interface is complex and the implant success is multi-factorial. This approach would also be useful for the identification of possible areas for future research and to highlight several complexities associated with the development of improved implant surfaces.
This study’s findings are encouraging, yet it has constraints. The antimicrobial tests were conducted with GNP in suspension, which may not entirely reflect the behaviour of GNP when adhered to a surface. Future research should include antimicrobial testing on both coated and uncoated titanium samples. Additionally, more in-depth studies are required to explore the in vivo osteogenic differentiation process and evaluate the long-term biocompatibility and effectiveness of GNP-coated implants.
Clinical Implications of the Study
Microbial contamination and infection pose significant challenges in implantology, often leading to implant failure. The localized antimicrobial properties of GNP, combined with their ability to enhance osteogenesis, make them a promising candidate for surface modification of titanium implants. By reducing infection risks and promoting better integration with bone tissue, GNP-coated implants could lead to improved clinical outcomes and longevity. This study offers useful information that may be used to enhance the safety and more effective implant materials, with GNP playing a crucial role in advancing biomedical implant technology.
Conclusion
Through this study, we show that coating titanium implants with gold nanoparticle (GNP) enables significant improvement to both osteogenic activity and antimicrobial effect. We also generated 20 nm spherical nGNPs through a green process that resulted in strong surface properties that favor the MG63 cell attachment, proliferation and differentiation. It observed these effects along with preferred focal adhesion dynamics such as increased vinculin expression and organization. Moreover, the antibacterial activity of the GNP coated titanium discs was effective against S. aureus and E. coli and could be used as a strong MIC value (indicating strong inhibition of microbial growth).
These findings highlight the potential of GNP coatings as a dual-functionality surface treatment for titanium implants, addressing two critical challenges in implantology: Osteointegration enhancement and infection prevention. The capacity of GNPs to either induce localized antimicrobial effects and promote bone cell growth simultaneously suggests that future development of such composite GNPs due to a more stable and long lasting implant has benefits to the reduction of implant failure and thereby improved patient outcomes.
Inaive future research should focus on in vivo validation of these results, in particular soon to assess whether GNPs coated implants are biocompatible for long term use, osteogenic differentiating and antimicrobial properties. In general, the integration of GNPs into titanium surface yields a promising advance in biomedical implant technology, increasing the safety, efficacy and clinical longevity of titanium base implants.
Acknowledgement
The author wants to thank KAHERS, Basic Research Lab, Belgaum.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
- Santosh Nelogi made substantial contributions to conception and design of the study, drafting the manuscript and revising it critically and have given final approval of the version to be published.
- Kiran Kaur have been involved in substantial contributions data collection and data analysis
- Prashant Kani have been involved in data collection and data analysis
- Dr Amit Porwal have been involved in data collection and data analysis
- Arvind Halgekar have been involved in data collection and data analysis
- Varky Santosh involved in data interpretation
References
- Tengvall P, Lundström. Physico-chemical considerations of titanium as a biomaterial. Clinical materials 1992;9:115-34.
CrossRef - Stanford CM, Surface modifications of dental implants. Australian Dental Journal 2008;53: S26–S33.
CrossRef - Parekh RB, Shetty O, Tabassum R. Surface modifications of endosseous dental implants.Int J Oral ImplantolClin Res 2012 ;3: 116-21.
CrossRef - Atieh MA, Alsabeeha NH, FaggionJr CM, Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis.Journal of periodontology 2013: 1586-98.
CrossRef - Tiwari PM. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 2011; 6: 14: 31-63.
CrossRef - Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. ChemSoc Rev. 2009; 38: 1759-82.
CrossRef - Khlebtsov N, Bogatyrev V, Dykman L, Khlebtsov B, Staroverov S, Shirokov A. Analytical and Theranostic Applications of Gold Nanoparticles and Multifunctional Nanocomposites. Theranostics.2013; 3: 167-80
CrossRef - Niu, Chenguang; Yuan, Keyong; Ma, Rui; Gao, Li; Jiang, Wenxin. Gold nanoparticles promote osteogenic differentiation of human periodontal ligament stem cells via the p38 MAPK signalling pathway. Molecular Medicine Reports; Athens Vol. 16, Iss.4, (2017).
CrossRef - Kawazoe, N., & Chen, G. (2015). Gold nanoparticles with different charge and moiety induce differential cell response on mesenchymal stem cell osteogenesis. Biomaterials, 54, 226-236.
CrossRef - Yi C, Liu D, Fong CC, Zhang J, Yang M. Gold Nanoparticles Promote Osteogenic Differentiation of Mesenchymal Stem Cells through p38 MAPK Pathway. ACS Nano. 2010; 4: 6439-48.
CrossRef - Choi SY, Song MS, Ryu PD, Lam AT, Joo SW, Lee SY. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the Wnt/beta-catenin signalling pathway. Int J Nanomedicine. 2015; 10: 4383-92.
CrossRef - Zhang D, Liu D, Zhang J, Fong C, Yang M. Gold nanoparticles stimulate differentiation and mineralization of primary osteoblasts through the ERK/MAPK signalling pathway. Mater SciEng C Mater Biol Appl. 2014; 42: 70-7.
CrossRef - Yao Y, Shi X, Chen F. The Effect of Gold Nanoparticles on the Proliferation and Differentiation of Murine Osteoblast: A Study of MC3T3-E1 Cells In Vitro. J NanosciNanotechnol.2014; 14: 4851-7.
CrossRef - Heo DN, Ko WK, Bae MS, Lee JB, Lee D-W, Byun W . Enhanced bone regeneration with a gold nanoparticle-hydrogel complex. J Mater Chem B Mater Biol Med. 2014; 2: 1584-93.
CrossRef - Heo DN, Ko WK, Lee HR, Lee SJ, Lee D, Um SH . Titanium dental implants surface-immobilized with gold nanoparticles as osteoinductive agents for rapid osseointegration. J Colloid Interface Sci. 2016; 469: 129-37.
CrossRef - Zabirnyk, O., Yezhelyev, M., & Seleverstov, O. (2007). Nanoparticles as a novel class of autophagy activators. Autophagy, 3(3), 278-281.
CrossRef - Cui Y, Zhao Y, Tian Y, Zhang Y, Lü X, Jiang X.The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli 2012.
CrossRef - Allarkar, R, Vargas-Reus, M and Ren, G. Nanometales as antimicrobials. Antimicrobial polymers 2011:327-350.
CrossRef - T.S, Zang, Y And Yu, H. Antibacterial activity and cytotoxicity of gold (i) and (iii) ions and gold nano particles. Biochemistry and pharmacology: open access 4(2015).
- Heo DN, Ko WK, Lee HR, Lee SJ, Lee D, Um SH, Lee JH, Woo YH, Zhang LG, Lee DW, Kwon K. Titanium dental implant surface-immobilized with gold nanoparticles as osteoinductive agents for rapid osseointegration. Journal of colloid and interface science 2016 ; 5: 1;469:129-37.
CrossRef - Cervino, G., Fiorillo, L., Iannello, G., Santonocito, D., Risitano, G., & Cicciù, M. (2019). Sandblasted and acid etched titanium dental implant surfaces systematic review and confocal microscopy evaluation. Materials, 12(11), 1763.
CrossRef - Vial S, Reis RL, Oliveira JM. Recent advances using gold nanoparticles as a promising multimodal tool for tissue engineering and regenerative medicine. CurrOpin Solid State Mater Sci. 2017;21:92–112.
CrossRef - Vieira S, Vial S, Reis RL, Oliveira JM. Nanoparticles for bone tissue engineering.BiotechnolProg. 2017;33:590–611.
CrossRef - Liang H, Xu X, Feng X, Ma L, Deng X, Wu S, Liu X, Yang C. Gold nanoparticles-loaded hydroxyapatite composites guide osteogenic differentiation of human mesenchymal stem cells through Wnt/β-catenin signalling pathway. Int J Nanomedicine. 2019;14:6151-6163.
CrossRef - Li, H., Pan, S., Xia, P., Chang, Y., Fu, C., Kong, W., … & Qi, Z. (2020). Advances in the application of gold nanoparticles in bone tissue engineering. Journal of biological engineering, 14, 1-15.
CrossRef - Handayani, C. Imawan, and S. Purbaningsih. Potensiek strakbeberapajenistumbuhansebagaiagenpered uksiuntuk bio sintesis nanopartikel perak. Seminar NasionalBiologi 2010; 558–567.
- Tarantola M, Pietuch A, Schneider D, Rother J, Sunnick E, Rosman C, Pierrat S, Sönnichsen C, Wegener J, Janshoff A. Toxicity of gold-nanoparticles: synergistic effects of shape and surface functionalization on micro mobility of epithelial cells. Nanotoxicology 2011;5:254-68.
CrossRef - Alkilany AM, Nagaria PK, Hexel CR, Shaw TJ, Murphy CJ, Wyatt MD. Cellular uptake and cytotoxicity of gold nanorods: molecular origin of cytotoxicity and surface effects. Small 2009;5: 701-8.
CrossRef - Male KB, Lachance B, Hrapovic S, Sunahara G, Luong JH. Assessment of cytotoxicity of quantum dots and gold nanoparticles using cell-based impedance spectroscopy. Analytical chemistry 2008;80:5487-93.
CrossRef - Comenge J, Sotelo C, Romero F, Gallego O, Barnadas A, Parada TG, Domínguez F, Puntes VF. Detoxifying antitumoral drugs via nanoconjugation: the case of gold nanoparticles and cisplatinPloS one.2012 ;7 : e47562.
CrossRef - Cho EC, Xie J, Wurm PA, Xia Y. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a 2/KI etchant. Nano letters 2009;9:1080-4.
CrossRef - Justynasiemieniec. Synthesis of silver and gold nanoparticles using methods of green chemistry Chemik 2013; 6710: 842–847.
- P. Mohanpuria, N. K. Rana, and S. K. Yadav, Biosynthesis of nanoparticles: technological concepts and future applications, Journal of Nanoparticle Research 2008;10:507–517.
CrossRef - A. Rajan, A. R. Rajan, and D. Philip, Elettariacardamomum seed-mediated rapid synthesis of gold nanoparticles and its biological activities. OpenNano 2017; 2: 1–8.
CrossRef - P. Singh, Y.-J. Kim, D. Zhang, and D.-C. Yang, “Biological synthesis of nanoparticles from plants and microorganisms,” Trends in Biotechnology 2016;34: 588–599.
CrossRef - P. D. Shankar, S. Shobana, I. Karuppusamy A review on the biosynthesis of metallic nanoparticles (gold and silver) using bio-components of microalgae: Formation mechanism and applications. Enzyme and Microbial Technology 2016; 95: 28–44.
CrossRef - S. Yallappa, J. Manjanna, and B. L. Dhananjaya, Photosynthesis of stable Au, Ag and Au-Ag alloy nanoparticles using J. Sambac leaves extract and their enhanced antimicrobial activity in presence of organic antimicrobials, SpectrochimicaActa Part A: Molecular and Biomolecular Spectroscopy 2015;137: 236–243.
CrossRef - Jadhav K, Rajeshwari HR, Deshpande S, Jagwani S, Dhamecha D, Jalalpure S, Subburayan K, Baheti D. Photosynthesis of gold nanoparticles: Characterization, biocompatibility, and evaluation of its osteoinductive potential for application in implant dentistry. Materials Science and Engineering: C. 2018 ;12:1;93:664-70.
CrossRef - Richert, L., Lavalle, P., Payan, E., Shu, X. Z., Prestwich, G. D., Stoltz, J. F., … & Picart, C. (2004). Layer by layer buildup of polysaccharide films: physical chemistry and cellular adhesion aspects. Langmuir, 20(2), 448-458.
CrossRef - Im GI, Qureshi SA, Kenney J, Rubash HE, Shanbhag AS. Osteoblast proliferation and maturation by bisphosphonates. Biomaterials. 2004 Aug 1;25(18):4105-15.
CrossRef - Murphy, C. J., Gole, A. M., Stone, J. W., Sisco, P. N., Alkilany, A. M., Goldsmith, E. C., & Baxter, S. C. (2008). Gold nanoparticles in biology: beyond toxicity to cellular imaging. Accounts of chemical research, 41(12), 1721-1730.
CrossRef - Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjugate chemistry 2004;15:897-900.
CrossRef - K. Das, S. Bose and A. Bandyopadhyay, J. Biomed. Mater. Res., Part A, 2009, 90, 225–237
CrossRef - H. Zhang, L. F. Cooper, X. N. Zhang, Y. Zhang, F. Deng, J. L. Song and S. Yang, RSC Adv., 2016, 6, 44062–4406
CrossRef - S. Di Cio and J. E. Gautrot, Acta Biomater., 2016, 30, 26–48 .
CrossRef - J. R. Liu, Y. L. Wang, W. I. Goh, H. Goh, M. A. Baird, S. Ruehland, S. Teo, N. Bate, D. R. Critchley, M. W. Davidson and P. Kanchanawong, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, E4864–E4873
Abbreviations
GNP: gold nanoparticles.
PM: Pterocarpus marsupium wood
S aureus : Staphylococcus aureus.
Ecoli :bacterium Escherichia.
MIC: minimum inhibitory concentration.
ALP assay: alkaline phosphatase activity.
G+v: Gram-positive bacteria.
G-v: Gram-negative bacteria.
PEG: polyethene glycol.
PBS: phosphate-buffered saline







