Manuscript accepted on :11-02-2025
Published online on: 06-03-2025
Plagiarism Check: Yes
Reviewed by: Dr. Rajat Goyal
Second Review by: Dr. Miss Ananya Naha
Final Approval by: Dr. Gul Ozcan
Kamal Upreti1* , Jossy George1
, Jossy George1 , Shitiz Upreti2
, Shitiz Upreti2 and Shubham Mahajan1
and Shubham Mahajan1
1Department of Computer Science, CHRIST (Deemed to be University), Delhi NCR, Ghaziabad, Uttar Pradesh, India.
2Department of IT & Management, Maharishi Markandeshwar (Deemed to be University), Mullana-Ambala, Haryana, India.
Corresponding Author E-mail: kamalupreti1989@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/3093
Abstract
PCOS is an endocrine illness that affects 6–10% of women worldwide. It can cause a variety of symptoms, including irregular menstruation periods, ovarian cysts, and hyperandrogenism. Its lack of defined biomarkers, overlapping symptoms, and heterogeneity make diagnosis difficult. By studying hormone profiles, identifying patterns difficult to see with conventional approaches, and offering great precision and accuracy, AI and ML techniques are transforming diagnostic difficulties. Hybrid models in the list include SWISS-AdaBoost and ensemble learning techniques that have accuracies up to 98% enabling early diagnosis along with appropriate treatment strategies. Early detection by technologies such as AI will prevent significant health complications that are PCOS-related, such as infertility, type II diabetes, or cardiovascular diseases. This study depicts the transformative role of the application of AI in diagnosing cases of PCOS and highlights the possibility of facilitating clinical decision-making, reducing potential diagnostic delays, and enhancing improvements in patient outcomes. Future research should hence be directed towards the establishment of AI within healthcare with consideration of validation, reliability, and ethical considerations to maximize its use in clinical practice.
Keywords
Artificial Intelligence; Endocrine disorders; Hyperandrogenism; Infertility; Polycystic ovaries; Rotterdam Criteria
Download this article as:| Copy the following to cite this article: Upreti K, George J, Upreti S, Mahajan S. Polycystic Ovary Syndrome Diagnosis: The Promise of Artificial Intelligence for Improved Clinical Accuracy. Biomed Pharmacol J 2025;18(1). |
| Copy the following to cite this URL: Upreti K, George J, Upreti S, Mahajan S. Polycystic Ovary Syndrome Diagnosis: The Promise of Artificial Intelligence for Improved Clinical Accuracy. Biomed Pharmacol J 2025;18(1). Available from: https://bit.ly/4iit7Ky |
Introduction
There are about 7 to 15% of female reproductive-age women who suffer from polycystic ovaries during their reproductive years on average according to Stein and Leventhal in 1935.1 PCOS, oligomenorrhea, and amenorrhea are not the only symptoms that women suffering from those conditions experience.2,3 The process of ovulation and the associated occurrence of ovarian cysts can also lead to hormonal imbalances, ovulation problems, and anovulations, which are also possible side effects. Hyperandrogenism is an illness that is associated with a number of symptoms including menstrual cycle irregularities, ovarian cysts, irregular periods, irregular periods, and irregular periods. The symptoms of this condition will be extremely diverse, so you might have to spend a lot of time looking for any symptoms that might be specific to your case in order to identify any severity. PCOS, in accordance with the World Health Organization, is estimated to affect around 116 million women all over the world.4 Those with this disorder have elevated levels of LH (luteinizing hormone), FSH (follicle stimulating hormone), and GHRH (gonadotropin-releasing hormone) as a result of a disproportionate ratio between these hormones.5,6 The combination of genetic predispositions and environmental factors can cause and exacerbate PCOS.7 Aside from obesity, type II diabetes, infertility, cardiovascular disease, mental health concerns, osteoporosis, and the development of comorbid conditions, the development of obesity, type II diabetes, infertility, cardiovascular disease, mental health concerns, osteoporosis, and the development of other comorbid conditions can all lead to complications that may worsen as they are left untreated.8 It can be possible to lose 5 percent of body weight by increasing physical activity and altering diet.9 Some patients who have not responded to conventional medicine may benefit from complementary and alternative medicine (CAM). This is often due to personal beliefs and monetary concerns.10 A multidisciplinary approach is crucial to managing PCOS effectively since it can have a variety of effects on women and have a potential severity.11 Fig. 1 shows the illustration about Polycystic Ovary Syndrome.
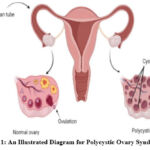 |
Figure 1: An Illustrated Diagram for Polycystic Ovary Syndrome11 |
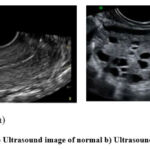 |
Figure 2: a) Ultrasound image of normal b) Ultrasound image of PCOS42 |
Fig. 2 is a real diagram of ultrasounds of two ovaries Fig. 2 a) shows the ultrasound image of a normal ovary whereas Fig. 2 b) shows the ultrasound image of a polycystic ovary. PCOS is diagnosed using the NIH (National Institute of Health’s) guidelines, a disorder marked by alterations in hormones during puberty.12 Among them include polycystic ovarian morphology, anovulation, elevated testosterone levels, insulin resistance, and irregular menstrual cycles. With the addition of ovaries with polycystic disorders as a diagnosis criterion, the Rotterdam criteria—which defined PCOS, also known as oligo/anovulation, elevated testosterone levels, and polycystic ovaries—improving the diagnosis even more in 2012.
Current Challenges in PCOS Diagnosis
Diagnosing PCOS is a challenging task for many reasons as shown in Fig.3. Different diagnostic criteria among guidelines, such as Rotterdam and NIH criteria, are variable. Overlapping symptoms with other conditions such as hypothyroidism and metabolic syndrome make the correct identification of the disease a very challenging task. Also, subjective assessments such as ultrasound imaging and clinical evaluation of hyperandrogenism have variability and bias. The lack of reliable biomarkers for early detection limits objective diagnostic tools. Moreover, variability in demographics and ethnicity imparts a different presentation of symptoms due to PCOS. And finally, psychosocial factors such as stigma and mental health issues coupled with low awareness often delay the diagnosis and treatment and heighten the impact of PCOS on patients. For these challenges, standardizing diagnostic approaches and innovative technologies, such as AI, should bring about accuracy and consistency.
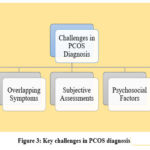 |
Figure 3: Key challenges in PCOS diagnosis |
While such traditional diagnostic criteria of NIH guidelines and the Rotterdam criteria have served as guidelines to help with the diagnosis of PCOS,12 the new innovations, such as technological advancements from AI (Artificial Intelligence) and ML (Machine Learning), open up the possibilities for increasing the accuracy in the early diagnosis. Such technological systems process vast amounts of medical data – medical, hormonal profiles, images from ultrasound, and clinical histories – to spot certain patterns that perhaps may not be so easily discerned through traditional methods. AI/ML models can be able to offer improving clinical decision-making while reducing diagnostic delays, potentially offering a more personalized therapy to a PCOS patient.
Pathophysiology and Symptoms of Pcos
Hyperandrogenism
A prevalent disorder in young girls and adolescents is hyperandrogenism, which is frequently brought on by physiological pubertal variance (see Fig.4). The most prevalent type is called polycystic ovarian syndrome (PCOS), which is frequently mislabeled. To prevent pathogenic causes and needless work-ups, systematic examination is essential. Strict guidelines, screening tests, lifestyle modifications, and drugs like glucose & estrogen-progesterone preparations are all part of PCOS treatment.13 The presence of luteinized ca cells in the ovarian stroma causes hyperthecosis, a disease that results in a continuous release of testosterone. This disorder is a severe variant of Polycystic Ovary Syndrome (PCOS) that frequently manifests as hyperandrogenism.14 The course of treatment includes metformin, Gonadotropin agonists, anti-androgen medication, or laparoscopic bilateral salpingo-oophorectomy. Surgical might prove necessary when hormone levels in the blood are high, however treatment usually results in a decrease in ovarian androgen output. The anti-androgenic effects of glucophage (MET) as well as quercetin (Q) on hormonal imbalances and hyperandrogenism in a human version of Pc caused by DHEA were compared. The findings demonstrated that for Polycystic rats, both treatments decreased body weight, serum free testosterone, luteinizing hormone, and the FSH ratio. In addition, MET and Q raised the E2/free T, which are number, lunar converter quantity, overall ethanol levels. The study found that Q is just as good as MET at lowering hyperandrogenism and enhancing the function of the hypothalamic-pituitary-ovarian axis.15
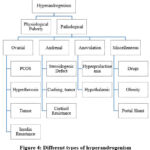 |
Figure 4: Different types of hyperandrogenism |
Menstrual Irregularities
Menstrual abnormalities following the first and second doses of the COVID-19 vaccination were investigated in this study. According to the study, 50–60% of women who were the adulthood had abnormalities, irrespective of the type of vaccine. The subsequent dosage resulted in a somewhat higher incidence. In half of the cases, the abnormalities resolved on their own in less than two months. According to the study,16 these characteristics should be taken into account when counseling women who will be receiving the vaccine. Periods lasting less than 21 days or more than 35 days are considered irregular menstrual cycles. These irregular cycles can upset the body’s hormonal balance or result in a number of health problems, including infertility, heart disease, type 2 diabetes, rheumatoid arthritis (RA), metabolic syndrome, anemia, osteoporosis, or psychological disorders.17 Pregnancy-related hypertension problems and unfavorable obstetric and newborn outcomes are also made more likely by these irregular periods. It makes sense to comprehend these elements while creating treatment and prevention plans. The study looked at how Forty female adolescent endurance athletes’ cycle of periods while weight of bones changed through bulimia.18 Runners who reported pathogenic conduct within their last month or who had a global score of ≥4.0 or who were concerned about their weight or shape were categorized as having disordered eating. According to the findings, runners who suffer from disordered eating have the majority had irregular menstruation, a longer span experiencing ovulation loss as few period cycles annually. The study backs the links between disordered eating and decreased cervical density possibly future irregular menstruation.
Polycystic Ovary Morphology
PCOS, or polycystic ovarian syndrome, is a metabolic, psychosocial, and reproductive disorder that strikes 5–18% of women. Genetic predisposition, menstrual or hypothalamic dysfunction, excessive androgen exposure, insulin resistance, and systems linked to obesity are the causes. The 2003 Rotterdam criteria, which include cystic ovarian morphology, irregular cycles, and hyperandrogenism, are used to make the diagnosis.19 Changes in lifestyle and medication, such as metformin, oral contraceptives, and anti-androgens, are part of the treatment. PCOS, a prevalent endocrine condition in women, is associated with hazards for cardiovascular disease, including pre-eclampsia, diabetes, and obesity. According to the 2023 International Evidence-Based PCOS Guideline, PCOS is a risk factor for cardiovascular disease.19 PCOS is a common endocrinopathy that affects women starting around puberty. It causes hirsutism, infertility, anovulatory menstrual periods, obesity, diabetes, hypertension, lipid abnormalities, and metabolic syndrome. It is a diverse illness that manifests differently in women & teenagers of adulthood.20
Metabolic Disturbances
Overweight, obesity, type 2 diabetes, and steatotic liver disease are metabolic illnesses that are becoming more common worldwide. They are leading to a considerable increase in morbidity and mortality. While the gut microbiota is essential for preserving homeostasis regulation, genetic and environmental factors also play a part in these illnesses. Obesity, inflammation, & the generation of electricity are some of the disease mechanisms. Uncertainty surrounds the gut microbiota’s function and potential therapeutic benefits.21 Within a person’s brain, microglia are resident immune cells that are activated by cerebral ischemia/reperfusion injury. These cells can both exacerbate and protect against disease processes. Immune cell metabolism in inflammatory diseases, such as cerebral infarction, has emerged as a key area of research interest. The many activities of microglia, metabolic changes, mitochondrial modifications, reactive oxygen species participation, and therapeutic approaches to reduce aggravating metabolic abnormalities are all covered.22
Psychological Consequences
Reactions such as ecological sadness, eco-guilt, and eco-anxiety are brought on by climate change. In order to evaluate these psychological effects and their connection to pro-environmental behavior (PEB), a study created questionnaires. The one-factor Eco-Guilt and Ecological Grief questionnaires showed a favorable correlation with PEB. The surveys are appropriate for evaluating negative emotional states associated with environmental issues or climate change.23 One of the study examined the effects of bullying victimization on psychological symptoms, suicidality, and self-harm in teenagers, with a particular emphasis on gender disparities. According to data from a cross-sectional study conducted in China, such behaviors were substantially correlated to multiple kinds of bullying. Domestic abuse was prevalent between females, but cyber is less prevalent. The results emphasize the necessity of focused intervention techniques to deal with bullying victimization.24 The survey, which was carried across the following two months of the conflict in Ukraine, revealed a sharp deterioration in the mental and physical well-being of the civilian populace, which was made worse by losses in housing, work, and money. This underscored the necessity of psychiatric assistance.25
Polycystic Ovary Syndrome (PCOS) is the most common endocrine disorder, affecting 6%-10% of women worldwide, although the difference in prevalence is largely due to the diagnostic parameters used.26 PCOS affects females throughout their lifetime, from adolescence to post-menopause, significantly reducing their quality of life due to its complex nature and wide-ranging health impacts. It contributes substantially to both morbidity and mortality, particularly among women of reproductive age. The review mainly aims at providing a full overview of the health effects of PCOS, beginning in reproductive years and through subsequent life stages.27 Fig. 5, below, illustrates the clinical manifestations of PCOS in different stages of a woman’s life. In this regard, all the symptoms and manifestations depend on age, hormonal status, and metabolic conditions within an individual.
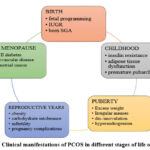 |
Figure 5: Clinical manifestations of PCOS in different stages of life of females |
These four major phenotypes can be captured according to the Rotterdam criteria: the presentation of the condition. The metabolic and reproductive consequences of these phenotypes vary.28,29 According to the Rotterdam Consensus Workshop, PCOS is classified as an ovarian dysfunction syndrome based on two cardinal features: hyperandrogenism and polycystic ovarian morphology (PCOM)15, dated 2003. It expanded the definition of PCOS and made it one of the most common endocrine disorders in women of reproductive age, with a global prevalence between 6% and 8% according to the NIH 1990 criteria.15 Fig. 6 depicts the four distinct phenotypes of PCOS.
 |
Figure 6: Four different phenotypes of polycystic ovarian syndrome (PCOS) |
The symptoms of PCOS are often very diverse and particularly challenging to diagnose, especially in adolescents and women at different ages in their lives. Because the symptoms of PCOS overlap with those of most other endocrine disorders, more than some basic diagnostic tests are necessary to differentiate it from hyperprolactinemia, thyroid disease, Cushing’s syndrome, and adrenal hyperplasia. Most investigations do include a mix of pelvic examination, ultrasound imaging, and hormone level analyses that include androgens as well as luteinizing hormone (LH).35,31 Fig. 7 summarizes the evolution of the diagnostic criteria for PCOS. In the 1990s, the NICHD established criteria that helped to rule out other causes of endocrinopathies and sources of hyperandrogenism for PCOS diagnoses.32 In 2003, the Rotterdam Criteria elaborated more on these and emphasized that at least two of the following three features must be present for diagnosis: polycystic ovaries, clinical or biochemical hyperandrogenism, and oligo /anovulation.33 The latter further criteria reflect the clinical variability of PCOS, allowing for several presentations that range widely from irregular periods, elevated androgens to polycystic ovaries that are identifiable upon ultrasound imaging.
PCOS Diagnosis in Adolescents and Menopausal Women
PCOS diagnosis becomes even more challenging in adolescence and during the menopausal transition. Actually, during the whole spectrum of puberty, adolescents may experience irregular menstrual cycles, which will sometimes make distinguishing between normal pubertal changes and PCOS difficult.33 According to the ESHRE/ASRM guidelines, PCOS can only be diagnosed in adolescents who have experienced persistent menstrual abnormalities for more than two years following menarche, in conjunction with elevated androgen levels or clinical signs of hyperandrogenism like acne and hirsutism.33 It is even more difficult to make a diagnosis of PCOS in postmenopausal women since many of the natural changes that occur during menopause may mimic PCOS symptoms, such as excess androgen and complicate the clinical picture. Therefore, an adaptive and dynamic diagnostic approach is the need of the hour for women of all ages.33
Materials and Methods
Systematic Review Process
This study aims at giving a comprehensive review of the literature covering the integration of Artificial Intelligence and Machine Learning techniques for the early and accurate detection of Polycystic Ovary Syndrome. This approach will result in a very stringent selection of relevant studies while using a systematic methodology and strictly framing an emphasis on AI-based techniques and their efficacy in the detection of PCOS as depicted in Fig. 7. The overall process follows a procedure of careful systematic literature search, selection criteria, data extraction, and synthesis of the findings to identify the current advancement and gaps in the area of research.
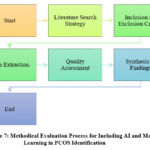 |
Figure 7: Methodical Evaluation Process for Including AI and Machine Learning in PCOS Identification |
Inclusion and Exclusion Criteria
In order to involve high-quality and relevant research, the review used a focus on articles in journals that were published in a peer-reviewed journal during 2016 to 2024, emphasizing the technique of AI/ML regarding the detection of PCOS. The eligible studies used a performance metric, which had accuracy, precision, recall, and F1-score while using clinical, biochemical, or imaging data. Also, the inclusion criterion rejected non-English-language research, studies that were found without performance metrics, review articles, editorials, and research not dealing with the diagnosis of PCOS.
Database Search Strategy
Databases like PubMed, IEEE Xplore, ScienceDirect, and Google Scholar were used to run a literature search as depicted in Table 1. Key terms such as “Polycystic Ovary Syndrome,” “PCOS,” “Artificial Intelligence,” “Machine Learning,” “AI-based diagnosis,” and “early detection of PCOS” were used; the Boolean operators AND, OR were applied for refining search results. Filters for dates, languages, and type of document ensured that pertinent studies were retrieved. These were performed using the major databases, with a publication date range between 2016 to 2024, peer-reviewed, and having performance metrics reported for the applications of AI/ML for PCOS. AI/ML techniques, characteristics of dataset characteristics and performance metrics were abstracted from all individual studies.
Table 1: Database Search Strategy for PCOS Detection
| Database | Search Query | Boolean Operators | Filters Applied | Results Retrieved |
| PubMed | (“Polycystic Ovary Syndrome” OR “PCOS”) AND (“Artificial Intelligence” OR “Machine Learning”) | AND, OR | Publication date: 2016–2024, English, Peer-reviewed | 527 |
| IEEE Xplore | (“AI-based diagnosis” OR “Machine Learning”) AND “Polycystic Ovary Syndrome” | AND, OR | Publication date: 2016–2024, English, Peer-reviewed | 336 |
| ScienceDirect | (“Early detection of PCOS” OR “Artificial Intelligence”) AND (“PCOS”) | AND, OR | Publication date: 2016–2024, English, Peer-reviewed | 488 |
| Google Scholar | (“PCOS” OR “Polycystic Ovary Syndrome”) AND (“AI-based diagnosis” OR “Machine Learning”) | AND, OR | Publication date: 2016–2024, English, Peer-reviewed | 1,286 |
Data Extraction and Evaluation Frameworks
Relevant information was systematically extracted from the chosen studies in terms of the study design, objectives, and the AI/ML techniques or algorithms applied. Main details such as dataset characteristics- size, source, and demographic diversity were noted with performance metrics such as accuracy, precision, recall, F1-score, and AUC. In addition, the main findings and contributions of the studies were documented for a complete understanding of their implications. A modified Newcastle-Ottawa Scale was used in order to evaluate the quality of the studies and the risks of bias. Comparative analyses were carried out to compare different datasets performance in models based on AI/ML. Where appropriate, the explainability of AI models was tested with frameworks such as SHAP (SHapley Additive exPlanations) and LIME (Local Interpretable Model-agnostic Explanations), which brought forth the interpretability of predictions and trust in the clinical applicability of these models.
The Role of Artificial Intelligence in Detecting PCOS
AI has been identified as a transformative tool in the detection of PCOS by analyzing complex clinical, biochemical, and imaging datasets with high precision. Machine learning and deep learning algorithms can identify subtle patterns in data that may not be recognized by traditional diagnostic methods, thereby allowing for early and accurate diagnosis. These technologies do not only enhance the accuracy of diagnosis but also provide for personalized treatment strategies in order to effectively deal with the heterogeneous nature of PCOS. Because polycystic ovarian syndrome is so common, has a large healthcare burden, is challenging to detect, and can be challenging to identify throughout the clinical, biochemical processes and radiological criteria, it presents an attractive environment for future AI-based solutions. It is very promising to target women with PCOS symptoms who have gone over 2 years without receiving a diagnosis using AI/ML.34 In addition to geographic differences, AI/ML can be used to resolve PCOS issues that may be caused by environmental influences.35 Other diseases, including PCOS, are expensive and require long diagnostic delays that can be addressed with AI technology. AI has a very high potential for diagnosing PCOS because of its heterogeneous nature, as well as its incorporation of clinical, biochemical, and radiological features.36 In addition to integrating these features into EHR systems, AI offers the potential to reduce diagnostic delays associated with PCOS by integrating these features into EHR (Electronic Health Record) systems. A significant level of sensitivity and precision for PCOS identification have been found in the corpus of research currently available on AI in PCOS. This suggests that a well-thought-out AI/ML based program might greatly improve our capacity to identify PCOS at an early stage, leading to financial savings and a lighter load on individuals and the healthcare system. Nonetheless, there are still a number of holes in the field of AI/ML-based PCOS identification. First, it should be emphasized that while assessing AI in PCOS, only 32% of research employed established criteria as reference standards, such as the Rotterdam, NIH, and Global PCOS criteria.37
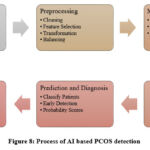 |
Figure 8: Process of AI based PCOS detection |
PCOS (Polycystic Ovary Syndrome) can be diagnosed using traditional and machine learning methods, demonstrating the trend towards technology integration in medical diagnostics as shown in Fig. 8.15 The traditional method of diagnosing PCOS relies primarily on clinical evaluations, where the healthcare provider collects a history of symptoms and performs hormone tests in order to determine the patient’s condition. PCOS is a condition in which multiple ovarian cysts are present, which are indicative of the presence of polycystic ovary syndrome, and can be detected visually using ultrasound examinations. As a contrast to this, machine learning provides a variety of algorithms for detecting PCOS more efficiently and with greater accuracy thanks to its use of a variety of algorithms. There are a number of classification techniques used to categorize patients into PCOS and non-PCOS groups based on data patterns identified in these datasets, such as Decision Trees, Logistic Regression, K-Nearest Neighbors, Naive Bayes, Random Forests, and Support Vector Machines. Additionally, advanced neural networks such as CNNs and MLPs are capable of analyzing complex datasets, including image data, using techniques like clustering, like K-means, which group similar patient data without prior labeling. As healthcare trends move to data-driven, algorithmic approaches for enhancing patient outcomes and streamlining diagnostic processes, this movement reflects a broader trend of leveraging technology to improve patient outcomes and simplify diagnostic processes in general.36
Early Detection and Overcoming Diagnostic Challenges using AI
Apart from all of the abovementioned factors, the complex nature of the disorder, considering its heterogeneous symptoms, makes diagnosis and accurate detection difficult. Complex nature can be considered one of the significant challenges for early detection and proper diagnosis. Traditional methods mostly rely on ultrasounds and assessment of hormone levels for the diagnosis, which is still unable to detect the full range of symptoms of PCOS, particularly in its earliest stages. It is at this point that the AI-driven tools enter the scenario: for both early detection and ongoing management of PCOS. AI and machine learning models can analyze tremendous quantities of information involved in datasets, including hormonal profiles, metabolic markers, and ultrasound images, identifying subtle patterns which may easily go unnoticed in most traditional methods applied by clinicians. These AI techniques can integrate clinical, biochemical, and imaging data toward a better understanding of the variability in PCOS, thereby possibly identifying signs that would be undetected otherwise. Machine learning models might increase diagnostic accuracy by focusing on the complex relationships between these markers of the disease, particularly for early detection of PCOS.
This is important because early interventions are also likely to avert lifelong health consequences such as infertility, diabetes, and cardiovascular disease associated with PCOS. Use of AI at the early stage of detection of this disorder will enable care providers to offer more individualized treatment strategies tailored to the specific phenotypes of PCOS for improving patient outcomes. AI tools, more especially deep learning models, can further differentiate PCOS from other diseases since they can identify something that even a human observer cannot, thereby leaving it with a more accurate diagnosis and thus less chance of overdiagnosis or underdiagnosis. The integration of AI and ML into diagnosis presents the possibility of revolutionizing the management process of PCOS. These technologies not only fill the gaps of conventional techniques but also give earlier and more accurate diagnoses, which have been considered a major medical tool for the betterment of outcomes in patients with PCOS across the varying phenotypes and stages in life. AI-guided diagnostic instruments can better take care of the complexity of PCOS, which allows health care strategies to be proactive rather than reactive and transform the entire horizon of management of PCOS.35,36
Results and Discussion
Performance Analysis of AI Methods for PCOS Diagnosis
Polycystic Ovary Syndrome (PCOS) is an endocrine disorder characterized with a high percentage of women of reproductive age. It has been ranked as one of the most prevalent conditions affecting women within this age category. The disorder manifests commonly with symptoms including obesity, acne, infertility, and hirsutism, thereby making it a multifaceted condition that impacts many aspects of a woman’s health.38 AI, where changes for better in healthcare systems, especially in diagnosis and management of conditions such as PCOS, has only recently transformed the possibilities of accurate diagnosis and treatment, especially when traditional methods are unable to thrive with the complex nature of the disorder.39 In a clinical study conducted on 541 patients from Kerala, India, AI tools were applied to predict PCOS using different machine learning and deep learning classifiers. These models obtained high-performance metrics; in all cases accuracy, precision, recall, and F1-score were well above 98%.40,41 Performance notwithstanding, explainable AI techniques such as the SHAP (SHapley Additive Values) method and the LIME algorithm were adopted. These tools ensure transparency and improve the interpretability of AI models, which is critical for their uptake in clinical settings.42 More advanced techniques of image processing used in the study included models such as DarkNet-19, AlexNet, SqueezeNet, and SVM (support vector machine), which were used to automate PCOS diagnosis. These models have dramatically improved diagnostic procedures in ultrasound images with a very high degree of accuracy. For example, DarkNet-19 obtained an accuracy of 99%, which may reduce the chances of late diagnosis and, therefore, avoid possible fatal consequences in patients with untreated PCOS.43,44 The impact of PCOS on fertility is also augmented since it ranks among the most common causes of infertility in women worldwide, affecting more than five million women.45
This heterogeneity in the presentation of symptoms of PCOS, such as irregular menstrual cycles and overproduction of androgen, also makes it difficult to diagnose. Traditional approaches towards diagnosis often do not capture the full spectrum of manifestations of this disorder.46 AI-driven methods are now fast becoming the backbone in both diagnosis and prognosis. AI-based decision support systems are increasingly applied to assist clinicians improve their ability at identification and management of PCOS.47 For example, a Support Vector Machine-based classifier was shown to be able to classify PCOS with a sensitivity of 96.92% compared to seven other AI approaches, thereby making it a good auxiliary tool for clinicians.48 The Random Forest models combined with the use of LIME further permitted the estimation of the risk of developing PCOS given the presence of certain factors significantly associated with positive and negative diagnoses, thus further enhancing AI model interpretability in clinical settings.49 The possibility of using AI and machine learning for the diagnosis of PCOS has led to the emergence of fast, accurate, and interpretable methods that have improved our ability to respond to the healthcare burden imposed by the disorder, also can be seen in Table 2. Beyond the facility provided for early detection, these technologies have also created a better understanding of the complex pathophysiology culminating into improved patient outcomes through more timely and effective interventions.50
Although promising, AI models for PCOS diagnosis need to be validated correctly for reliability. This document has not sufficiently considered methods used to avoid overfitting and generalize well on unseen data in the validation part. Including a Section 5 with validation techniques would add methodological strength to this study. Moreover, a method such as k-fold cross-validation would prevent overfitting to a specific dataset in the first place because this approach divides the dataset into various folds, and then trains and tests the model on different folds. It also tests the high accuracy that occurs with models using external validation where the model is tested on independent datasets of other populations and environments. This would make the AI models robust and reliable for practical use in real-world clinical environments, thus applicable to support the diagnosis and management of PCOS. This is an important step toward bridging the development gap for AI-based tools to routine practice in healthcare settings with a significant improvement in diagnostic precision and thus overall patient care.
Table 2: Parametric Graph for Accuracy, Precision, Recall and F-1 Score for PCOS detection
| Study Reference | Techniques and Tools Used | Dataset and Population | Performance Metrics | Key Findings and Contributions |
| [41] | ML and DL classifiers, XAI techniques | 541 patients from Kerala, India | Accuracy: 98%, Precision: 97%, Recall: 98%, F1-Score: 98% | Utilized a multi-stack ML approach and XAI to enhance transparency in model predictions. Demonstrated high efficacy in PCOS diagnosis, aiding medical decision-making. |
| [42] | DarkNet-19, AlexNet, SqueezeNet, SVM | Not specified | Accuracy: 99% for DarkNet-19 | Focused on automation of PCOS diagnosis through advanced image processing and classification algorithms, improving speed and accuracy of diagnoses to prevent delayed detection and related complications. |
| [43] | Various ML techniques, PCA | Survey of 541 women | Most accurate: RFC at 89.02% | Employed statistical tools and PCA for feature reduction and utilized several ML algorithms to identify early markers for PCOS, enhancing early detection capabilities. |
| [44] | SVM and other AI techniques | 541 females, metabolic and biochemical features | Prediction Efficacy: 96.92% by SVM | Highlighted the use of SVM which outperformed other AI techniques in diagnosing PCOS, aiding in more effective prognosis and diagnosis strategies. |
| [45] | RF, LIME | Polycystic ovary syndrome dataset available online | Accuracy: 86.03%, Sensitivity: 86.32%, Specificity: 85.37% | Applied LIME to explain RF model predictions, focusing on interpreting PCOS risk factors. Identified critical features influencing the presence or absence of PCOS, enhancing understanding of key diagnostic indicators. |
| [46] | CatBoost, YOLOv2 | Tabular and ultrasound scan data | F1-Score: 88.68% for Tabular data, 85.86% for images | Developed a dual approach using CatBoost and YOLOv2 for analyzing tabular and image data to predict PCOD, creating tools for clinical use that aid in saving patient data for future diagnostic use. |
| [47] | Deep learning with Resnet, U-Net | Full-eye images of 721 Chinese women | Average AUC: 0.979, Classification Accuracy: 0.929 | Proposed a novel deep learning algorithm to explore scleral changes for auxiliary PCOS detection, showing high accuracy and demonstrating deep learning’s potential in PCOS detection. |
| [48] | XGBRF, CatBoost | PCOS dataset from Kaggle | Accuracy: 95% (CatBoost), 89% (XGBRF) | Integrated boosting algorithms for early PCOS identification, supported by data re-sampling to handle outliers and imbalance, enhancing classification performance significantly. |
| [49] | Noninvasive and invasive models | Not specified | Prediction Accuracy: 81%-90.1% | Examined the efficacy of both noninvasive and invasive predictors in PCOS diagnosis, emphasizing the importance of various clinical signs and metabolic parameters in enhancing predictive accuracy. |
| [50] | CNN with VGGNet16, XGBoost, ensemble models | 594 ovary USG images | Accuracy: 99.89% | Utilized a CNN with advanced ensemble techniques to enhance the accuracy and efficiency of PCOS diagnosis through USG images, setting a high standard for future diagnostic procedures. |
| [51] | Bayesian and Logistic Regression classifiers | Clinical and metabolic features | Accuracy: 93.93% (Bayesian), 91.04% (Logistic Regression) | Demonstrated that Bayesian classifiers outperform logistic regression in automating PCOS detection, focusing on clinical and metabolic markers to streamline diagnostic processes. |
| [52] | Ensemble classifiers, feature selection | Kaggle PCOS dataset | Accuracy: 98.89%, Sensitivity: 100% | Highlighted the impact of ensemble classifiers and feature selection on improving the diagnosis of PCOS, using a combination of methods to achieve high accuracy and sensitivity in detection. |
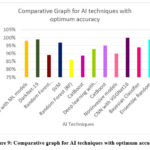 |
Figure 9: Comparative graph for AI techniques with optimum accuracy |
There are various Artificial Intelligence (AI) techniques available at the present time, and the Fig.9. shows the optimum accuracy percentages for different AI techniques in diagnosing Polycystic Ovary Syndrome (PCOS). From algorithms such as Machine Learning (ML) to convolutional neural networks (CNNs) such as VGGNet16, these techniques range from Machine Learning (ML) to Deep Learning (DL) and other algorithms. Its robustness in clinical decision support is reflected by its high accuracy of 98% with Explainable AI (XAI) using ML models. Among the four classifiers, DarkNet-19, a deep learning model, achieved the highest accuracy with 99%, and its performance is one of the best of the bunch. Support Vector Machines (SVM) achieve a higher accuracy of 96.92% than the traditional Random Forest Classifier (RFC) of 89.02%. It is worth mentioning that Random Forest (RF) was proven to have an accuracy of 86.03% for the implementation on one hand and CatBoost for the implementation on the other hand had accuracies of 88.68% and 95% for the implementation on the other. ResNet achieves an accuracy rate of 92.90% in deep learning. In non-invasive diagnostic settings, non-invasive models are 90.10% accurate and Bayesian classifiers are 93.93% accurate. Combining multiple models can enhance diagnostic precision, as demonstrated by the Ensemble Random Forest’s 98.89% accuracy. Its high-resolution image analysis capability demonstrates its capability for high-resolution PCOS diagnosis thanks to its accuracy of 99.89%.
AI Applications in Early PCOS Detection
PCOS is a hormonal disorder that affects mostly women during their reproductive years, causing irregular periods, cysts in the ovaries, and fertility problems. As a result of its complexity, vague symptoms, and unknown causes, PCOS is difficult to diagnose, but it is vital to treating the condition and preventing long-term health issues.51 A wide range of huge datasets, including medical histories, hormonal profiles, and imaging results, have been analyzed with machine learning (ML) models as a means of enhancing diagnosis. A focus on relevant data subsets allows these models to improve diagnostic accuracy by using advanced feature selection techniques.52 SWISS-AdaBoost has been demonstrated to be superior to traditional classifiers such as SVM, KNN, and Random Forest in a number of studies. The SWISS-AdaBoost classifier unquestionably has a higher accuracy of 97.81% and an AUC of 99.08%. To identify PCOS, hybrid machine learning models include many methods, including SVM, Random Forest (RF), and XGboosting more accurately, achieving 93.8% accuracy in preliminary tests.53 To address data imbalances, XGBRF and CatBoost, specifically by using Synthetic Minority Over-sampling Techniques (SMOTE), have been used in studies to identify key clinical parameters to detect PCOS early.54 IFFOA-ANN and adaptive k-means models have also been developed for ultrasound imaging follicle detection, improving the diagnostic process by identifying ovarian disorders accurately.55 A combination of image and clinical data has been shown to increase diagnosis accuracy, with studies showing that deep learning frameworks like Inception have been adapted for the assessment of ovary ultrasound images, reducing false positives and improving model performance.56 Furthermore, studies have shown that GLCM methods for detecting features in pre-processed ultrasound images can be accurate up to 99%.57 PCOS diagnostics have been further improved by integrating ensemble learning algorithms. PCOS is a hormonal condition that mainly affects women during their reproductive years, which gives them symptoms like disturbed menstrual cycles, ovarian cysts, and difficulty conceiving. Since the case is rather complex, with symptoms and causes being pretty vague, diagnosing them is not an easy affair. But it’s essential to detect it early in order to manage it without creating other serious medical conditions like diabetes and cardiovascular diseases.58 ML models have been shown to be instrumental in achieving better diagnostic accuracies while analyzing huge amounts of data including medical histories, hormonal profiles, and imaging results. It is because these models can focus on appropriate subsets of relevant data with the help of refined techniques of feature selection so as to improve diagnostic precision.59
Hybrid AI models, like SWISS-AdaBoost, were found to outperform traditional classifiers like SVM, KNN, and Random Forest when an accuracy up to 97.81% and an AUC of 99.08% was achieved.55 Hybrid models combining techniques like SVM, Random Forest (RF), and XGBoost also demonstrated preliminary accuracy rates at 93.8% for PCOS diagnosis.55 Other models like XGBRF and CatBoost have used Synthetic Minority Over-sampling Techniques (SMOTE) to surmount the class imbalance problem in datasets, thus maximally enhancing the detection rate of critical clinical parameters, subsequently giving an indication toward early identification of PCOS.56 Other techniques, IFFOA-ANN and adaptive k-means, have also been suggested that enable enhancement of the ultrasound imaging technique in detecting follicles for better diagnosis and identification of ovarian disorders.57 The integration of clinical and imaging data also increases the accuracy of diagnosis. For instance, some of the recent frameworks have transformed deep learning in the domain of assessing ovarian ultrasound with decreased false positives and improving performance in the overall model.58 Follow-up studies indicated that by using GLCM methods for feature detection in pre-processed ultrasound images, these can be as accurate as 99%.59 Ensemble learning algorithms and fine-tuning diagnostic accuracy improve diagnostics with advancements in PCOS.
Feature selection techniques implemented along with classifiers such as Random Forest and XGBoost outperform many other methods, high levels of accuracy, and sensitivity in the detection of PCOS at an early stage.60 Machine learning models adapted using techniques like chi-square evaluation and Bayesian classifiers enhance the reliability of these models when utilized with clinical factors.61 These developments indicate how machine learning techniques and feature selection algorithms may be utilized to improve the precision and reliability of PCOS diagnosis, thus motivating clinicians to apply AI for more aggressive management of women with PCOS.62,63 Some hybrid AI techniques are presented in Fig 10, which have been shown to exhibit optimal accuracy for diagnosing PCOS. SWISS-AdaBoost is one of the best models with accuracy of 97.81%, while DarkNet-19 demonstrates its strengths with accuracy of 99%.55 Other techniques like SVM, Random Forest, and XGBoost report the accuracy of around 93.80%. The specific techniques, IFFOA-ANN and XGBRF, have found accuracy as high as 97.50% and 95%, respectively.56,57 However, improvement in integrating dataset demand in specific techniques like joint fusion types of Inception model report an accuracy of 84.81%.58
The combination of models, such as an ensemble, has outstanding accuracy of 98.87% with LR, RF, DT, NB, SVM, KNN, XGBoost, AdaBoost classifiers.59 Other ensemble techniques are also found in RF with AdaBoost for the accuracy of 92%, and HRFLR with CatBoost for the accuracy of 94%.60 The RF performs in great accuracy of 98.89% when trained alone.51 Also, the GNB achieved remarkable robustness with a 100% accuracy rate in some other studies.52 Deep learning architectures like CNN combined with VGGNet16 and XGBoost as a meta-learner have achieved an impressive rate of accuracy near perfection with 99.89% and thus exhibited effectiveness in diagnosing PCOS.53
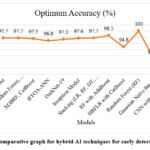 |
Figure 10: Comparative graph for hybrid AI techniques for early detection of PCOS |
This study can be made clearer by combining the performance metrics of different AI models for diagnosing PCOS into one table. A reader can compare each model side by side, including key metrics such as accuracy, precision, recall, and F1-score, and distinguish its good and weak points. This controlled comparison will give a clearer sense of how different AI models differ in their performance to improve the diagnostic challenges of PCOS. This side-by-side format would help readers better understand how other AI models approach the challenges involved in diagnosing PCOS. For example, it would include performance metrics of any models used, such as SVM and Random Forest and DarkNet-19, to actually go deeper into why some models apparently outperformed the others. In addition, the discussion would be carried about their practical implications concerning computational efficiency, reliability, and suitability for clinical application. Such an approach will make the study clearer and provide depth into how AI could be exploited in the earlier detection of PCOS accurately.
Impact of PCOS on women’s health
There are numerous systems within the body affected by polycystic ovaries syndrome (PCOS), best known as an endocrine disorder affecting many aspects of women’s reproductive health as shown in Fig. 11. All things considered, PCOS is a complex illness that affects women who are fertile. A key feature of PCOS is insulin resistance, which results in elevated insulin levels, which are caused by insulin’s diminished ability to stimulate glucose intake and suppress hepatic glucose production. Hormones such as luteinizing hormone (LH) further exacerbate androgen production in ovarian theca cells, which reduces sex hormone-binding globulin, enhancing free androgens and causing hirsutism symptoms.
 |
Figure 11: Various impacts of PCOS on women’s health |
The pathophysiology of resistance to insulin in PCOS can be significantly influenced by genetic and epigenetic variables which includes factors that affect glucose transporter 4 (GLUT4).36 Moreover, there is a connection between insulin resistance and more general metabolic diseases like obesity, dyslipidemia, and increased risk for heart disease. PCOS is characterized by metabolic disturbances that tend to be worsened by obesity, particularly visceral fat, which promotes insulin resistance and metabolic disorders associated with PCOS, but which are not universal in people with PCOS. Fig. 12. Fat around the abdominal area and visceral adipose tissue contributes to the formation of visceral fat in women. Triglyceride levels in the patient’s blood and HDL (high-density lipoprotein) levels in his or her lipid profile are elevated in PCOS patients. This type of pattern is caused by obesity and insulin resistance, both of which contribute to aging and contribute to an increase in symptoms. Additionally, PCOS can negatively affect endocrine function, including sleep apnea and excessive sleepiness during the day, which can indicate poor reproductive health as a result of PCOS.18
 |
Figure 12: Women with PCOS have increased visceral fat and belly fat |
Abbreviations
Hypertriglyceridemia, renal disease, and endothelial dysfunction, Low-density lipoprotein, free fatty acids (FFAs), and polycystic ovarian syndrome (PCOS).
The hormones related to PCOS are not affected by obesity in the same way that obesity hormones are affected by obesity. Several epidemiological studies have found that irregular sleep is associated with diabetes and cardiovascular disease. There is a high risk of cardiovascular events associated with PCOS because it has an increased propensity for cardiovascular events as a result of its inflammatory characteristics and impaired fibrinolysis. Aside from its negative impact on physical health, PCOS also negatively impacts emotional health, according to research. Consequently, those with PCOS tend to experience mental health problems such as depression, anxiety, and stress, along with obesity and weight gain, at a higher rate than the general population. Aside from PCOS, a number of other factors can contribute to the occurrence of the symptoms of the condition. There are a number of factors that can aggravate PCOS, such as body image issues, fertility problems, chronic health conditions, and other factors. There are many underlying mental disorders associated with PCOS, as well as profound ramifications for the mental health of the patient. The management process will be complicated as a result, particularly when it comes to implementing and maintaining lifestyle changes that will be essential to a successful outcome.28
Conclusion
Millions of women worldwide suffer from the complicated endocrine condition known as polycystic ovarian syndrome, or PCOS. Conventional diagnostic techniques, such imaging and clinical assessments, are imprecise and inconsistent. With AI-driven methods reaching accuracies of over 98%, AI and machine learning have become a game-changing strategy. These tools look for minuscule patterns in complex datasets. In addition to increasing diagnostic precision, early AI-assisted PCOS identification allows for customized therapies to reduce long-term health concerns such as infertility, type II diabetes, cardiovascular disease, and mental health issues. Additionally, explainable AI methods like SHAP and LIME improve the interpretability of AI models, which builds clinician trust and makes it easier to incorporate them into clinical practice. Despite AI’s enormous potential, there are still issues to be resolved, such as the requirement for uniform validation, dealing with dataset biases, and guaranteeing robustness over a range of demographics. To guarantee scalability and dependability, future work should concentrate on improving AI models, integrating them with Electronic Health Record (EHR) systems, and testing them in actual clinical settings.
Acknowledgment
The authors wish to express their gratitude to the Centre for Research Projects (CRP) at CHRIST (Deemed to be University), Bangalore Central Campus, Bengaluru, India, for generously supporting this research with Seed Money for the academic year 2023-24 (Project Number CU: CRP: SMSS-2351).
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
George JP designed the research;
George JP and Upreti S performed the research;
Mahajan S contributed to data & sample collection;
Upreti K and Mahajan S contributed analytic tools and analyzed the data;
Upreti K, George JP , Upreti S and Mahajan S wrote the paper;
Reference
- Collée J, Mawet M, Tebache L, Nisolle M, Brichant G. Polycystic ovarian syndrome and infertility: overview and insights of the putative treatments. Gynecological Endocrinology. 2021 Oct 3;37(10):869-74.
CrossRef - Bulsara J, Patel P, Soni A, Acharya S. A review: Brief insight into Polycystic Ovarian syndrome. Endocrine and metabolic science. 2021 Jun 30;3:100085.
CrossRef - Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nature Reviews Endocrinology. 2018 May;14(5):270-84.
CrossRef - Bharathi RV, Swetha S, Neerajaa J, et al. An epidemiological survey: Effect of predisposing factors for PCOS in Indian urban and rural population. Middle East Fertility Society Journal. 2017 Dec 1;22(4):313-6.
CrossRef - El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G. Poly cystic ovarian syndrome: an updated overview. Frontiers in physiology. 2016 Apr 5;7:124.
CrossRef - Sadeghi HM, Adeli I, Calina D, et al. Polycystic ovary syndrome: a comprehensive review of pathogenesis, management, and drug repurposing. International journal of molecular sciences. 2022 Jan 6;23(2):583.
CrossRef - Bednarska S, Siejka A. The pathogenesis and treatment of polycystic ovary syndrome: What’s new?. Advances in Clinical and Experimental Medicine. 2017 Mar 1;26(2):359-67.
CrossRef - Ganie MA, Vasudevan V, Wani IA, Baba MS, Arif T, Rashid A. Epidemiology, pathogenesis, genetics & management of polycystic ovary syndrome in India. Indian Journal of Medical Research. 2019 Oct 1;150(4):333-44..
CrossRef - Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism. 2019 Mar 1;92:108-20.
CrossRef - Damone AL, Joham AE, Loxton D, Earnest A, Teede HJ, Moran LJ. Depression, anxiety and perceived stress in women with and without PCOS: a community-based study. Psychological medicine. 2019 Jul;49(9):1510-20.
CrossRef - Nautiyal H, Imam SS, Alshehri S, et al. Polycystic Ovarian Syndrome: A Complex Disease with a Genetics Approach. Biomedicines 2022 Feb 24;10(3):540.
CrossRef - Meczekalski B, Niwczyk O, Kostrzak A, Maciejewska-Jeske M, Bala G, Szeliga A. PCOS in Adolescents—Ongoing riddles in diagnosis and treatment. Journal of Clinical Medicine. 2023 Feb 3;12(3): 1221.
CrossRef - Yadav V, Sharma Y. Hyperandrogenism. Indian Journal of Pediatrics. 2023 Oct;90(10):1018-24.
CrossRef - Meczekalski B, Szeliga A, Maciejewska-Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecological Endocrinology. 2021 Aug 3;37(8):677-82.
CrossRef - Mahmoud AA, Elfiky AM, Abo-Zeid FS. The anti-androgenic effect of quercetin on hyperandrogenism and ovarian dysfunction induced in a dehydroepiandrosterone rat model of polycystic ovary syndrome. Steroids. 2022 Jan 1;177:108936..
CrossRef - Laganà AS, Veronesi G, Ghezzi F, et al. Evaluation of menstrual irregularities after COVID-19 vaccination: Results of the MECOVAC survey. Open Medicine. 2022 Mar 9;17(1): 475-84..
CrossRef - Attia GM, Alharbi OA, Aljohani RM. The Impact of Irregular Menstruation on Health: A Review of the Literature. Cureus. 2023 Nov:15(11).
CrossRef - Barrack MT, Van Loan MD, Rauh M, Nichols JF. Disordered eating, development of menstrual irregularity, and reduced bone mass change after a 3-year follow-up in female adolescent endurance runners. International journal of sport nutrition and exercise metabolism. 2021 Jun 7;31(4):337-44.
CrossRef - Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nature reviews Disease primers. 2016 Aug 11;2(1):1-8.
CrossRef - Benham JL, Goldberg A, Teede H, Tay CT. Polycystic ovary syndrome: associations with cardiovascular disease. Climacteric. 2024 Jan 2;27(1):47-52.
CrossRef - Lentscher JA, Decherney AH. Clinical presentation and diagnosis of polycystic ovarian syndrome. Clinical obstetrics and gynecology. 2021 Mar 1;64(1):3-11.
CrossRef - Portincasa P, Khalil M, Graziani A, Frühbeck G, Baffy G, Garruti G, Di Ciaula A, Bonfrate L. Gut microbes in metabolic disturbances. Promising role for therapeutic manipulations?. European Journal of Internal Medicine. 2024 Jan 1;119:13-30.
CrossRef - Takeda H, Yamaguchi T, Yano H, Tanaka J. Microglial metabolic disturbances and neuroinflammation in cerebral infarction. Journal of Pharmacological Sciences. 2021 Jan 1;145(1):130-9.
CrossRef - Ágoston C, Urban R, Nagy B, et al. The psychological consequences of the ecological crisis: Three new questionnaires to assess eco-anxiety, eco-guilt, and ecological grief. Climate risk management. 2022 Jan 1;37:100441.
CrossRef - Yang B, Wang B, Sun N, et al. The consequences of cyberbullying and traditional bullying victimization among adolescents: Gender differences in psychological symptoms, self-harm and suicidality. Psychiatry research. 2021 Dec 1;306:114219.
CrossRef - Kokun O. The Ukrainian population’s war losses and their psychological and physical health. Journal of Loss and Trauma. 2023 Jul 4;28(5):434-47.
CrossRef - Elasam AN, Ahmed MA, Ahmed AB, et al. The prevalence and phenotypic manifestations of polycystic ovary syndrome (PCOS) among infertile Sudanese women: a cross-sectional study. BMC Women’s Health. 2022 May 13;22(1):165.
CrossRef - Bellver J, Rodríguez-Tabernero L, Robles A, et al. Polycystic ovary syndrome throughout a woman’s life. Journal of assisted reproduction and genetics. 2018 Jan;35: 25-39.
CrossRef - Yesiladali M, Yazici MG, Attar E, Kelestimur F. Differentiating polycystic ovary syndrome from adrenal disorders. Diagnostics. 2022 Aug 24;12(9): 2045.
CrossRef - Witchel SF, Burghard AC, Tao RH, Oberfield SE. The diagnosis and treatment of PCOS in adolescents: an update. Current opinion in pediatrics. 2019 Aug 1;31(4): 562-9.
CrossRef - Ilie IR, Georgescu CE. Polycystic ovary syndrome-epigenetic mechanisms and aberrant microRNA. Advances in clinical chemistry. 2015 Jan 1;71: 25-45.
CrossRef - Maloy S, Hughes K, editors. Brenner’s encyclopedia of genetics. Academic Press; 2013 Mar 3.
- Sagvekar P, Dadachanji R, Patil K, Mukherjee S. Pathomechanisms of polycystic ovary syndrome: multidimensional approaches. Front Biosci (Elite Ed). 2018 Mar 1;10(03): 384-422.
CrossRef - Lentscher JA, Decherney AH. Clinical presentation and diagnosis of polycystic ovarian syndrome. Clinical obstetrics and gynecology. 2021 Mar 1;64(1): 3-11.
CrossRef - Khanna VV, Chadaga K, Sampathila N, Prabhu S, Bhandage V, Hegde GK. A distinctive explainable machine learning framework for detection of polycystic ovary syndrome. Applied System Innovation. 2023 Feb 23;6(2): 32.
CrossRef - Sumathi M, Chitra P, Sheela S, Ishwarya C. Study and implementation of automated system for detection of PCOS from ultrasound scan images using artificial intelligence. The Imaging Science Journal. 2024 Oct 2;72(7): 828-39.
CrossRef - Denny A, Raj A, Ashok A, Ram CM, George R. i-hope: Detection and prediction system for polycystic ovary syndrome (pcos) using machine learning techniques. In TENCON 2019-2019 IEEE Region 10 Conference (TENCON) 2019 Oct 17 (pp. 673-678). IEEE.
CrossRef - Gibson-Helm M, Teede H, Dunaif A, Dokras A. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. The Journal of Clinical Endocrinology & Metabolism. 2017 Feb 1;102(2): 604-12.
- VanHise K, Chan JL, Wertheimer S, et al. Regional variation in hormonal and metabolic parameters of white and black women with PCOS in the United States. The Journal of Clinical Endocrinology & Metabolism. 2023 Mar 1;108(3): 706-12.
CrossRef - Rotterdam ESHRE/ASRM‐Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Human reproduction. 2004 Jan 1;19(1): 41-7
CrossRef - Roy DG, Alvi PA. Artificial intelligence in diagnosis of polycystic ovarian syndrome. In Contemporary Issues in Communication, Cloud and Big Data Analytics: Proceedings of CCB 2020 2022 (pp. 453-463). Springer Singapore.
CrossRef - Çiçek İB, Küçükakçalı Z, Yağın FH. Detection of risk factors of PCOS patients with Local Interpretable Model-agnostic Explanations (LIME) Method that an explainable artificial intelligence model. The Journal of Cognitive Systems. 2021 Dec 30;6(2): 59-63.
CrossRef - Wagh P, Panjwani M, Amrutha S. Early detection of PCOD using machine learning techniques. In Artificial Intelligence and Speech Technology 2021 Jun 29 (pp. 9-20). CRC Press.
CrossRef - Lv W, Song Y, Fu R, et al. Deep learning algorithm for automated detection of polycystic ovary syndrome using scleral images. Frontiers in Endocrinology. 2022 Jan 27;12: 789878.
CrossRef - Bhat SA. Detection of polycystic ovary syndrome using machine learning algorithms(Doctoral dissertation, Dublin, National College of Ireland).
- Zigarelli A, Jia Z, Lee H. Machine-aided self-diagnostic prediction models for polycystic ovary syndrome: observational study. JMIR Formative Research. 2022 Mar 15;6(3): 29967.
CrossRef - Suha SA, Islam MN. An extended machine learning technique for polycystic ovary syndrome detection using ovary ultrasound image. Scientific Reports. 2022 Oct 12;12(1): 17123.
CrossRef - Mehrotra P, Chatterjee J, Chakraborty C, Ghoshdastidar B, Ghoshdastidar S. Automated screening of polycystic ovary syndrome using machine learning techniques. In2011 Annual IEEE India Conference 2011 Dec 16 (pp. 1-5). IEEE.
CrossRef - Danaei Mehr H, Polat H. Diagnosis of polycystic ovary syndrome through different machine learning and feature selection techniques. Health and Technology. 2022 Jan;12(1): 137-50.
CrossRef - Reka S, Karthik Sainadh Reddy D, Dhiraj I, T Praba S. Hybrid Machine Learning Approach for Early Diagnosis of Polycystic Ovary Syndrome with Stable Features. Journal of Intelligent & Fuzzy Systems.(Preprint): 1-2.
CrossRef - Swamy SR, KS NP. Hybrid machine learning model for early discovery and prediction of polycystic ovary syndrome. In2022 Second International Conference on Advanced Technologies in Intelligent Control, Environment, Computing & Communication Engineering (ICATIECE) 2022 Dec 16 (pp. 1-8). IEEE.
CrossRef - Nilofer NS. Follicles classification to detect polycystic ovary syndrome using GLCM and novel hybrid machine learning. Turkish Journal of Computer and Mathematics Education (TURCOMAT). 2021 Apr 19;12(7): 1062-73.
- Alamoudi A, Khan IU, Aslam N, et al. A deep learning fusion approach to diagnosis the polycystic ovary syndrome (pcos). Applied Computational Intelligence and Soft Computing. 2023;2023(1): 9686697.
CrossRef - Alshakrani S, Hilal S, Zeki AM. Hybrid Machine Learning Algorithms for Polycystic Ovary Syndrome Detection. In 2022 International Conference on Data Analytics for Business and Industry (ICDABI) 2022 Oct 25 (pp. 160-164). IEEE.
CrossRef - Elmannai H, El-Rashidy N, Mashal I, et al. Polycystic ovary syndrome detection machine learning model based on optimized feature selection and explainable artificial intelligence. 2023 Apr 21;13(8): 1506.
CrossRef - Dutta P, Paul S, Majumder M. An efficient SMOTE based machine learning classification for prediction & detection of PCOS.
- Subha R, Nayana BR, Radhakrishnan R, Sumalatha P. Computational intelligence for early detection of infertility in women. Engineering Applications of Artificial Intelligence. 2024 Jan 1;127: 107400.
CrossRef - Rahman MM, Islam A, Islam F, et al. Empowering early detection: A web-based machine learning approach for PCOS prediction. Informatics in Medicine Unlocked. 2024 Jan 1;47:101500.
CrossRef - Tiwari S, Kane L, Koundal D, et al. SPOSDS: A smart Polycystic Ovary Syndrome diagnostic system using machine learning. Expert Systems with Applications. 2022 Oct 1;203:
CrossRef - Nasim S, Almutairi MS, Munir K, Raza A, Younas F. A novel approach for polycystic ovary syndrome prediction using machine learning in bioinformatics. IEEE Access. 2022 Sep 12;10: 97610-24.
CrossRef







