Manuscript accepted on :11-02-2025
Published online on: 28-02-2025
Plagiarism Check: Yes
Reviewed by: Dr. Foram joshi
Second Review by: Dr. B Kirthika
Final Approval by: Dr. Kapil Joshi
Arvind Mewada1 , Sushil Kumar Maurya2
, Sushil Kumar Maurya2 and Mohd. Aquib Ansari1*
and Mohd. Aquib Ansari1*
1School of Computer Science Engineering and Technology, Bennett University, Greater Noida, India.
2Department of Computer Science and Engineering, ITER, Siksha ’O’ Anusandhan University, Bhubaneswar, India.
Corresponding Author E-mail: mohd.aquib@bennett.edu.in
DOI : https://dx.doi.org/10.13005/bpj/3081
Abstract
Diabetes mellitus (DM) is a chronic metabolic disorder condition that requires continuous monitoring and early detection to prevent serious complications such as diabetic retinopathy (DR) and diabetic foot (DF) disease. In recent years, medical imaging technologies coupled with machine learning techniques have made progress in the automated detection of DM-related complications using retina or foot images. This article proposes a novel Ens-DRDF model that integrates the detection of diabetic retinopathy and diabetic foot ulcers using advanced machine learning and image processing techniques. The process involves removing the optic disc and blood vessels, followed by feature extraction, segmentation, and classification. Fuzzy clustering aids lesion differentiation, enhancing image quality for improved DR diagnosis.
Keywords
Deep CNN; Diabetic Retinopathy; Fuzzy Clustering; K-Nearest Neighbors (KNN); Medical Image Processing; Retinal Lesion Detection
Download this article as:| Copy the following to cite this article: Mewada A, Maurya S. K, Ansari M. A. Seeing Beyond: Advanced Image and Thermal Analysis for Early Detection of Diabetic Retinopathy and Diabetes. Biomed Pharmacol J 2025;18(March Spl Edition). |
| Copy the following to cite this URL: Mewada A, Maurya S. K, Ansari M. A. Seeing Beyond: Advanced Image and Thermal Analysis for Early Detection of Diabetic Retinopathy and Diabetes. Biomed Pharmacol J 2025;18(March Spl Edition). Available from: https://bit.ly/3QBtX9x |
Introduction
Diabetic retinopathy (DR) and diabetic foot (DF) are global health concerns that significantly impact patients’ quality of life. Diabetic retinopathy, a leading cause of blindness in adults, is a direct complication of prolonged diabetes.1 Early detection and timely treatment are crucial for preventing vision loss and managing diabetes-related complications.2 Traditional diagnostic methods often rely on manual examinations by specialists, which are time-consuming, expensive, and susceptible to human error.3 As a result, an urgent need is to develop automated, reliable, and cost-effective screening techniques that leverage recent advancements in artificial intelligence and image processing technologies to address these limitations.4
The primary objective of this research is to develop a robust, automated detection system for DR and DF using a combination of image processing and thermal imaging techniques.5 Advanced machine learning algorithms aim to accurately detect early-stage DR lesions, including exudates, micro-aneurysms, and haemorrhages from retinal images.6 Additionally, thermal imaging of the foot is explored to identify signs of diabetic neuropathy and detect DF.7,8 This dual approach targets comprehensive screening for both ocular and systemic manifestations of diabetes, ultimately enhancing diagnostic precision and patient outcomes.9,10
This paper aims to develop a state-of-the-art approach to detect diabetes complications using deep learning strategies. Here, we present the following novel contributions to the fields of diabetic retinopathy and diabetic foot ulcer detection:
We proposed a novel Ens-DRDF model that integrates the detection of diabetic retinopathy and diabetic foot ulcers and achieves high sensitivity and specificity in identifying early-stage DM.
We introduced advanced preprocessing methods, such as removing the optic disc and blood vessel segmentation, to enhance feature extraction from retinal images. Employed fuzzy clustering to aid in lesion differentiation, enhancing image quality for improved DR diagnosis.
Thermal imaging to capture subtle temperature variations in the foot, combined with a K-Nearest Neighbors (KNN) classifier, enables the non-invasive detection of DF, offering a novel diagnostic tool for screening neuropathic complications.
By combining retinal and foot thermal images, the research introduces a hybrid diagnostic system that leverages the strengths of both modalities to provide a comprehensive screening solution for diabetic complications.
The structure of this paper is organised as follows: Section 2 describes related work on existing research in DR and DF detection. Section 3 presents the Proposed Model, outlining the methodologies used, system architecture, and retinal and thermal imaging data integration. Section 4 focuses on Experimentation and Model Evaluation, detailing the experimental setup, performance metrics, and evaluation results of the proposed models, followed by a discussion of their implications. Finally, Section 5 provides the Conclusion and Future Scope, summarising the key findings and suggesting directions for future research in automated diabetes and retinopathy screening.
Related Work
Convolutional Neural Networks (CNNs) have been widely applied to detect retinal abnormalities from fundus images automatically. These systems are capable of identifying signs of diabetic retinopathy, such as haemorrhages, neovascularisation, exudates, and microaneurysms.5 Recent advancements include using transfer learning and fine-tuning approaches to improve classification accuracy, such as the technique presented in the study using a deep forest algorithm combined with a Bat-based feature selection method.1,11,20,21,22,23,24 Another study focused on using radionics analysis on ultra-wide optical coherence tomography angiography scans to automate the grading of diabetic retinopathy, achieving higher diagnostic precision.2
While CNNs remain the backbone of these diagnostic models, recent works have also explored optimisation techniques to enhance detection efficiency. An example is improved multithresholding Tunicate Swarm Algorithm and Hybrid Butterfly Optimization for automated detection of DR severity.12 EdgeSVDNet, a novel model utilising Singular Value Decomposition and deep learning, has been proposed to classify vision-threatening diabetic retinopathy accurately.10 Thermal imaging techniques have been applied to measure variations in foot temperature to diagnose diabetes mellitus. Research has established a correlation between peripheral vascular disease and diabetic complications such as neuropathy, which manifests as abnormal foot temperatures.7 Several machine learning algorithms, including support vector machines (SVMs) and neural networks, have been employed to classify thermal foot images, distinguishing between normal and diabetic patterns.9 One study introduced a decision fusion-based model, which effectively combined thermal and visible imaging modalities for the early detection of diabetes.13 The traditional approach to diagnosing diabetic retinopathy relies on dilated fundus examinations and retinal imaging, necessitating manual assessment by trained ophthalmologists.14 This process is labour-intensive, time-consuming, and susceptible to inter-observer variability, making it challenging for large-scale screening programs.4 Physical examinations, patient histories, nerve conduction studies, and monofilament testing are typically used to assess diabetic peripheral neuropathy. However, these methods often lack the sensitivity required for early diagnosis and are unsuited for broad-population screening.15 On the other hand, thermography offers a non-invasive way to detect diabetic foot complications. However, traditional thermal analysis techniques are subjective and lack the precision required for early-stage detection.8
To address these limitations, recent studies have proposed the integration of multiple imaging modalities and optimisation algorithms to improve diagnostic accuracy. One approach combines retinal and thermal images to develop a hybrid system capable of diagnosing ocular and systemic diabetic complications.11 Incorporating techniques like contrast-limited adaptive histogram equalisation has enhanced the quality of fundus images further, enabling more accurate identification of DR features.16 Overall, the research community has significantly progressed in developing automated systems for diabetic retinopathy and diabetes mellitus detection. By leveraging the power of deep learning, image processing, and hybrid imaging modalities, these systems have the potential to transform current diagnostic practices and improve patient outcomes. Further research is needed to refine these models and expand their applicability to real-world clinical settings.17, 25
Problem Formulation
Despite the advancements in automated diabetic retinopathy (DR) and diabetes foot (DF) detection, several challenges persist. Current DR detection algorithms rely heavily on large volumes of annotated data to train deep learning models. Obtaining high-quality labelled retinal images requires significant manual effort from experts, making it resource-intensive and susceptible to human biases. Moreover, these models are computationally demanding, requiring high-end hardware, which limits their applicability in resource-constrained environments. For widespread adoption in clinical settings, models must be lightweight, efficient, and capable of operating with limited labelled data without compromising diagnostic accuracy.
On the other hand, thermal imaging presents a promising non-invasive alternative for DM detection by identifying temperature variations in the foot associated with neuropathic complications. However, existing thermal imaging-based methods suffer from inconsistencies in capturing accurate temperature variations due to varying room conditions and patient positioning. Additionally, interpreting thermal images can be challenging, leading to potential misclassification when used alone without supplementary information. As a result, the standalone use of thermal imaging for DM detection lacks robustness and reproducibility, making it less reliable for clinical diagnostics.
High Dependency on Annotated Data: Deep learning models for DR detection depend on large-scale annotated datasets to perform well. However, acquiring diverse and well-labeled data for different stages of DR is challenging, especially in underserved regions.
Computational Inefficiency: The complex architectures of existing DR detection models demand significant computational resources, making real-time deployment difficult in scenarios with limited processing power.
Thermal Imaging Limitations: While thermal imaging is a non-invasive option for detecting diabetic foot complications, current models struggle with inconsistent image quality and sensitivity to environmental changes, reducing their effectiveness.
Lack of Integrated Diagnostic Systems: Most research independently focuses on retinal or thermal imaging. There is limited work on developing an integrated system that can leverage the strengths of both imaging modalities to provide a comprehensive diagnostic solution for diabetes-related complications.
The goal is to develop a lightweight, efficient, and integrated diagnostic system that combines deep learning-based retinal image analysis with thermal imaging techniques for comprehensive screening of diabetic retinopathy and diabetes mellitus. The system should address the limitations of current methods by reducing dependency on large, annotated datasets, improving computational efficiency, and enhancing the reliability of thermal imaging. By integrating multiple imaging modalities, the aim is to achieve robust and accurate detection of both ocular and systemic complications of diabetes, facilitating early diagnosis and timely intervention.
Materials and Methods
The proposed Ens-DRDF model aims to develop an efficient and integrated system for early detection of diabetic retinopathy (DR) and diabetes foot (DF) using sophisticated image processing techniques and machine learning algorithms. The model addresses the limitations of existing approaches by combining retinal image analysis with thermal foot imaging, thereby providing a comprehensive diagnostic solution for diabetes-related complications. The architecture is divided into two subsystems: DR detection from retinal images and DF detection using thermal foot imaging. Each subsystem employs advanced methodologies to enhance feature extraction and classification, ensuring high accuracy and efficiency.
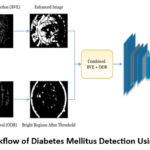 |
Figure 1: Workflow of Diabetes Mellitus Detection Using Retinopathy |
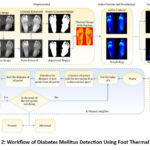 |
Figure 2: Workflow of Diabetes Mellitus Detection Using Foot Thermal Images |
Diabetic Mellitus Detection using Retinal Image Processing
The proposed model for diabetic retinopathy (DR) detection, illustrated in Figure 1, focuses on identifying lesions in retinal fundus images using advanced image processing techniques combined with machine learning classification for accurate diagnosis. The methodology starts by capturing the retinal image, followed by blood vessel extraction to create an “Extracted Vessel Map” that highlights vascular structures. These are then suppressed to isolate other abnormalities. Concurrently, the optic disc (OD), which can interfere with lesion detection, is identified based on intensity and shape characteristics and suppressed to minimise false positives. Image enhancement uses techniques like histogram equalisation to adjust contrast and brightness, followed by denoising and sharpening using median and Gaussian filters. This enhances the clarity of lesions and microstructures like microaneurysms and haemorrhages. Lesion detection employs region growing, edge detection, and thresholding to isolate potential lesion areas. Identified regions are further processed using bandpass filters for exudates (EXS), contrast enhancements for haemorrhages (HEMS), and target detection for microaneurysms (MAS). In the post-processing phase, morphological operations and additional classifiers refine the detected lesions, minimising false positives and ensuring accurate mapping onto the original image. Finally, the model’s performance is evaluated using standard metrics such as sensitivity, specificity, and accuracy to validate its effectiveness in identifying DR-related abnormalities and aiding early diagnosis.
Diabetes Mellitus Detection using Thermal Imaging
Figure 2 illustrates an advanced method for automatically detecting diabetes mellitus using thermal variations in the foot. The process begins with Data Acquisition, where thermal images of the patient’s feet are captured, highlighting patterns indicative of diabetes-related complications. Next, Data Preprocessing is applied to clean and normalise the images, reducing noise and enhancing quality for accurate analysis. The images are then segmented into smaller patches, enabling the model to focus on specific areas with significant thermal variations.
In the Feature Extraction phase, key metrics such as mean temperature, standard deviation, entropy, contrast, and correlation are computed, capturing critical thermal characteristics. These features are fed into classifiers like Random Forest, Decision Tree, Gradient Boosting, Naive Bayes, and K-Nearest Neighbors (K-NN), each detecting patterns to distinguish between normal and diabetic states. The testing and evaluation phase assesses classifier performance to ensure reliable predictions. The process concludes with a Decision-Making step, categorising patients as normal or diabetic, resulting in a non-invasive, efficient diagnostic tool for early diabetes detection and management. This approach combines thermal imaging and machine learning to enhance early diagnosis and treatment of diabetes-related complications.
Ensemble Model for Diabetic Mellitus Detection
By combining the diabetes mellitus score using retinal image processing and the diabetes mellitus score using thermal imaging into an ensemble model, we improved the overall diagnosis accuracy. We took a high-level approach to design this ensemble model using a Weighted Average Ensemble. We combined the prediction probabilities from both models (retinal image processing and thermal imaging) using a weighted average, giving more weight to the model with higher confidence or historical accuracy.
Final Prediction = α∗ Retinal Model Score + (1 −α) ∗ Thermal Model Score
where α is a weight coefficient (tuned during validation). Each model is assigned a weight α based on their individual performance. More reliable models are given higher weights. By adjusting the weights, we can optimise the performance of the ensemble model, making it more accurate and robust than using any one model alone.
The final diagnostic system integrates the outputs from both the retinal and thermal imaging subsystems. By combining the strengths of these two modalities, the system offers a comprehensive assessment of diabetes-related complications. The integrated model capitalises on the high sensitivity of retinal imaging for detecting ocular complications while utilising the non-invasive nature of thermal imaging to assess systemic complications. This synergy results in a robust screening tool capable of thoroughly evaluating diabetes-related health issues.
Results
The proposed Ens-DRDF model for diabetic retinopathy (DR) and diabetes foot ulcer (DF) detection was evaluated through experiments using retinal fundus images and thermal foot images. The results were systematically documented to demonstrate the effectiveness of each processing step, highlighting improvements in lesion detection, feature extraction, and overall classification accuracy. This section provides a detailed analysis of the experimental results for DR and DF detection systems, followed by a discussion on system performance and validation metrics.
Dataset Description
Diabetic Retinopathy research utilises a (Diabetic Retinopathy)18 dataset of approximately 1,200 high-quality fundus images captured with a 3CCD camera, featuring resolutions of 1440×960, 2240×1488, and 2304×1536 pixels. This dataset supports identifying critical pathological features such as exudates and microaneurysms, enhancing automated lesion recognition algorithms and diagnostic accuracy. Similarly, a dataset of over 1,000 thermal images for Type 2 Diabetes Mellitus, obtained via a FLIR E60 camera, includes clinical data like blood glucose levels. 19 This combination allows researchers to detect physiological changes linked to diabetes, improving early detection and treatment strategies through robust machine learning algorithms.
Diabetic Retinopathy Detection Results
The detection module for Diabetic Retinopathy (DR) consists of several key steps: extracting blood vessels, removing the optic disc, detecting lesions, and enhancing images. Each step is illustrated in Figure 1, which shows the process from the original image to the final output of the model. This experiment uses SGD-based deep CNN with the mentioned parameters: learning rate (0.001), Momentum (1.00), Weight Decay (1e-4), Dampening (0), Nesterov Momentum (True), Batch Size (16), Optimizer (Adam), Loss Function (Cross-Entropy), and Activation (ReLu). This output is then compared with the existing model, as illustrated in Table 1.
Table 1: Performance Comparison of Diabetic Retinopathy Detection Models
| Year | Lesions | Method | Accuracy | Sensitivity | Specificity |
| 2010 | Exudates | Morphological operations | 93.75% | 94.25% | 91.47% |
| 2011 | Exudates, Microaneurysms | Contrast enhancement, intensity threshold | 87.25% | 89.41% | 80.85% |
| 2013 | Exudates | Vessel detection, intensity threshold, area threshold | 82.25% | 85.25% | 81.25% |
| 2014 | Exudates | Median filtering, dynamic thresholding, image addition | 91.25% | 94.50% | 94.10% |
| 2015 | Exudates | Feature extraction, segmentation using Fuzzy C-means,PSO optimisation | 95.25% | 92.25% | 90.25% |
| 2020 | Exudates | Green channel extraction, intensity difference | 89.25% | 84.25% | 73.25% |
| 2021 | Exudates, Microaneurysms | Moat operator | 92.25% | 85.75% | 97.95% |
| ** | Exudates, Haemorrhage, Microaneurysms, Neovascularization | Fuzzy C-means (FCM), Median Filtering, Gamma Correction, Advanced Segmentation Techniques | 96.25% | 98.10% | 94.22% |
First, the module extracts blood vessels using techniques that enhance contrast and shape. This step is crucial for clearly identifying blood vessels and assists in locating the optic disc and other potential issues. Next, the optic disc is removed by recognising its shape and brightness. This removal helps reduce incorrect results in the subsequent lesion-detection phase. After identifying a candidate region, further enhancements are applied to improve the visibility of lesions. The final output highlights the detected lesions in the retinal image, demonstrating the model’s ability to identify important markers of DR, such as microaneurysms and haemorrhages.
Previous models have made significant advancements in identifying specific lesions linked to diabetic retinopathy. For example, in 2010, a model achieved an accuracy of 93.75%, with a sensitivity of 94.25% and specificity of 91.47%. In 2011 and 2013, various techniques were utilised, resulting in accuracy rates of 87.25% and 82.25%, respectively; the 2013 study reported a sensitivity of 85.25%, although specificity data was not provided. Between 2013 and 2015, advanced techniques led to 82.25%, 91.25%, and 95.25% accuracy. Additionally, studies during this period showed detection rates of microaneurysms with accuracies of 90% and 98%. In 2020 and 2021, research focused on detecting exudates, raising sensitivity to 97.5%. In 2021, sensitivity and accuracy were achieved by around 95.1% using complex classification methods despite some gaps in specificity reporting.
Ens-DRDF model distinguishes itself with a comprehensive approach, employing various image processing techniques to effectively identify different lesion types, including exudates, haemorrhages, microaneurysms, and neovascularisation. The model accurately identifies diabetic retinopathy with a strong accuracy of 96.25%. It also has a sensitivity of 98.1%, effectively identifying true positive cases, which is essential for timely diagnosis and treatment. Moreover, the model achieves a specificity of 94.22%, effectively reducing false positives.
Table 2: Performance Comparison of Diabetes Mellitus Detection Models
| Model | Class | Accuracy (%) | Precision (%) | Sensitivity (%) | F1-Score (%) | Specificity (%) |
| J48 | DM | 93.41 | 94.40 | 95.72 | 95.55 | 84.44 |
| RF | DM | 90.42 | 92.74 | 94.26 | 93.49 | 80.00 |
| NB | DM | 91.62 | 92.86 | 95.90 | 94.36 | 80.00 |
| KNN* | DM | 93.59 | 92.59 | 95.23 | 93.89 | 91.83 |
Figure 3 illustrates the accuracies acquired from J48, RF, NB and KNN classifiers. Key features of the proposed model include broad lesion coverage, allowing it to identify various lesion types and enhance its diagnostic ability compared to models that focus on a single type. With an accuracy of 96.25%, the model ranks among the top performers, ensuring reliable detection. The 98.1% sensitivity reflects its effectiveness in detecting actual cases of diabetic retinopathy, which is vital for effective treatment. Finally, a specificity of 94.22% indicates that the proposed model provides trustworthy results by minimising false positives.
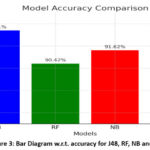 |
Figure 3: Bar Diagram w.r.t. accuracy for J48, RF, NB and KNN |
Ens-DRDF model offers a thorough and accurate method for detecting diabetic retinopathy. It often surpasses previous models in performance thanks to effective image processing and classification techniques. The Ens-DRDF model can lead to better patient outcomes by accurately and promptly identifying various lesion types.
Diabetes Mellitus Detection Results
Table 2 presents the noteworthy performance of various models in the detection of diabetes mellitus (DM), including the J48 Decision Tree, Random Forest (RF), and Naive Bayes (NB). These models aim to identify the presence of diabetes mellitus and the coarseness of granulation (CG), achieving varying degrees of success. The J48 Decision Tree, for instance, achieved an accuracy of 93.41% for both DM and CG, with sensitivity and precision metrics of 95.72% and 94.40% for DF, respectively. Although the Random Forest model had slightly lower overall metrics than the J48 Decision Tree, it still demonstrated solid performance, achieving an accuracy of 90.42%. The Naıve Bayes model recorded an overall accuracy of 91.62%, maintaining competitive precision and sensitivity values. However, there is potential for improvement in reducing false positives, as these models often exhibit a trade-off between sensitivity and specificity, with specificity values generally ranging from 80% to 84.44%. The proposed model for diabetes mellitus detection employs a K-Nearest Neighbors (KNN) technique, which significantly enhances performance measures. It achieves an impressive accuracy rate of 93.59%, 92.59% precision and 95.23% sensitivity.
Additionally, the Ens-DRDF model’s specificity of 91.83% signifies a notable reduction in false positives compared to existing models. With an Area Under the Curve (AUC) of 1 and an F1-Score of 93.89%, the Ens-DRDF model underscores its robustness and reliability in accurately identifying diabetes mellitus. This comprehensive performance highlights the excellent trade-off between accuracy, sensitivity, and specificity, establishing it as a valuable tool for diabetes mellitus identification. Ens-DRDF model demonstrates balanced performance metrics, achieving 93.59% accuracy, 92.59% precision, 95.23% sensitivity, and 91.83% specificity. In contrast, current models often compromise sensitivity and specificity to provide reliable detection. With a specificity of 91.83%, the Ens-DRDF model significantly outperforms many existing models, such as the J48 Decision Tree and Naive Bayes, which report specificity values between 80% and 84%. This enhancement indicates that the Ens-DRDF model effectively reduces false positives, critical for accurate medical diagnosis. Ens-DRDF model excels at detecting true positive instances of diabetes mellitus, achieving a sensitivity of 95.23%. This high sensitivity enables the model to identify abnormalities early, essential for prompt intervention and treatment. The robustness and dependability of the Ens-DRDF model are illustrated by its F1-Score of 93.89% and an AUC of 1. These metrics demonstrate strong overall recall and accuracy and a remarkable ability to discriminate between classes. Ens-DRDF approach integrates advanced image processing techniques with the K-Nearest Neighbors (KNN) algorithm. This combination leverages KNN’s pattern recognition and classification strengths, particularly in medical imaging contexts. Unlike certain models that focus on specific traits or types of lesions, the Ens-DRDF model is designed to identify a wide variety of features related to diabetes mellitus. Its extensive detection capabilities enhance its clinical utility. In summary, the Ens-DRDF model’s key advantages include balanced and superior performance metrics, especially in terms of specificity and sensitivity, its strong F1-Score and AUC, innovative methodological approach, and broad lesion identification capabilities. Collectively, these elements establish the Ens-DRDF model as a significantly more accurate and reliable tool for diabetes mellitus detection compared to existing models.
Performance Evaluation and Validation
The model’s performance was validated using a confusion matrix to quantify the detection accuracy, sensitivity, and specificity for DR and DF. The confusion matrix for DR detection showed high precision in distinguishing between healthy and affected regions, with minimal false positives due to effective optic disc removal and lesion refinement techniques. The K-Nearest Neighbors (KNN) classifier demonstrated robust performance for DF detection, accurately classifying temperature anomalies based on spatial pixel patterns and neighbourhood similarity.
Overall, the system achieved commendable results, demonstrating the potential to be a reliable tool for non-invasive and early detection of diabetic complications. By combining retinal image processing and thermal imaging, the integrated system ensures a comprehensive assessment, supporting timely diagnosis and intervention for diabetes management
Discussion
This section discusses the performance of the proposed models based on the AUC-ROC curve.
Diabetic Retinopathy AUC-ROC Curve
The ROC (Receiver Operating Characteristic) curve in Figure 4 illustrates the trade-off between sensitivity (True Positive Rate) and false positive rate (1 – Specificity) for diabetic retinopathy detection.
AUC (Area Under the Curve) Value: The model achieves an AUC of 0.965, considered excellent. AUC values range from 0.5 (no discrimination) to 1.0 (perfect discrimination). An AUC of 0.965 indicates that the model is highly effective at distinguishing between positive (patients with diabetic retinopathy) and negative cases (patients without diabetic retinopathy).
Curve Shape: The rapid ascent of the ROC curve towards the top-left corner signifies high sensitivity and low false positive rates, demonstrating the model’s strong diagnostic performance.
Interpretation: The Y-axis represents the True Positive Rate (proportion of correctly identified positive cases), while the X-axis shows the False Positive Rate (proportion of incorrectly labelled negative cases). A higher AUC value close to 1.0 indicates excellent separability between patients with and without diabetic retinopathy.
Conclusion: The model effectively differentiates between the two classes, but some misclassifications (false negatives) suggest room for improvement, especially in detecting certain borderline cases.
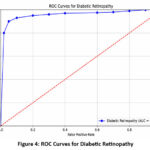 |
Figure 4: ROC Curves for Diabetic Retinopathy |
Diabetes Mellitus AUC-ROC Curve
The ROC curve shown in Figure 5 for diabetes mellitus detection highlights the model’s diagnostic capability.
AUC Value: An AUC of 1.0 indicates perfect model performance, meaning the model can flawlessly distinguish between individuals with and without diabetes mellitus.
Curve Shape: The curve hugs the top and left borders of the plot, which signifies ideal specificity (no false positives) and sensitivity (no false negatives).
Interpretation: The Y-axis represents the True Positive Rate (proportion of correctly detected positives), while the X-axis represents the False Positive Rate (proportion of real negatives misclassified as positives).
Conclusion: The model achieves perfect classification, with zero false positives and false negatives, indicating its complete reliability in identifying diabetes mellitus cases.
 |
Figure 5: ROC Curves for DF |
Comparative Analysis
Diabetic Retinopathy Model: The AUC of 0.965 indicates a high classification accuracy. The few false negatives (FN) in the confusion matrix suggest minor gaps in detecting all positive cases, which could be addressed with further optimisation.
Diabetes Mellitus Model: An AUC of 1.0 denotes a flawless classification with no misclassifications, as confirmed by a confusion matrix showing zero false positives (FP) or false negatives.
Overall Performance: Both models show strong diagnostic capabilities. While the diabetic retinopathy model has a minor scope for enhancement, the diabetes mellitus model operates at an ideal level. The AUC-ROC curves serve as visual confirmation of the model’s efficacy, complementing the numerical insights provided by the confusion matrices.
Conclusion
This research significantly advances medical diagnostics by utilising thermal imaging and the K-Nearest Neighbors (K-NN) algorithm for the automated detection of diabetes mellitus and diabetic retinopathy. By identifying distinct heat patterns indicative of diabetes, the proposed model offers a non-invasive, accurate, and cost-effective diagnostic tool that enhances early detection and management of diabetic complications. Validation tests confirm the model’s accuracy and reliability, demonstrating its potential to improve patient outcomes. Future research could integrate advanced machine learning techniques, such as deep learning, to enhance diagnostic accuracy and robustness. Here, the model relies heavily on a high-quality dataset for the training and testing process. In addition, thermal images are used in experimentations where consistency could be affected due to patient positioning and environmental factors. To compete with these problems, the expanded dataset to include a wider variety of thermal and retinal images could be used to improve the model’s generalizability across different populations. The development of wearable technology equipped with thermal imaging capabilities represents an exciting direction, allowing for real-time health monitoring and proactive diabetes management.
Additionally, creating real-time diagnostic applications could revolutionise patient care by enabling immediate assessments during routine check-ups. Longitudinal studies assessing the long-term effectiveness of these diagnostic methods will provide valuable insights into their practical benefits. By pursuing these avenues, researchers can significantly advance diabetes diagnostics, ultimately leading to better health management and improved quality of life for individuals with diabetes.
Acknowledgment
We thank Bennett University, Greater Noida and Shiksha ‘O’ Anusandhan University, Bhubaneswar, for supporting our research.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
Arvind Mewada: Conceptualization, Methodology, Writing – Original Draft; Shushil Kumar Maurya: Data Collection, Analysis, Review & Editing; Mohd. Aquib Ansari: Visualization, Validation, Writing – Review & Editing.
Reference
- Modi P, Kumar Y. Smart detection and diagnosis of diabetic retinopathy using bat-based feature selection algorithm and deep forest technique. Comput Ind Eng. 2023;182:109364.
CrossRef - Soren VN, Prajwal HS, Sundaresan V. Automated grading of diabetic retinopathy and radiomics analysis on ultra-wide optical coherence tomography angiography scans. Image Vis Comput. 2024;151:105292.
CrossRef - Shahriari MH, Sabbaghi H, Asadi F, Hosseini A, Khorrami Z. Artificial intelligence in screening, diagnosis, and classification of diabetic macular edema: A systematic review. Surv Ophthalmol. 2023;68(1):42-53.
CrossRef - Silva PS, Zhang D, Jacoba CMP, et al. Automated machine learning for predicting diabetic retinopathy progression from ultra-widefield retinal images. JAMA Ophthalmol. 2024;142(3):171-178.
CrossRef - Hasan DA, Zeebaree SR, Sadeeq MA, Shukur HM, Zebari RR, Alkhayyat AH. Machine learning-based diabetic retinopathy early detection and classification systems: A survey. In: 2021 1st Babylon International Conference on Information Technology and Science (BICITS). IEEE; 2021:16-21.
CrossRef - Ali G, Dastgir A, Iqbal MW, Anwar M, Faheem M. A hybrid convolutional neural network model for automatic diabetic retinopathy classification from fundus images. IEEE J Transl Eng Health Med. 2023;11:341-350.
CrossRef - Arora AS. Automated prediction of diabetes mellitus using infrared thermal foot images: Recurrent neural network approach. Biomed Phys Eng Express. 2024;10(2):025025.
CrossRef - Meenakshi RM, Padmapriya N, Venkateswaran N, Shperling S, Leshno A. Classification of retinal vascular diseases using ensemble decision tree in thermal images. Int J Pattern Recognit Artif Intell. 2023;37(12).
CrossRef - Munadi K, Saddami K, Oktiana M, et al. A deep learning method for early detection of diabetic foot using decision fusion and thermal images. Appl Sci. 2022;12(15):7524.
CrossRef - Bilal A, Liu X, Baig TI, Long H, Shafiq M. EdgeSVDNet: 5G-enabled detection and classification of vision-threatening diabetic retinopathy in retinal fundus images. Electronics. 2023;12(19):4094.
CrossRef - Ohri K, Kumar M. Supervised fine-tuned approach for automated detection of diabetic retinopathy. Multimed Tools Appl. 2024;83(5):14259-14280.
CrossRef - Bhimavarapu U. Optimized automated detection of diabetic retinopathy severity: Integrating improved multi-thresholding tunicate swarm algorithm and improved hybrid butterfly optimization. Health Inf Sci Syst. 2024;12(1):42.
CrossRef - Thirunavukkarasu U, Umapathy S, Ravi V, Alahmadi TJ. Tongue image fusion and analysis of thermal and visible images in diabetes mellitus using machine learning techniques. Sci Rep. 2024;14(1):14571.
CrossRef - Pavithra KC, Kumar P, Geetha M, Bhandary SV. Computer-aided diagnosis of diabetic macular edema in retinal fundus and OCT images: A review. Biocybern Biomed Eng. 2023;43(1):157-188.
CrossRef - Gunawardhana PL, Jayathilake R, Withanage Y, Ganegoda GU. Automatic diagnosis of diabetic retinopathy using machine learning: A review. In: 2020 5th International Conference on Information Technology Research (ICITR). IEEE; 2020:1-6.
CrossRef - Pour AM, Seyedarabi H, Jahromi SHA, Javadzadeh A. Automatic detection and monitoring of diabetic retinopathy using efficient convolutional neural networks and contrast-limited adaptive histogram equalization. IEEE Access. 2020;8:136668-136673.
CrossRef - Bhandari S, Pathak S, Jain SA. A literature review of early-stage diabetic retinopathy detection using deep learning and evolutionary computing techniques. Arch Comput Methods Eng. 2023;30(2):799-810.
CrossRef - Wang Z, Yang J. Diabetic retinopathy detection via deep convolutional networks for discriminative localization and visual explanation. In: Workshops at the Thirty-Second AAAI Conference on Artificial Intelligence; 2018.
- Hernandez-Contreras DA, Peregrina-Barreto H, de Jesus Rangel-Magdaleno J, Renero-Carrillo FJ. Plantar thermogram database for the study of diabetic foot complications. IEEE Access. 2019;7:161296-161307.
CrossRef - Rusia MK, Singh DK, Ansari MA. Human face identification using LBP and Haar-like features for real-time attendance monitoring. In: 2019 Fifth International Conference on Image Information Processing (ICIIP). IEEE; 2019:612-616.
CrossRef - Ansari MA, Singh DK. An approach for human-machine interaction using dynamic hand gesture recognition. In: 2019 IEEE Conference on Information and Communication Technology. IEEE; 2019:1-6.
CrossRef - Tiwari M, Singh N, Mewada A, Ansari MA. Machine learning-empowered breast cancer diagnosis: Insights from Coimbra dataset analysis. Recent Adv Comput Sci Commun. 2024.
CrossRef - Akram M, Adnan M, Ali SF, et al. Uncertainty-aware diabetic retinopathy detection using deep learning enhanced by Bayesian approaches. Sci Rep. 2025;15(1):1342.
CrossRef - Zhang J, Dai Y, Lee CH. Healthcare with smart contact lenses: Innovations, challenges, and future perspectives. In: Advanced Sensors for Smart Healthcare. Elsevier; 2025:521-545.
CrossRef - Kumar RR, Shankar SV, Jaiswal R, Ray M, Budhlakoti N, Singh KN. Advances in deep learning for medical image analysis: A comprehensive investigation. J Stat Theory Pract. 2025;19(1):9.
CrossRef







