Abdel-Nasser El-Shorbagi1,2* , Sachin Chaudhary1
, Sachin Chaudhary1 , Hitesh Kumar3
, Hitesh Kumar3 , Harish Chandra Verma4
, Harish Chandra Verma4 , Prabhash Nath Tripathi5
, Prabhash Nath Tripathi5 , Aditi Giri5
, Aditi Giri5 , Garima Agarwal5
, Garima Agarwal5 , Shweta Dumoga5
, Shweta Dumoga5 and Ramesh Kumar Gupta6
and Ramesh Kumar Gupta6
1Department of Medicinal Chemistry, College of Pharmacy, University of Sharjah, Sharjah, United Arab Emirates.
2Faculty of Pharmacy, University of Assiut, Assiut, Egypt.
3Department of Pharmaceutics, RV Institute of Pharmacy, Bijnor, Uttar Pradesh, India.
4Department of Pharmaceutical Chemistry, College of Pharmacy, Teerthanker Mahaveer University, Moradabad, Uttar Pradesh, India.
5Department of Pharmaceutical Technology, Meerut Institute of Engineering and Technology, NH-58, Baghpat Road Crossing, Bypass Road, Meerut, Uttar Pradesh, India.
6Department of Pharmacology, Amity Institute of Pharmacy, Amity University, Lucknow, Uttar Pradesh, India.
Corresponding Author E-mail: aelshorbagi@sharjah.ac.ae
DOI : https://dx.doi.org/10.13005/bpj/2949
Abstract
Doxorubicin that is on WHO's list of essential medicines and other anthracycline analogues, in general, are natural metabolites isolated from Streptomycetaceae, or semi-synthetized derivatives stated as first-generation anticancer agents. The tetracyclic scaffold attached mostly to amino sugar is known to be effective against solid tumors compared to other anticancer agents. The mechanism had been stated as intercalating agent at the minor groove of DNA strands during the step of releasing supercoiled DNA. Along with their anticancer activity, anthracyclines possess antimicrobial effects of notable MIC values. Cardiotoxicity represents the main challenge for both of medical care for treatment of cancers and drug discoverers. This exertion deals with careful structural investigation of the three-dimensional, fully optimized drugs in use. Drug-candidates in clinical studies, and leads failed in last developments. The aim is to find a structural gate to guard against or reduce the cardiac side effects. It deals also, with the topological features differentiating between antibacterial and anticancer agents bearing the tetracyclic scaffold features as well as between the topoisomerases as target molecules.
Keywords
Anticancer Activity; Anthracycline; Cardiac Toxicity; Doxorubicin; Fully Optimized 3D Structure; Gene; Topoisomerase Top2A; Topoisomerase Top2B
Download this article as:| Copy the following to cite this article: El-Shorbagi A. N, Chaudhary S, Kumar H, Verma H. C, Tripathi P. N, Giri A, Agarwal G, Dumoga S, Gupta R. K. Structural Variance of Doxorubicin and Anthracycline Analogues as Topoisomerase Alpha and Beta (Top2a and Top2b) Inhibitors and Potential Design of Analogue Candidates of Less Side Effects on Cardiomyocytes. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: El-Shorbagi A. N, Chaudhary S, Kumar H, Verma H. C, Tripathi P. N, Giri A, Agarwal G, Dumoga S, Gupta R. K. Structural Variance of Doxorubicin and Anthracycline Analogues as Topoisomerase Alpha and Beta (Top2a and Top2b) Inhibitors and Potential Design of Analogue Candidates of Less Side Effects on Cardiomyocytes. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/4dkhVKy |
Introduction
Scaffolds especially those derived from natural sources are of vital biological importance as antineoplastic agents. Anthracyclines dates back to 1960’s and their primary isolation revolutionized the treatment modalities for many cancers, namely solid tumors and neoplastic types. Early agents known were doxorubicin and daunorubicin, categorized as antibiotics having high affinity for Gram-positive bacteria but exhibited significant cytotoxicity which led to their exploitation as anticancer drugs.1-4
Anthracyclines are currently used in combination for treatment of breast cancer with the standard CMF (cyclophosphamide, methotrexate, and fluorouracil) regimen5, as well as other combination.6,7 The enclosure of an anthracycline has been proved to decrease mortality rates in women. Anthracyclines are also used for different clinical indications.8,9
Several anthracyclines obtained via biosynthesis or chemical modifications. An alarming aspect of their clinical use is the toxic risks associated with their accumulation. The aim has always been the development of safer anthracyclines, with broader spectrum of activity against different tumors, as well as enhanced selectivity. Most of the first, second and third generations share an amino-sugar moiety essential for DNA binding and intercalation.10-12 The main drawback associated with the use of anthracyclines lies in their considerable affinity to the cardiac tissue, lead to cardiotoxicity.13 In order to avoid and/or minimize the risk of cardiomyopathy and congestive heart failure, this work relates the extent of cardiotoxicity and structural differences in each drug by considering the topology of the fully optimized 3D-structure. The physicochemical data of the optimized structures are also considered. Anthracyclines bind to topoisomerases II and DNA resultant in a ternary complex, and preventing re-ligation.14,15 The cardiotoxicity involves production of free-radical, which results in damaging DNA, proteins, and lipids and leads to cellular dysfunction.16 Thorough, investigation of occurrence in cells and organs of topoisomerases mentioned that doxorubicin targets both of topoisomerases namely, alpha (Top2A) and beta (Top2B). Human topoisomerase TOP2A is encoded by the Top2A–gene on chromosome-17q21-22, and Top2B is encoded by the Top2B-gene on chromosome-3p24. Cardio-myocytes express Top2B but not TopP2A.15-18
In area of drug discovery scientists usually locking in the main pharmacophore that fits the pharmaco-dynamic interactions. Derivatization on the skeleton rely on addition, removal, and/or modification of adaptable entities on the skeleton that lead to selectivity and specificity whenever isoforms found. Considering cardiomyocytes that express Top2B but not Top2A and the inhibitor’s level of effects and side effects.19-22 Topoisomerase-2 necessitates ATP to act. It makes a temporary break and rejoin via trans-esterification utilizing phosphate-diester (Figure 1). Thus, inhibition of topoisomerase II preventing DNA repair and causing DNA damage and cell-death. 23
Several strategies are mentioned for tumor selectivity such as (a) – It can be boosted if aided by specific transporters to malignant tissues in specific organs,24-27 (b) – Liposomal formulations as well as binding to polymers (31), and (c) – Prodrug preparations that are favorably activated intracellular.28-33 These tactics were used to increase selectivity and reduce undesirable effects.34,35 This work is a thorough structural and topological investigation of anthracyclines. Molecular modeling of each selected approved drug or candidate structure studied as fully optimized at full self-consistent field (SCF) levels by using MOPAC36,37; a general molecular orbital package implemented with molecular mechanics software MMXPC.38 This study is to find the structural modalities differentiating between toxic anthracyclines, safer anthracyclines, anthracyclines bearing both antibacterial and anticancer effects, and the related tetracyclines. It is a continuation of our interest in compounds bearing anti-cancer, anti- inflammatory and antimicrobial activities.24,39-52 The answer for topology differences between approved antimicrobial and antineoplastic tetracyclic structures can be gathered in throughout the article.
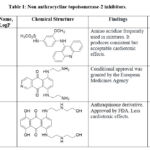 |
Table 1: Non anthracycline topoisomerase-2 inhibitors.
|
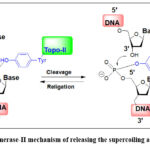 |
Figure 1: Topoisomerase-II mechanism of releasing the supercoiling and rejoining DNA.
|
Structural Characteristics of Drugs Approved Candidates of Anthracyclines
The structural differences of derivatives in medical use mainly attributed to three substructures in the tetracyclic skeleton namely C-4 (Ring A), C-7 and C-9 (Ring D). The entities involved are amino-sugar (Figure 2, R1), the minor differences of aglycone moiety (Figure 2, R2) and the whole acyl group (Figure 2, R) at C-9.53-76 The important physicochemical data for these drugs and/or candidates are considered as calculated partition coefficient.54,55-76
The calculated partition coefficients of clinically used analogs range from CLogP: 1.734 for the more lipid soluble, like idarubicin to CLogP: 0.648 for the less lipid soluble one like doxorubicin. Lipid solubility is important for effects and side effects as well.56-76
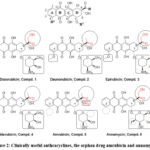 |
Figure 2: Clinically useful anthracyclines, the orphan drug amrubicin and annamycin. |
Brief Biosynthesis of Anthracyclines
Biosynthesis of doxorubicin involves many steps reported in conventional articles. It starts by a three carbons unit; propanoyl-CoA; (Figure 3) which combines by a decarboxylation coupling with malonyl-CoA. Malonyl-Co-A is, repeatedly added via carriers after losing carbon dioxide in each step to provide a skeleton of 21 carboxylic acid bearing 10 carbonyl groups.2,79-81 The poly-carbonyl 21-carbon acid manipulated successively by different enzymes to yield tricyclic; alkanoic acid, then to the aglycone; rhodomycinone. Rhodomycinone undergoes structural modifications and coupling with amino-sugar, via several bio-transformations to provide doxorubicin.82,83
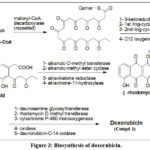 |
Figure 3: Biosynthesis of doxorubicin. |
Medical Importance of Anthracyclines in Cancer Treatment
Anthracyclines are still very important for treatment of acute lymphocytic and chronic myelogenous leukemia; the disease in which the bone marrow makes too many white blood cells. Doxorubicin also, succeeded to show a substantial efficacy against solid tumors.84 Several types of solid tumors are responsive to doxorubicin, as breast carcinoma, small-cell lung carcinoma, and ovarian carcinoma.85
Pharmacological Effects and Toxicological Profiles of Anthracyclines
Not only disrupting topoisomerase-II-mediate DNA repair, anthracyclines also, produce free radicals by reversible quinonoid transformation. Free radicals damaging membrane, DNA and proteins. In addition, reactive oxygen can make epoxidation of fatty acids leading to membrane and DNA damage, oxidative stress, and triggering apoptosis.86,87 Alternatively, doxorubicin can enter the nucleus and poison topoisomerase-II, also resulting in DNA damage, cell cycle control and cell death.88-91
The cumulative dose-dependent cardiac toxicity of doxorubicin represents unwanted health problem from essential medicines. There are some differences between the anthracycline’s congeners in toxicological profile and upon comparing their ability to induce topoisomerase II-mediated DNA cleavage.92-94 Cardiac toxicity as reported in several articles attributed to the cellular oxidative stress induced by free radicals. Unfortunately, doxorubicin preferentially interacts with cardiomyocytes, and the side effects resulted as reflection of free radicals on DNA, protein and lipid as reported elsewhere (Figure 4).95-97
Oxidative stress resulted as reflection of coordination reactions between metals such as iron and functionalities of the co-planar system at rings B and C (Figure 4). The complex of metal and anthracyclines catch soluble oxygen that accordingly producing superoxide. Superoxide dismutase: the natural cellular antioxidant change catalyzes the disproportionation of superoxide to be converted into two less damaging species.
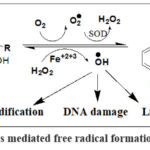 |
Figure 4: Anthracyclines mediated free radical formation and biological effects. |
Comparative Cardiac Toxicity and Potential Chemical Modifications to Decrease Side Effects
The relevant chemical entities involved in oxidative stress-are mentioned on tetracyclic structure of anticancer as well as antibacterial activities. The group of drugs and candidates realized for elaboration of topology and SAR study (Figure 5). The structural entities responsible for chelation and free radical production are highlighted. Compounds are mentioned by numbers and names (Figure 2 and Table 2). An anthracycline; Compound 14; Mutamycin E (Figure 5) fulfills the features of doxorubicin except the C-9 alpha-hydroxy acetyl group. This compound as active but not considered for further preclinical effects as anticancer. On the other-hand, tetracycline (Figure 5, Compound 15) which has no amino-sugar attachment at C-7 is in use as antibacterial medicine with high safety. The two compounds 14 and 15 are included with anthracyclines to elaborate the important topological differences on biological activity. The differences are important to verify the clinical usefulness of tetracyclic structures as anticancer and/or antibacterial activity.
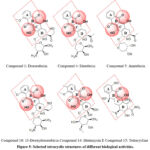 |
Figure 5: Selected tetracyclic structures of different biological activities.
|
 |
Table 2: Important investigational and experimental anthracyclines.
|
Doxorubicin (Compound 1, Figure 2) is in wide use especially in solid tumors and used admixed with other agents to reduce as possible the side effects on heart (98). In addition to the entities that are highlighted for their contribution in free radical formation (Figure 4), doxorubicin has three main groups represent the differences with congeners. The most important entities are:
A coplanar C-4-methoxy group.
Amino-sugar (pyran ring) substituent attached to C-7 of three groups a methyl, a hydroxyl and one amino group.
A hydroxy acetyl group-oriented beta as a substituent at C-9.
Daunorubicin has the same structural entities at C-4 and C-7 but having acetyl instead of hydroxyl-acetyl at C-9. This small difference led to a product of less side effect on heart.98-100
The (Figure 6) shows a graphical representation of doxorubicin G1 as coplanar four fused rings part C, the substituent at C-7 as part A and the substituent at C-9 as part B. Doxorubicin G2 as optimized at full self-consistent field (SCF) levels by using MOPAC; a general molecular orbital package implemented with molecular mechanics software MMXPC.36-38 In the general substituted tetracyclic system, the structure part C is planar. Part B (the C-9 substituent) appears perpendicular up with the planar C and the amino-sugar; part A appears perpendicular down to the planar system. The following monographs are introduced for comparative purposes between tetracyclic structures of anticancer activity concomitant with severe side effect on heart with those having less side effect and with the others bearing antimicrobial activity rather than cytotoxicity.
Table 3: Important used drugs, investigational, and experimental tetracyclic structures optimized at full self-consistent field (SCF) levels by using MOPAC.
|
Compound |
% PSA |
% UnSA |
SE |
Str |
bnd |
DM |
HF |
CLogP |
|
Compound 1: Doxorubicin |
37.72 |
14.25 |
-97.6 |
1.480 |
9.203 |
7.433 |
-383,43 |
0.648 |
|
Compound 2: Daunorubicin |
35.26 |
14.25 |
-99.2 |
1.422 |
8.306 |
5.598 |
-346.62 |
0.959 |
|
Compound 3: Epirubicin |
37.84 |
14.35 |
-98.5 |
1.509 |
8.875 |
7.52 |
-384.24 |
0.648 |
|
Compound 4: Idarubicin |
35.72 |
16.02 |
-84.4 |
1.373 |
7.091 |
6.595 |
-310.49 |
1.734 |
|
Compound 5: Amrubicin |
45.36 |
26.55 |
-73.4 |
1.688 |
5.960 |
6.245 |
-201.03 |
0.113 |
|
Compound 6: Annamycin |
37.76 |
14.04 |
-110.0 |
1.871 |
8.839 |
5.608 |
-369.53 |
1.599 |
|
Compound 10: 13-deoxy-doxorubicin |
30.66 |
13.30 |
-103.1 |
1.290 |
7.265 |
2.758 |
-397.34 |
1.444 |
|
Compound 11: Esorubicin |
34.89 |
13.88 |
-95.6 |
1.394 |
7.440 |
5.655 |
-343.02 |
0.879 |
|
Compound 12: Zorubicin |
27.45 |
20.97 |
-128.6 |
13.850 |
12.000 |
13.886 |
-303.78 |
3.032 |
|
Compound 14: Mutamycin E |
32.68 |
14.22 |
-115.0 |
1.930 |
9.837 |
6.179 |
-447.55 |
2.613 |
|
Compound 15: Tetracycline |
44.55 |
15.61 |
-143.7 |
1.717 |
7.152 |
5.215 |
-238.7 |
0.911 |
PSA = Polar Surface Area, UnSA = Unsaturated Surface Area, SE = Standard Entropy, Str = stretching, Bnd = bending, DM = dipole moment, HF = heat of formation, and ClogP = Calculated partition coefficient for n-octanol/water obtained from chemdraw 8 ultra.
Candidates of analogs as anticancer compounds, in addition to compd. 14 a natural product (101) bearing antibacterial and cytotoxic activities and tetracycline are studied as 3D-optized structures at full SCF and outlined in (Table 4).101,102 A very important value gathered from the table is the tetracycline having very high percentage of polar surface area (% PSA, 44.6%). The candidates failed in clinical development like compound 10 and compound 12 bearing the least percentage of polar surface area (% PSA 22.5 and 30.6%). It also indicates the importance of the ketonic-function of substituent at C-9.103-106
The methods by which anthracyclines can be prepared are semi synthesis, genetically engineered Streptomyces peucetius and from total synthesis.107-110
The attempts to reduce the incidence of cardiotoxicity can be made if several modifications can be applied.
First is to remove the methoxy substituent at C-4 of the skeleton. The drugs and candidate such as compounds 4, 5 and 6 are of less cardiotoxic effect. Methoxy group on aromatic systems donates electrons and, in these cases, it may increase the chelation power of the keto-enol systems undergoing the chelation with iron. Other groups can be tried.111,112
Second is the ketonic group (C=O) of the C-9 substituent appear of high importance for tumoricidal action (Topoisomerase IIα) but also in the pathogenesis of cardiotoxicity (Topoisomerase IIβ). Replacement by (CH2) decreases the effect topoisomerase IIα but increases the cardiotoxicity; topoisomerase IIβ. Compd. 10 bears less effects than doxorubicin as anticancer but high side effects on heart. The physicochemical properties of compound 10 appears of less percentage of polar surface area % PSA and less dipole moment than all the clinically useful anthracyclines.113,114
Third is the sugar at C-7: In doxorubicin there are three substituents on pyran ring methyl, hydroxyl, and amino groups of specific stereochemistry. Changing the stereochemistry of a single group in pyran provided a potent with less cardiotoxic derivative compound 3. Amino group of pyran at C-7 which has been rigorously mentioned as essential for activity, two derivatives (compound 5 and 6) one of which is orphan drug, compound 5 having no amino group in the C-7 pyran entity. The presence of a powerful hydrogen bond acceptor and donor such as NH2 or OH groups.115-118
Fourth is the possible absence of one of the parts at C-7 and C-9 mentioned as A and B in the topology graph (Figure 6) decreases the cytotoxicity relative to all of the anthracyclines considered for developments like compound 14.
Fifth, is the absence of both parts at C-7 and C-9 mentioned as A and B in the topology graph (Figure 6) abolishing the cytotoxic effect and emerging the antibacterial activity. The compound 15, physico-chemical data in Table 3, demonstrates a high percentage polar surface area of compd. 15 (Table 4) than all the derivatives % PSA exceeding 44%. In silico calculation of ADME (Table 4) showed many differences between compound 15 and others in partition coefficient, water solubility, bioavailability score, and even in not being a good substrate for oxidase enzymes.
Table 4: Important used drugs, investigational, and experimental tetracyclic structures calculated in-silico- for pharmacokinetics by Swiss ADME.
|
No. |
Compound |
MR |
Consensus Log P |
Pgp substrate |
Bioavail-ability Score |
Lead-likeness #violations |
|
1 |
Doxorubicin |
132.66 |
0.52 |
Yes |
0.17 |
1 |
|
2 |
Daunorubicin |
131.5 |
1.18 |
Yes |
0.17 |
1 |
|
3 |
Epirubicin |
132.66 |
0.5 |
Yes |
0.17 |
1 |
|
4 |
Idarubicin |
125.01 |
1.14 |
Yes |
0.55 |
1 |
|
5 |
Amrubicin |
120.2 |
0.82 |
Yes |
0.55 |
1 |
|
6 |
Annamycin |
137.59 |
0.99 |
Yes |
0.17 |
1 |
|
7 |
13-deoxy-dextrorubicin |
134.18 |
0.95 |
Yes |
0.17 |
1 |
|
8 |
Esorubicin |
136.2 |
1.76 |
Yes |
0.55 |
1 |
|
9 |
Zorubicin |
167.5 |
2.24 |
No |
0.17 |
2 |
|
10 |
Mutamycine E |
132.12 |
0.56 |
Yes |
0.17 |
1 |
|
11 |
Tetracycline |
110.22 |
-0.56 |
No |
0.11 |
1 |
MR = Molar refractivity, Consensus Log P = It is method is similar (but not identical) to the ClogP method in Swiss ADME.
Sixth is fail in development of the highly potent compound 6. The full output of the in-silico calculation of ADME (Table 4) showed that compound 6 has four deviations when investigated for drug-likeness namely being alkyl halide, iodine derivative, the molecular weight, and the bone marrow toxicity.
There are several analogs related to doxorubicin appear with less side effects on heart and Table 5 introduces the comparative data between these derivatives and doxorubicin as well as between each other.119-152
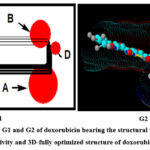 |
Figure 6: Topology G1 and G2 of doxorubicin bearing the structural units for anticancer activity and 3D-fully optimized structure of doxorubicin. |
Table 5: Comparative cardiac effects versus doxorubicin and/or each other.
|
Compound |
Comparative cardiotoxicity |
References / Findings |
|
Daunorubicin; Compound 2 versus doxorubicin |
By contrast, daunorubicin was approximately half as cardiotoxic when compared with doxorubicin. Daunorubicin was less cardiotoxic among survivors of childhood cancer. |
Ref (119, 120)
1- C-9 acetyl instead of hydroxyl-acetyl 2- Sugar at C-7 substituents differently arranged 3- Methoxy-substituent at C-7. |
|
Epirubicin; Compound 3 versus doxorubicin |
Clinical trials demonstrated safety comparable to that of doxorubicin in early and advanced breast cancer. Epirubicin has been favored over doxorubicin for lower cardiac toxicity |
Ref (92, 121-123) It is the diastereomer of doxorubicin. Sugar C5’ alpha-hydroxyl group. 1- C-9 acetyl instead of hydroxyl-acetyl 2- Sugar at C-7 substituents differently arranged 3- Methoxy-substituent at C-7. |
|
Idarubicin; Compound 4 versus doxorubicin
|
It is more cytotoxic than doxorubicin, explained by higher hepatic penetration because of high lipophilicity. Orally active. It is less cardiotoxic than doxorubicin in phase II clinical trials. |
Ref (124) & Ref. (92, 100, 125-127)
1- C-9 acetyl instead of hydroxyl-acetyl 2- Amino-sugar at C-7. 3- No substituent at C-4. |
|
Idarubicin; Compound 4 vesus Epirubicin |
A significantly lower accumulation in cardiomyocytes was obtained with epirubicin and idarubicin compared with carminomycin and doxorubicin. |
Ref. (126-129) 1- C-9 acetyl instead of hydroxyl-acetyl 2- Amino-sugar at C-7. 3- No substituent at C-4. |
|
Esorubicin; Compound 11 (CLogP: 0.879) versus doxorubicin |
For human solid tumors in vitro in clonogenic assay appeared to be more potent on a weight basis than DOX. ESO has been reported to have decreased cardiac toxicity in preclinical models as compared to DOX. |
Ref. (130-132) Experimental 1- C-9 hydroxyl-acetyl, similar 2- Deoxy-sugar at C-7 substituents (a hydroxyl group is missed from the C-7 entity) 3- Methoxy-substituent at C-7, similar |
|
Amrubicin; Compound 5 versus doxorubicin
N.B. Not approved FDA but approved in Japan |
There was no significant cardiac toxicity, and concluded that amrubicin has efficacy Amrubicin showed lower cardiotoxicity at equivalent dosages. This seems to be due to the restricted distribution of the active metabolite in non-tumor tissues. |
Ref (133, 134), (58, 135)
1- C-9 acetyl instead of hydroxyl-acetyl 2- No amino group and no methyl group in the sugar at C-7. 3- No substituent at C-4.
|
|
Annamycin; Compound 6 versus doxorubicin |
Annamycin have little to no cardiac toxicity. It is formulated in a nano-molecular bi-lamellar liposomal system. Side effect (136) Bone marrow toxicity delaying its development. |
Ref (137), (62), (63, 136, 137) 1- C-9 hydroxyl-acetyl 2- Sugar at C-7 the substituents with iodine and no amino group. 3- No substituent at C-7.
|
|
Zorubicin; Compound 12 versus doxorubicin |
Toxicity appears high grade granulo-cytopenia, thrombo-cytopenia,
Cardiotoxicity appears like DOX. |
Ref (138-141)
It is the phenylhydrazone of daunorubicin. 1- C-9 acetyl instead of hydroxyl-acetyl 2- Amino-sugar at C-7. 3- Methoxy substituent at C-4. |
|
Amsacrine; Compound 7 versus doxorubicin |
Amsacrine in clinical trials it developed occasional instances of acute cardiac arrhythmias and cardiomyopathy. Amsacrine-related cardiac events are less common than those related to anthracycline chemotherapeutic agents. |
Ref. (142-146) Acridine derivative
|
|
Pixantrone; Compound 8 versus doxorubicin |
It is of reduced cardiotoxic potential compared with doxorubicin and mitoxantrone |
Ref. (66, 67, 147, 148) Benzo(g)isoquinoline derivative
|
|
Mitoxantrone; Compound 9; versus doxorubicin |
Active DNA intercalating agent with low cardiotoxic potential. |
Ref. (125, 149-151) Aza-anthraquinone derivative |
|
SP1049C; Compound 13; doxorubicin in P-glyco-protein versus doxorubicin
|
P-glycoprotein for increasing cellular uptake, transport, and half-lives of drugs. It is not approved, yet. It reduces the relative cardiotoxity to about half of that of doxorubicin. |
Ref. (126, 127, 152, 153)
|
Conclusion
Anthracyclines have showed a great deal of cytotoxic activity since their incorporation in cancer treatment protocols, with considerable attempts to ameliorate their structure to overcome their evident cardiotoxicity. The approaches towards making better anthracyclines as anticancer agents are slow. In this work the structures of drugs in clinical use, the orphan drugs, the candidates bearing high cytotoxic activity and examples of tetracyclic structures bearing weak and/or cytotoxicity are collected for investigation. Important findings have been introduced in different points around the topological 3D-feature (Figure 6). Taking in consideration the points mentioned about the substitutions around main tetracyclic structure may help in introducing a selective and potent anticancer with much less cardiotoxicity from anthracycline-scaffold which still very important in treatment of solid tumors.
Acknowledgement
The authors would like to acknowledge University of Sharjah, Sharjah, United Arab Emirates.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article
Conflicts of Interest
The authors do not have any conflict of interest.
Ethical Approvals
This research did not involve human participants, animal subjects, or any material that requires ethical approval
Authors’ Contributions
All authors made a significant and equal contribution to this work.
References
- Lown JW. Discovery and development of anthracycline antitumour antibiotics. Chem. Soc. Rev., 1993; 22: 165-176.
CrossRef - Kantola J, Kunnari T, Hautala A, Hakala J, Ylihonko K, Mäntsälä P. Elucidation of anthracyclinone biosynthesis by stepwise cloning of genes for anthracyclines from three different Streptomyces spp. Microbiol., 2000; 146(1): 155-163.
CrossRef - Aubel-Sadron G, Londos-Gagliardi D. Daunorubicin and doxorubicin, anthracycline antibiotics, a physicochemical and biological review. Biochimie., 1984; 66(5): 333-352.
CrossRef - Fujiwara A, Hoshino T, Westley JW. Anthracycline antibiotics. Crit. Rev. Biotechnol., 1985; 3(2): 133-157.
CrossRef - Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer- the results of 20 years of follow-up. N. Engl. J. Med., 1995; 332(14): 901-906.
CrossRef - Levine MN, Bramwell VH, Pritchard KI, Norris BD, Shepherd LE, Abu-Zahra H, Findlay B, Warr D, Bowman D, Myles J, Arnold A. Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer. National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol., 1998; 16(8): 2651-2658.
CrossRef - Poole CJ, Earl HM, Hiller L, Dunn JA, Bathers S, Grieve RJ, Spooner DA, Agrawal RK, Fernando IN, Brunt AM, O’Reilly SM. Epirubicin and cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy for early breast cancer. N. Engl. J. Med., 2006; 355(18): 1851-1862.
CrossRef - Adams SF, Marsh EB, Elmasri W, Halberstadt S, VanDecker S, Sammel MD, Bradbury AR, Daly M, Karlan B, Rubin SC. A high response rate to liposomal doxorubicin is seen among women with BRCA mutations treated for recurrent epithelial ovarian cancer. Gynecol. Oncol., 2011; 123(3): 486-91.
CrossRef - Saffi J, Agnoletto MH, Guecheva TN, Batista LF, Carvalho H, Henriques JA, Stary A, Menck CF, Sarasin A. Effect of the anti-neoplastic drug doxorubicin on XPD-mutated DNA repair-deficient human cells. DNA Repair., 2010; 9(1): 40-47.
CrossRef - Weiss RB. The anthracyclines: will we ever find a better doxorubicin? Semin. Onclol., 1992; 19: 670-686.
- Chen SY, Du LD, Zhang YH. Pilot study of intravesical instillation of two new generation anthracycline antibiotics in prevention of superficial bladder cancer recurrence. Chin. Med. J., 2010; 123(23): 3422-6.
- Lown JW, Hsiao-Hsiung C, Plambeck JA, Acton EM. Diminished superoxide anion generation by reduced 5-iminodaunorubicin relative to daunorubicin and the relationship to cardiotoxicity of the anthracycline antitumor agents. Biochem. Pharmacol., 1979; 28(17): 2563-2568.
CrossRef - Kremer LC, Caron HN. Anthracycline cardiotoxicity in children. N. Engl. J. Med., 2004; 351: 120-121.
CrossRef - Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart, 2018; 104 (12): 971-977.
CrossRef - Lang AJ, Mirski SE, Cummings HJ, Yu Q, Gerlach JH, Cole SP. Structural organization of the human TOP2A and TOP2B genes. Gene, 1998; 221(2): 255-266.
CrossRef - Unverferth BJ, Magorien RD, Balcerzak SP, Leier CV, Unverferth DV. Early changes in human myocardial nuclei after doxorubicin. Cancer, 1983; 52(2): 215-221.
CrossRef - Sawyer DB. Anthracyclines and heart failure. N. Engl. J. Med., 2013; 368(12): 1154-1156.
CrossRef - Beretta GL, Zunino F. Molecular mechanisms of anthracycline activity. Top. Curr. Chem., 2008; 283: 1-19.
CrossRef - Zunino F, Capranico G. DNA topoisomerase II as the primary target of anti-tumor anthracyclines. Anti-cancer drug design, 1990; 5(4): 307-317.
- Ashley N, Poulton J. Mitochondrial DNA is a direct target of anti-cancer anthracycline drugs. Biochem Biophys. Res. Commun., 2009; 378(3): 450-455.
CrossRef - Corremans R, Adão R, De Keulenaer GW, Leite‐Moreira AF, Brás‐Silva C. Update on pathophysiology and preventive strategies of anthracycline‐induced cardiotoxicity. Clin. Exp. Pharmacol., 2019; 46(3): 204-215.
CrossRef - Sadurska E. Current views on anthracycline cardiotoxicity in childhood cancer survivors. Pediatr. Cardiol., 2015; 36: 1112-1119.
CrossRef - El-Shorbagi AN, Chaudhary S, Chaudhary A, Agarwal G, Tripathi PN, Dumoga S, Aljarad AA, Omer E, Mahmoud F, Gupta RK, Mohamed MH. Marine Antineoplastic Templates: Clinical Trials (I–III) and Motifs Carried via Antibodies to Target Specific Cancerous Tissues. Biomed. Pharmacol. J., 2022; 15(2): 579-603.
CrossRef - Pereira NA, Chan KF, Lin PC, Song Z. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. Taylor & Francis- InMAbs., 2018; 10(5): 693-711.
CrossRef - Wallace PM, Senter PD. Selective activation of anticancer prodrugs by monoclonal antibody-enzyme conjugates. Methods Find Exp Clin Pharmacol., 1994; 16(7): 505-512.
- Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science, 2013; 341 (6151): 1192-1198.
CrossRef - Koudelka S, Knotigova PT, Masek J, Prochazka L, Lukac R, Miller AD, Neuzil J, Turanek J. Liposomal delivery systems for anti-cancer analogues of vitamin E. J Control Release., 2015; 207: 59-69.
CrossRef - Park JW, Benz CC, Martin FJ. Future directions of liposome-and immunoliposome-based cancer therapeutics. WB Saunders-InSeminars in oncology, 2004; 31: 196-205.
CrossRef - Ito K, Hamamichi S, Asano M, Hori Y, Matsui J, Iwata M, Funahashi Y, Umeda IO, Fujii H. Radiolabeled liposome imaging determines an indication for liposomal anticancer agent in ovarian cancer mouse xenograft models. Cancer Sci., 2016;107(1): 60-67.
CrossRef - Delplace V, Couvreur P, Nicolas J. Recent trends in the design of anticancer polymer prodrug nanocarriers. Polym. Chem., 2014; 5(5): 1529-44.
CrossRef - Bildstein L, Dubernet C, Couvreur P. Prodrug-based intracellular delivery of anticancer agents. Adv. Drug Deliv. Rev., 2011; 63(1-2): 3-23.
CrossRef - Li G, Sun B, Li Y, Luo C, He Z, Sun J. Small‐molecule prodrug nano assemblies: an emerging nanoplatform for anticancer drug delivery. Small. 2021;17(52): 2101460.
CrossRef - Rafiyath SM, Rasul M, Lee B, Wei G, Lamba G, Liu D. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysis. Exp. Hematol. Oncol., 2012; 1: 1-9.
CrossRef - Houba PH, Leenders RG, Boven E, Scheeren JW, Pinedo HM, Haisma HJ. Characterization of novel anthracycline prodrugs activated by human β-glucuronidase for use in antibody-directed enzyme prodrug therapy. Biochem. Pharmacol., 1996; 52(3): 455-463.
CrossRef - Dewar MJ. Concerning criticisms of MINDO/3 by Pople and Hehre. J. Am. Chem. Soc., 1975; 97(22): 6591.
CrossRef - Bingham RC, Dewar MJ, Lo DH. Ground states of molecules. XXVII. MINDO/3 calculations for carbon, hydrogen, oxygen, and nitrogen species. J. Am. Chem. Soc., 1975; 97(6): 1302-1306.
CrossRef - Seiter K. Toxicity of topoisomerase II inhibitors. Expert Opin. Drug Saf., 2005; 4: 219-234.
CrossRef - Abdu-Allah HH, Abdel-Moty SG, El-Awady R, El-Shorbagi AN. Design, and synthesis of novel 5-aminosalicylate (5-ASA)–4-thiazolinone hybrid derivatives with promising antiproliferative activity. Bioorg. Med. Chem. Lett., 2016; 26(7): 1647-1650.
CrossRef - Ashmawy NS, El-Labbad EM, Hamoda AM, El-Keblawy AA, El-Shorbagi AN, Mosa KA, Soliman SS. The anti-Candida activity of Tephrosia apollinea is more superiorly attributed to a novel steroidal compound with selective targeting. Plants, 2022; 11(16): 2120.
CrossRef - El Bialy SA, Abdelal AM, El‐Shorbagi AN, Kheira SM. 2, 3‐Bis (5‐alkyl‐2‐thiono‐1, 3, 5‐thiadiazin‐3‐yl) propionic Acid: one‐pot domino synthesis and antimicrobial activity. Archiv der Pharmazie: Int. J. Pharm. Chem., 2005; 338(1): 38-43.
CrossRef - El-Naggar M, El-Shorbagi AN, Elnaggar DH, Amr AE, Al-Omar MA, Elsayed EA. Synthesis, characterization, and cytotoxic evaluation of some newly substituted diazene candidates. J. Chem., 2018; 2018.
CrossRef - El-Shorbagi AN, Chaudhary S. Monobactams: A unique natural scaffold of four-membered ring skeleton, recent development to clinically overcome infections by multidrug-resistant microbes. Lett. Drug Des. Discov. Title., 2019;16(12): 1305-1320.
CrossRef - El-Shorbagi AN, Chaudhary S, Chaudhary A, Agarwal G, Tripathi PN, Dumoga S. Beta-Lactamases Inhibitors: A Perspective on the Existing and the Potential Admixtures to Synergize Beta-lactams Versus Resistant Superbugs. Biomed. Pharmacol. J., 2022; 15(4): 1797-1819.
CrossRef - El-Shorbagi AN, El-Naggar M, Tarazi H, Chaudhary S, Abdu-Allah H, Hersi F, Omar H. Bis-(5-substituted-2-thiono-1, 3, 5-thiadiazinan-3-yl) butane as a scaffold of anti-proliferative activity, blended by a multi-component process. Med. Chem. Res., 2018; 27:1103-1110.
CrossRef - Ramadan WS, Saleh EM, Menon V, Vazhappilly CG, Abdu-Allah HH, El-Shorbagi AN, Mansour W, El-Awady R. Induction of DNA damage, apoptosis and cell cycle perturbation mediate cytotoxic activity of new 5-aminosalicylate–4-thiazolinone hybrid derivatives. Biomed Pharmacother., 2020; 131: 110571.
CrossRef - Sameh S, Alshaimaa MH, Abdel-Nasser A, Shorbagi E, El-Keblawy AA. Novel betulin derivative is responsible for the anticancer folk use of Ziziphus spina-christi from the hot environmental habitat of UAE. J. Ethnopharmacol., 2018; 11; 403-408.
CrossRef - Vazhappilly CG, Saleh E, Ramadan W, Menon V, Al-Azawi AM, Tarazi H, Abdu-Allah H, El-Shorbagi AN, El-Awady R. Inhibition of SHP2 by new compounds induces differential effects on RAS/RAF/ERK and PI3K/AKT pathways in different cancer cell types. Invest. New Drugs., 2019; 37: 252-261.
CrossRef - El-Shorbagi AN, Chaudhary S, Chaudhary A, Agarwal G, Tripathi PN, Dumoga S, Aljarad AA, Omer E, Mahmoud, F, Gupta RK, Mohamed MH. Marine antineoplastic templates: Clinical trials (I–III) and motifs carried via antibodies to target specific cancerous tissues. Biomed. Pharmacol. J., 2022; 15(2): 579-603.
CrossRef - El-shorbagi AN, Sakai SI, El-gendy MA, Omar N, Farag HH. Imidazo [2, 1-b] benzothiazoles. I. Chem. Pharm. Bull., 1988; 36(12): 4760-4768.
CrossRef - Emara S, Razee S, El-Shorbagi AN, Masujima T. Flow injection method for the determination of methotrexate with a column-packed oxidizing agent. Analyst, 1996; 121(2): 183-188.
CrossRef - Aboul‐Fadl T, El‐Shorbagi AN. New carriers for representative peptides and peptide drugs. Arch. Pharm., 1997; 330(11): 327-32.
CrossRef - Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev., 2004; 56(2): 185-229.
CrossRef - Dahan A, Wolk O, Kim YH, Ramachandran C, Crippen GM, Takagi T, Bermejo M, Amidon GL. Purely in silico BCS classification: Science-based quality standards for the world’s drugs. Mol. Pharmaceutics., 2013; 10(11): 4378-4390.
CrossRef - Machatha SG, Yalkowsky SH. Comparison of the octanol/water partition coefficients calculated by ClogP®, ACDlogP, and KowWin® to experimentally determined values. Int. J. Pharm., 2005; 294(1-2): 185-92.
CrossRef - Friche E, Jensen PB, Roed H, Skovsgaard T, Nissen NI. In vitro circumvention of anthracycline-resistance in Ehrlich ascites tumour by anthracycline analogues. Biochem. Pharmacol., 1990; 39(11): 1721-1726.
CrossRef - Ohmori H, Yamato T, Asahi T. Phase I study of amrubicin hydrochloride (SM-5887) for superficial bladder cancer in intravesical chemotherapy. Gan To Kagaku Ryoho. Cancer & Chemotherapy., 2001; 28(4): 475-82.
- von Pawel J, Jotte R, Spigel DR, O’Brien ME, Socinski MA, Mezger J, Steins M, Bosquée L, Bubis J, Nackaerts K, Trigo JM. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J. Clin. Oncol., 2014; 32(35): 4012-4019.
CrossRef - Kaira K, Sunaga N, Tomizawa Y, Yanagitani N, Shimizu K, Imai H, Utsugi M, Iwasaki Y, Iijima H, Tsurumaki H, Yoshii A. A phase II study of amrubicin, a synthetic 9-aminoanthracycline, in patients with previously treated lung cancer. Lung Cancer, 2010; 69(1): 99-104.
CrossRef - Monsuez JJ, Charniot JC, Vignat N, Artigou JY. Cardiac side-effects of cancer chemotherapy. Int. J. Cardiol., 2010; 144(1): 3-15.
CrossRef - Zou Y, Priebe W, Ling YH, Perez-Soler R. Organ distribution and tumor uptake of annamycin, a new anthracycline derivative with high affinity for lipid membranes, entrapped in multilamellar vesicles. Cancer Chemother. Pharmacol., 1993; 32(3): 190-196.
CrossRef - Wetzler M, Thomas DA, Wang ES, Shepard R, Ford LA, Heffner TL, Parekh S, Andreeff M, O’Brien S, Kantarjian HM. Phase I/II trial of nanomolecular liposomal annamycin in adult patients with relapsed/refractory acute lymphoblastic leukemia. Clin. Lymphoma Myeloma Leuk., 2013; 13(4): 430-434.
CrossRef - Trevino AV, Woynarowska BA, Herman TS, Priebe W, Woynarowski JM. Enhanced topoisomerase II targeting by annamycin and related 4-demethoxy anthracycline analogues. Mol. Cancer Ther. 2004; 3(11): 1403-1410.
CrossRef - Ketron AC, Denny WA, Graves DE, Osheroff N. Amsacrine as a topoisomerase II poison: importance of drug–DNA interactions. Biochem., 2012; 51(8): 1730-1739.
CrossRef - Jangir DK, Dey SK, Kundu S, Mehrotra R. Assessment of amsacrine binding with DNA using UV–visible, circular dichroism and Raman spectroscopic techniques. J. Photochem. Photobiol. B., 2012; 114: 38-43. CrossRef
- Pettengell R, Coiffier B, Narayanan G, de Mendoza FH, Digumarti R, Gomez H, Zinzani PL, Schiller G, Rizzieri D, Boland G, Cernohous P. Pixantrone dimaleate versus other chemotherapeutic agents as a single-agent salvage treatment in patients with relapsed or refractory aggressive non-Hodgkin lymphoma: a phase 3, multicentre, open-label, randomised trial. Lancet Oncol., 2012; 13(7): 696-706.
CrossRef - Cavalletti E, Crippa L, Mainardi P, Oggioni N, Cavagnoli R, Bellini O, Sala F. Pixantrone (BBR 2778) has reduced cardiotoxic potential in mice pretreated with doxorubicin: comparative studies against doxorubicin and mitoxantrone. Invest. New Drugs., 2007; 25(3): 187-195.
CrossRef - Petrylak DP, Tangen CM, Hussain MH, Lara Jr PN, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med., 2004; 351(15): 1513-1520.
CrossRef - Tannock IF, De Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med., 2004; 351(15): 1502-1512.
CrossRef - Unverferth DV, Unverferth BJ, Balcerzak SP, Bashore TA, Neidhart JA. Cardiac Evaluation of Mitoxantrone. Cancer Treat. Rep., 1983; 67(4): 343-350.
- Gambliel HA, Burke BE, Cusack BJ, Walsh GM, Zhang YL, Mushlin PS, Olson RD. Doxorubicin and C-13 deoxydoxorubicin effects on ryanodine receptor gene expression. Biochem Biophys Res Commun., 2002; 291(3): 433-438.
CrossRef - Braich T, Ahmann FR, Garewal HS, Robertone A, Salmon SE. Phase II trial of 4′-deoxydoxorubicin (esorubicin) in hormone-resistant prostate cancer. Invest. New Drugs., 1986; 4: 193-196.
CrossRef - Giaccone G, Donadio M, Calciati A. Phase II study of esorubicin in the treatment of patients with advanced sarcoma. Oncology., 1989; 46(5): 285-287.
CrossRef - Ringenberg QS, Propert KJ, Muss HB, Weiss RB, Schilsky RL, Modeas C, Perry MC, Norton L, Green M. Clinical cardiotoxicity of esorubicin (4′-deoxydoxorubicin, DxDx): Prospective studies with serial gated heart scans and reports of selected cases: A Cancer and Leukemia Group B report. Invest. New Drugs., 1990; 8: 221-226.
CrossRef - Marsoni S, Wittes R. Clinical development of anticancer agents- A National Cancer Institute perspective. Cancer Treat Rep., 1984; 68(1): 77-85.
- Linassier C, Barin C, Calais G, Letortorec S, Bremond JL, Delain M, Petit A, Georget MT, Cartron G, Raban N, Benboubker L. Early secondary acute myelogenous leukemia in breast cancer patients after treatment with mitoxantrone, cyclophosphamide, fluorouracil, and radiation therapy. Ann. Oncol., 2000; 11(10): 1289-1294.
CrossRef - Danson S, Ferry D, Alakhov V, Margison J, Kerr D, Jowle D, Brampton M, Halbert G, Ranson M. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br. J. Cancer., 2004; 90(11): 2085-91.
CrossRef - Alakhova DY, Zhao Y, Li S, Kabanov AV. Effect of doxorubicin/pluronic SP1049C on tumorigenicity, aggressiveness, DNA methylation and stem cell markers in murine leukemia. PloS one, 2013; 8(8): e72238.
CrossRef - Kantola J, Kunnari T, Hautala A, Hakala J, Ylihonko K, Mäntsälä P. Elucidation of anthracyclinone biosynthesis by stepwise cloning of genes for anthracyclines from three different streptomyces spp. Microbiol., 2000; 146(1): 155-163.
CrossRef - Lomovskaya N, Doi-Katayama Y, Filippini S, Nastro C, Fonstein L, Gallo M, Colombo AL, Hutchinson CR. The Streptomyces peucetius dpsY and dnrX genes govern early and late steps of daunorubicin and doxorubicin biosynthesis. J. Bacteriol., 1998; 180(9): 2379-2386.
- Scotti C, Hutchinson CR. Enhanced antibiotic production by manipulation of the Streptomyces peucetius dnrH and dnmT genes involved in doxorubicin (adriamycin) biosynthesis. J. Bacteriol., 1996; 178(24): 7316-7321.
CrossRef - Dickens ML, Priestley ND, Strohl WR. In vivo and in vitro bioconversion of epsilon-rhodomycinone glycoside to doxorubicin: functions of DauP, DauK, and DoxA. J. Bacteriol., 1997; 179(8): 2641-2650.
CrossRef - Jiang H, Hutchinson CR. Feedback regulation of doxorubicin biosynthesis in Streptomyces peucetius. Res. J. Microbiol., 2006; 157(7): 666-674.
CrossRef - Primeau AJ, Rendon A, Hedley D, Lilge L, Tannock IF. The distribution of the anticancer drug Doxorubicin in relation to blood vessels in solid tumors. Clin. Cancer Investig. J., 2005; 11(24): 8782-8788.
CrossRef - Duggan ST, Keating GM. Pegylated liposomal doxorubicin: a review of its use in metastatic breast cancer, ovarian cancer, multiple myeloma and AIDS-related Kaposi’s sarcoma. Drugs, 2011; 71: 2531-2558.
CrossRef - Gewirtz D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol., 1999; 57(7): 727-741.
CrossRef - Doroshow JH. Role of hydrogen peroxide and hydroxyl radical formation in the killing of Ehrlich tumor cells by anticancer quinones. Proc Natl Acad SCI journal., 1986; 83(12): 4514-4518.
CrossRef - Pawłowska J, Tarasiuk J, Wolf CR, Paine MJ, Borowski E. Differential ability of cytostatics from anthraquinone group to generate free radicals in three enzymatic systems: NADH dehydrogenase, NADPH cytochrome P450 reductase, and xanthine oxidase. Oncol. Res., 2003; 13(5): 245-252.
CrossRef - Fogli S, Nieri P, Cristina Breschi MA. The role of nitric oxide in anthracycline toxicity and prospects for pharmacologic prevention of cardiac damage. The FASEB Journal, 2004; 18(6): 664-675.
CrossRef - Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science, 1984; 226(4673): 466-468.
CrossRef - Oakman C, Moretti E, Galardi F, Santarpia L, Di Leo A. The role of topoisomerase IIα and HER-2 in predicting sensitivity to anthracyclines in breast cancer patients. Cancer Treat. Rev., 2009; 35(8): 662-667.
CrossRef - Khasraw M, Bell R, Dang C. Epirubicin: is it like doxorubicin in breast cancer? A clinical review. The Breast, 2012; 21(2): 142-149.
CrossRef - Yamaguchi N, Fujii T, Aoi S, Kozuch PS, Hortobagyi GN, Blum RH. Comparison of cardiac events associated with liposomal doxorubicin, epirubicin and doxorubicin in breast cancer: a Bayesian network meta-analysis. Eur. J. Cancer., 2015; 51(16): 2314-2320.
CrossRef - Mukai H, Kogawa T, Matsubara N, Naito Y, Sasaki M, Hosono A. A first-in-human Phase 1 study of epirubicin-conjugated polymer micelles (K-912/NC-6300) in patients with advanced or recurrent solid tumors. Invest. New Drugs., 2017; 35: 307-314.
CrossRef - Carvalho FS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, Oliveira PJ. Doxorubicin‐induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med. Res. Rev., 2014; 34(1): 106-135.
CrossRef - Goffart S, von Kleist-Retzow JC, Wiesner RJ. Regulation of mitochondrial proliferation in the heart: power-plant failure contributes to cardiac failure in hypertrophy. Cardiovasc. Res., 2004; 64(2): 198-207.
CrossRef - Henninger C, Fritz G. Statins in anthracycline-induced cardiotoxicity: Rac and Rho, and the heartbreakers. Cell Death Dis., 2018; 8(1): e2564.
CrossRef - Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology., 2010; 115(2): 155-162.
CrossRef - Creutzig U, Zimmermann M, Bourquin JP, Dworzak MN, Fleischhack G, Graf N, Klingebiel T, Kremens B, Lehrnbecher T, von Neuhoff C, Ritter J. Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: results from Study AML-BFM 2004. Blood, 2013; 122(1): 37-43.
CrossRef - Platel D, Pouna P, Bonoron-Adèle S, Robert J. Comparative cardiotoxicity of idarubicin and doxorubicin using the isolated perfused rat heart model. Anti-cancer drugs., 1999; 10(7): 671-676.
CrossRef - Hopp DC, Rabenstein J, Rhea J, Smith C, Romari K, Clarke M, Francis L, Irigoyen M, Milanowski D, Luche M, Carr GJ. Mutactimycin E, a new anthracycline antibiotic with gram-positive activity. J. Antibiot., 2008; 61(11): 675-679.
CrossRef - Eliopoulos GM, Eliopoulos GM, Roberts MC. Tetracycline therapy: update. Clin. Infect. Dis., 2003; 36(4): 462-467.
CrossRef - Mordente A, Meucci EL, Silvestrini A, Martorana GE, Giardina BR. New developments in anthracycline-induced cardiotoxicity. Curr. Med. Chem., 2009; 16(13): 1656-1672.
CrossRef - Goldberg K. CALGB chairman Schilsky to step down after 15 years leading cooperative group. Cancer Lett., 2008; 34: 1-4.
- Slørdal L, Spigset O. Heart failure induced by non-cardiac drugs. Drug Saf., 2006; 29: 567-586.
CrossRef - Berman E, McBride M. Comparative cellular pharmacology of daunorubicin and idarubicin in human multidrug-resistant leukemia cells. Blood, 1992; 79: 3267-3273.
CrossRef - Launchbury AP, Habboubi N. Epirubicin and doxorubicin: a comparison of their characteristics, therapeutic activity, and toxicity. Cancer treatment reviews., 1993; 19(3): 197-228.
CrossRef - Malla S, Niraula NP, Singh B, Liou K, Sohng JK. Limitations in doxorubicin production from Streptomyces peucetius. Microbiol. Res., 2010; 165(5): 427-435.
CrossRef - Niraula NP, Kim SH, Sohng JK, Kim ES. Biotechnological doxorubicin production: pathway and regulation engineering of strains for enhanced production. Appl. Microbiol. Biotechnol., 2010; 87: 1187-1194.
CrossRef - Parker KA, Kallmerten J. Efficient, regiospecific synthesis of anthracycline intermediates: total synthesis of daunomycin. J. Am. Chem. Soc., 1980; 102(18): 5881-5886.
CrossRef - Mendelsohn LD. ChemDraw 8 ultra, windows, and macintosh versions. J. Chem. Inf. Comput., 2004; 44(6): 2225-2226.
CrossRef - Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep., 2017; 7(1): 42717.
CrossRef - Daina A, Michielin O, Zoete V. iLOGP: a simple, robust, and efficient description of n-octanol/water partition coefficient for drug design using the GB/SA approach. J. Chem. Inf. Model., 2014; 54(12): 3284-3301.
CrossRef - Daina A, Zoete V. A boiled egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem., 2016; 11(11): 1117-1121.
CrossRef - Meredith AM, Dass CR. The increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. Journal of Pharmacy and Pharmacology., 2016; 68(6): 729-741.
CrossRef - Patel AG, Kaufmann SH. How does doxorubicin work?. Elife., 2012; 1: e00387.
CrossRef - Kauffmann-Guerrero D, Huber RM. Orphan drugs in development for the treatment of small-cell lung cancer: Emerging data on lurbinectedin. Lung Cancer (Auckl), 2020; 11: 27-31.
CrossRef - Metro G, Cappuzzo F. Emerging drugs for small-cell lung cancer. Expert Opin Emerg Drugs., 2009; 14(4): 591-606.
CrossRef - Feijen EA, Leisenring WM, Stratton KL, Ness KK, Van Der Pal HJ, Van Dalen EC, Armstrong GT, Aune GJ, Green DM, Hudson MM, Loonen J. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity. JAMA Oncol., 2019; 5(6): 864-871.
CrossRef - Feijen EA, Leisenring WM, Stratton KL, Ness KK, van der Pal HJ, Caron HN, Armstrong GT, Green DM, Hudson MM, Oeffinger KC, Robison LL. Equivalence ratio for daunorubicin to doxorubicin in relation to late heart failure in survivors of childhood cancer. J. Clin. Oncol., 2015; 33(32): 3774-3780.
CrossRef - Moebus V, Jackisch C, Lueck HJ, Du Bois A, Thomssen C, Kurbacher C, Kuhn W, Nitz U, Schneeweiss A, Huober J, Harbeck N. Intense dose-dense sequential chemotherapy with epirubicin, paclitaxel, and cyclophosphamide compared with conventionally scheduled chemotherapy in high-risk primary breast cancer: mature results of an AGO phase III study. J Clin Oncol., 2010; 28(17): 2874-2880.
CrossRef - Möbus V, Lück HJ, Ladda E, Klare P, Schmidt M, Schneeweiss A, Grischke EM, Wachsmann G, Forstbauer H, Untch M, Marmé F. Phase III randomised trial comparing intense dose-dense chemotherapy to tailored dose-dense chemotherapy in high-risk early breast cancer (GAIN-2). Eur. J. Cancer., 2021; 156: 138-148.
CrossRef - Bonilla L, Ben-Aharon I, Vidal L, Gafter-Gvili A, Leibovici L, Stemmer SM. Dose-dense chemotherapy in nonmetastatic breast cancer: a systematic review and meta-analysis of randomized controlled trials. J Natl Cancer Inst., 2010; 102(24): 1845-1854.
CrossRef - Favelier S, Boulin M, Hamza S, Cercueil JP, Cherblanc V, Lepage C, Hillon P, Chauffert B, Krausé D, Guiu B. Lipiodol trans-arterial chemoembolization of hepatocellular carcinoma with idarubicin: first experience. Cardiovasc Intervent Radiol., 2013; 36: 1039-46.
CrossRef - Dorr RT, Shipp NG, Lee KM. Comparison of cytotoxicity in heart cells and tumor cells exposed to DNA intercalating agents in vitro. Anti-cancer drugs., 1991; 2(1): 27-34.
CrossRef - Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J., 2016; 37(36): 2768-2801.
CrossRef - Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, Jones A. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC cancer, 2010; 10: 1-4.
CrossRef - Andersson BS, Eksborg S, Vidal RF, Sundberg M, Carlberg M. Anthraquinone-induced cell injury: acute toxicity of carminomycin, epirubicin, idarubicin and mitoxantrone in isolated cardiomyocytes. Toxicology, 1999; 135(1): 11-20.
CrossRef - Roth GS, Teyssier Y, Abousalihac M, Seigneurin A, Ghelfi J, Sengel C, Decaens T. Idarubicin vs doxorubicin in transarterial chemoembolization of intermediate stage hepatocellular carcinoma. World J Gastroenterol., 2020; 26(3): 324.
CrossRef - Salmon SE, Young L, Soehnlen B, Liu R. Antitumor activity of esorubicin in human tumor clonogenic assay with comparisons to doxorubicin. J. Clin. Oncol., 1984; 2(4): 282-286.
CrossRef - Wadler S, Fuks JZ, Wiernik PH. Phase I and II agents in cancer therapy: I. Anthracyclines and related compounds. J. Clin. Pharmacol., 1986; 26(7): 491-509.
CrossRef - Le Bot MA, Bégué JM, Kernaleguen D, Robert J, Ratanasavanh D, Airiau J, Riché C, Guillouzo A. Different cytotoxicity, and metabolism of doxorubicin, daunorubicin, epirubicin, esorubicin and idarubicin in cultured human and rat hepatocytes. Biochem. Pharmacol., 1988; 37(20): 3877-3887.
CrossRef - Yamaoka T, Hanada M, Ichii S, Morisada S, Noguchi T, Yanagi Y. Cytotoxicity of amrubicin, a novel 9‐aminoanthracycline, and its active metabolite amrubicinol on human tumor cells. Jpn. J. Cancer Res., 1998; 89(10): 1067-1073.
CrossRef - López-González A, Diz P, Gutierrez L, Almagro E, Palomo AG, Provencio M. The role of anthracyclines in small cell lung cancer. Ann. Transl. Med., 2013; 1(1): 5.
- Gupta S, Gouw L, Wright J, Chawla S, Pitt D, Wade M, Boucher K, Sharma S. Phase II study of amrubicin (SM-5887), a synthetic 9-aminoanthracycline, as first line treatment in patients with metastatic or unresectable soft tissue sarcoma: durable response in myxoid liposarcoma with TLS-CHOP translocation. Invest. New Drugs., 2016; 34: 243-252Booser DJ, Esteva FJ, Rivera E, Valero V, Esparza-Guerra L, Priebe W, Hortobagyi GN. Phase II study of liposomal annamycin in the treatment of doxorubicin-resistant breast cancer. Cancer Chemother. Pharmacol., 2002; 50: 6-8.
CrossRef - Shepard R. Liposomal annamycin-a new generation anthracycline that overcomes MDR and has No cardiac toxicity for the second line treatment of R/R AML. Clin. Lymphoma Myeloma Leuk., 2018; 18: S197-198.
CrossRef - Jelić S, Kovčin V, Milanović N, Kreačić M, Pendjer I, Jovanović V, Ristović Z, Oprić M, Mitrović L. Randomized study of zorubicin versus zorubicin-cisplatin in undifferentiated carcinoma of the nasopharynx (UCNT). Ann. Oncol., 1997; 8(8): 739-744.
CrossRef - Zagotto G, Gatto B, Moro S, Sissi C, Palumbo M. Anthracyclines: recent developments in their separation and quantitation. J Chromatogr B Biomed Sci Appl., 2001; 764(1-2): 161-171.
CrossRef - Wojtacki J, Lewicka-Nowak E, Lesniewski-Kmak K. Anthracycline-induced cardiotoxicity: clinical course, risk factors, pathogenesis, detection and prevention-review of the literature. Med. Sci. Monit., 2000; 6(2): 411-420.
- Frank N, Olson R, Walsh GM, Talley T, Cusack B. Effect of a non-cardiotoxic Doxorubicin analog, 13-deoxy, 5-imino doxorubicin on decatenation of DNA by Topoisomerase II. Cancer Res., 2013; 73(8_Supplement): 3428.
CrossRef - Weiss RB, Grillo-Lopez AJ, Marsoni S, Posada Jr JG, Hess F, Ross BJ. Amsacrine-associated cardiotoxicity: an analysis of 82 cases. J. Clin. Oncol., 1986; 4(6): 918-928.
CrossRef - Blasiak J, Gloc E, Drzewoski J, Wozniak K, Zadrozny M, Skórski T, Pertynski T. Free radical scavengers can differentially modulate the genotoxicity of amsacrine in normal and cancer cells. Mutat Res Genet Toxicol Environ Mutagen., 2003; 535(1): 25-34.
CrossRef - Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment, and prevention. Drug saf., 2000; 22: 263-302.
CrossRef - Thomas X, Le Q, Fiere D. Anthracycline-related toxicity requiring cardiac transplantation in long-term disease-free survivors with acute promyelocytic leukemia. Annals of hematology., 2002; 81: 504-507.
CrossRef - Temming P, Qureshi A, Hardt J, Leiper AD, Levitt G, Ancliff PJ, Webb DK. Prevalence and predictors of anthracycline cardiotoxicity in children treated for acute myeloid leukaemia: retrospective cohort study in a single centre in the United Kingdom. Pediatr Blood Cancer., 2011; 56(4): 625-630.
CrossRef - Hasinoff BB, Wu X, Patel D, Kanagasabai R, Karmahapatra S, Yalowich JC. Mechanisms of action and reduced cardiotoxicity of pixantrone; a topoisomerase II targeting agent with cellular selectivity for the topoisomerase IIα isoform. J Pharmacol Exp Ther., 2016; 356(2): 397-409.
CrossRef - Herbrecht R, Cernohous P, Engert A, Le Gouill S, Macdonald D, Machida C, Myint H, Saleh A, Singer J, Wilhelm M, van der Jagt R. Comparison of pixantrone-based regimen (CPOP-R) with doxorubicin-based therapy (CHOP-R) for treatment of diffuse large B-cell lymphoma. Ann. Oncol., 2013; 24(10): 2618-2623.
CrossRef - Damiani RM, Moura DJ, Viau CM, Caceres RA, Henriques JA, Saffi J. Pathways of cardiac toxicity: comparison between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch. Toxicol., 2016; 90: 2063-2076.
CrossRef - Henderson BM, Dougherty WJ, James VC, Tilley LP, Noble JF. Safety assessment of a new anticancer compound, mitoxantrone, in beagle dogs: comparison with doxorubicin. I. Clinical observations. Cancer Treat. Rep., 1982; 66(5):1139-1143.
- Herman EH, Zhang J, Rifai N, Lipshultz SE, Hasinoff BB, Chadwick DP, Knapton A, Chai J, Ferrans VJ. The use of serum levels of cardiac troponin T to compare the protective activity of dexrazoxane against doxorubicin-and mitoxantrone-induced cardiotoxicity. Cancer Chemother. Pharmacol., 2001; 48: 297-304.
CrossRef - Valle JW, Armstrong A, Newman C, Alakhov V, Pietrzynski G, Brewer J, Campbell S, Corrie P, Rowinsky EK, Ranson M. A phase 2 study of SP1049C, doxorubicin in P-glycoprotein-targeting pluronics, in patients with advanced adenocarcinoma of the esophagus and gastroesophageal junction. Invest. New Drugs., 2011; 29: 1029-1037.
CrossRef - Amin ML. P-glycoprotein inhibition for optimal drug delivery. Drug target insights., 2013; 7: DTI-S12519.
CrossRef








