Manuscript accepted on :10-09-2024
Published online on: 27-09-2024
Plagiarism Check: Yes
Reviewed by: Dr. Amit Panaskar
Second Review by: Dr. Anjaneyulu Vinukonda
Final Approval by: Dr. Eman Refaat Youness
Deepali Jagdale1* , Kaustubh Battellu1
, Kaustubh Battellu1 , Sampada Bhosale2
, Sampada Bhosale2 , Yashashree Sawant1
, Yashashree Sawant1 and Gayatri Nadar2
and Gayatri Nadar2
1Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Bharati Vidyapeeth College of Pharmacy, Sec-8, C.B.D. Belapur, Navi Mumbai, India.
2Department of Pharmacology, Faculty of Pharmacy, Bharati Vidyapeeth College of Pharmacy, Sec-8, C.B.D. Belapur, Navi Mumbai, India.
Corresponding Author E-mail:deepali.jagdale@bvcop.in
DOI : https://dx.doi.org/10.13005/bpj/2954
Abstract
Ferrocene is a remarkable organometallic compound with an iron ion sandwiched between two cyclopentadienyl rings. This unique molecular structure and diverse characteristics such as improved solubility, altered reactivity, and enhanced biological activity make them potential candidates for drug development and diverse applications including cancer therapy. Additionally, ferrocene-based compounds exhibit a lower tendency to induce severe side effects, making them a safer option for cancer treatment. They also have shown potential in overcoming resistance encountered by platinum compounds in treating certain types of cancer. The three primary metabolic pathways for ferrocene include oxidation, cyclization catalyzed by acid, and hydroxylation, forming quinone methide, cyclic indene, and allylic alcohol, respectively. Building on this foundation, researchers have delved deeper into synthesizing and assessing novel ferrocene derivatives to enhance their effectiveness in addressing cancer and other illnesses. This review comprehensively examines potential derivative reactions, highlighting the possibilities for tailoring these compounds to achieve specific therapeutic objectives.
Keywords
Cholesterol; Ferrocene; Ferrocene derivatives; Organometallics; Steroid
Download this article as:| Copy the following to cite this article: Jagdale D, Battellu K, Bhosale S, Sawant Y, Nadar G. Exploring the Versatility of Ferrocene and its Derivatives: A Comprehensive Review. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Jagdale D, Battellu K, Bhosale S, Sawant Y, Nadar G. Exploring the Versatility of Ferrocene and its Derivatives: A Comprehensive Review. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/3XBGdd4 |
Introduction
Organometallics are the metallic complexes of organic compounds. Organometallics have a greater diversity of stereochemistry than organic compounds, ranging from linear to octahedral and even beyond (30 stereoisomers exist for an octahedral complex with six different ligands). Kinetic properties of the organometallics can be controlled using rational ligand design. Additionally, their metal atoms have a low oxidation state, without any charges, are kinetically stable, and are highly lipophilic. Organometallic compounds offer abundant opportunities for creating innovative categories of pharmaceutical compounds, potentially showcasing distinctive mechanisms of action specific to metals. This arises from their fundamental distinctions compared to traditional coordination metal complexes1.
Organometallics employed for medical applications contain Fe, Ru, Co, Zr, Pt, Ti, V, Nb, and Mo. Among them, platinum compounds are most commonly employed. Despite enormous success, platinum compounds have two major side effects: they are ineffective against platinum-resistant tumors and have serious adverse effects, such as nephrotoxicity. The latter drawback arises from the medication’s focal point being DNA, a ubiquitous component in all cells. Furthermore, due to the particular chemical structure of platinum complexes, there are few opportunities for rational improvements that could increase its tumor specificity and lower undesirable side effects2. In contrast, ferrocene has fewer side effects and can be used to prevent platinum-resistant tumors. Historically, ferrocene’s therapeutic potential has been studied because it was the first organometallic substance for which anti-proliferative effects were noted2,3.
Ferrocene
Ferrocene (Figure 1) was first identified in 19514,5. Later, Wilkinson and co-workers, and Goermen and co-workers determined its precise structure6,7. Woodward and co-workers named these novel iron compounds ferrocene because of their similarity with benzene8. Modern organometallic chemistry was founded as a result of the elucidation of the ferrocene molecule’s structure, which was a significant discovery in the history of chemistry. Today, the words “sandwich compound” and “metallocene” describe a considerably wider variety of compounds containing various metals in addition to ferrocene and its derivatives9. Due to its intriguing chemistry, ferrocene immediately caught the interest of the scientific and technical world9,10. Without delay, chemists began to develop synthetic approaches that developed ferrocene derivatives and explored their uses in various scientific fields11. Ferrocene has several applications in materials science, including sensors12-21, catalysts19,22-27, electroactive materials28-33, and aerospace materials34-35, because of its advantageous electrical characteristics and ease of functionalization. Ferrocenes are well-liked molecules for biological applications due to their stability in aqueous and aerobic media and their variety of possible derivatives35-42.
There are ongoing studies on the uses of ferrocenes in pharmaceutical applications. Numerous studies have demonstrated that some ferrocene derivatives are highly effective both in vitro and in vivo against a variety of diseases, including bacterial and fungal infections43-44, malaria40, 45-47, human immunodeficiency virus (HIV) infection48, and cancer36-42. The anticancer efficacy of ferrocene compounds having amine or amide groups against lymphocytic leukemia P-388.48 led Brynes and coworkers49 to report the anticancer potential of ferrocene derivatives in the late 1970s. Since then, different ferrocene compounds have been synthesized and their anticancer abilities were evaluated. Ge X and coworkers50 studied ferrocene appended iridium (III) complexes for anticancer activity. Shen-Zhen Ren and coworkers synthesized and evaluated COX-2 inhibition activity of ferrocene-pyrazole derivatives51. Ferrocene-modified analogs such as ferrocene phenol hybrid52, Imatinib and Nilitinib53 have been studied for anticancer activity.
It was significant enough to demonstrate that adding a ferrocene group to the right carrier might increase an agent’s antitumor activity.
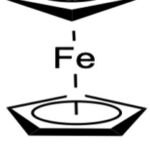 |
Figure 1: Ferrocene. |
Ferrocene Derivatives
Formyl Derivative of Ferrocene
Tang J54
synthesized Formyl ferrocene through the reaction of ferrocene, CH(OEt)3, and anhydrous
solvent
(anhydrous
AlCl3), followed by stirring to obtain the desired product.
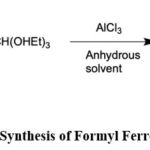 |
Scheme 1: Synthesis of Formyl Ferrocene |
Chalcone Derivative of Formyl Ferrocene
Song QB55 synthesized ferrocene chalcone derivatives (Scheme 2). To synthesize compound 3, formyl ferrocene underwent a reaction with bromoacetophenone (2). Subsequently, compound 3 was subjected to treatment with Pd (0) and ArB(OH)2, resulting in the formation of compounds 4a-4c. Various derivatives can be synthesized by using substituted Ar.
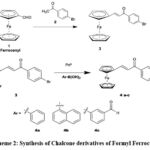 |
Scheme 2: Synthesis of Chalcone derivatives of Formyl Ferrocene |
Alternatively, acetyl ferrocene (5) was reacted with bromobenzaldehyde (6). After this, compound 7 was treated with Pd (0) and ArB(OH)2, forming compound 8. Various chalcone derivatives (Scheme 3) can be synthesized by substituting Ar in ArB(OH)2.
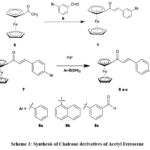 |
Scheme 3: Synthesis of Chalcone derivatives of Acetyl Ferrocene |
Furan Containing Derivatives of Ferrocene
Moynahan EB56 successfully produced Ferrocene derivatives incorporating a furan ring (Table 1). The condensation of ferrocene carboxy hydrazide with 5-nitro-2-furaldehyde and 5-nitro-2-acetylfuran led to compounds 9a and 9b, respectively. Ferrocene carboxy hydrazide was condensed with ethyl-5-nitro-2-furimidate hydrochloride, resulting in the synthesis of N-ferrocenecarboxamido-5-nitro-2-furamidine, designated as compound 10.
Acetylferrocene readily undergoes condensation with 2-furan aldehyde and 5-methyl-2-furaldehyde, forming compounds 11a and 11b, respectively. However, this reaction failed under various conditions with 5-nitro-2-furanaldehyde to yield 11c. Alternatively, ferrocene carboxaldehyde can be condensed with 2-acetylfuran and 2-acetyl-5-nitrofuran, producing compounds 12a and 12b respectively, which are isomeric to 11a and 11c.
The reaction of compounds 11a, 12a, and 12b with phenylhydrazine resulted in the formation of pyrazolines 13, 14a, and 14b, respectively55. However, when chalcone 12a was subjected to a reaction with phenylhydrazine at room temperature, a compound presumed to be 15 was obtained instead of the expected pyrazoline 14a.
 |
Table 1: Reactants for furan derivatives of ferrocene |
The reaction of hydrazine or acetyl hydrazine with 11a led to pyrazoline 16a or 16b formation. Reaction of isomeric chalcones 12a/12b with hydrazine led to pyrazoline 17a/17b56.
Cholesterol Derivative of Ferrocene
Cholesterol derivatives of ferrocene were synthesized by Lewkowski J and co-workers57 in 2004 and then by Váradi M, and Skoda-Földes R58 in 2022. α-(Ferrocenyl)-amino methane phosphonous acid derivatives (19a-19d) were synthesized by joining the steroid moiety and the ferrocene group with the help of amino phosphonous acid (Scheme 4).
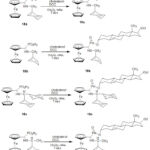 |
Scheme 4: Synthesis of Cholesterol derivatives of Ferrocene |
Diester Derivative of Ferrocene
1,1′-Bis-(chlorocarbonyl)ferrocene (20) derivatives were synthesized by Medina59. 20 after reaction with cholesterol in the presence of benzene and triethylamine, resulted in the formation of 1,1′-diester cholesterol derivative (21).
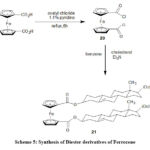 |
Scheme 5: Synthesis of Diester derivatives of Ferrocene |
Steroid Derivative of Ferrocene
Estradiol after esterification (22) with dicarboxylic acid (succinic acid) was converted to 17β- hemisuccinate (24). Cais M60 used amino methyl ferrocene (23) as a conjugating reagent to form an amide bond with the carboxylic acid.
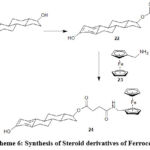 |
Scheme 6: Synthesis of Steroid derivatives of Ferrocene |
Steroid Derivative of Ferrocene with Glycine as Linker Group and with Two Cholesteryl Groups
Utilizing an N-protected glycine ester, product 25 was synthesized via the acylation of cholesterol. Subsequently, following the removal of the protecting group, the amino derivative 26 was linked to ferrocene derivatives 1-chlorocarbonyl ferrocene(27) and 1,1′-bis(chlorocarbonyl)ferrocene (20) through an amide bond, resulting in the formation of conjugates 28 and 29, respectively61.
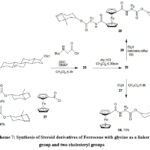 |
Scheme 7: Synthesis of Steroid derivatives of Ferrocene with glycine as a linker group and two cholesteryl groups |
Similarly, compound 32 containing two cholesteryl moieties was produced starting from 1,1′-bis(chlorocarbonyl)ferrocene 2062.
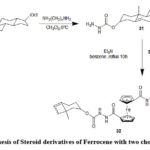 |
Scheme 8: Synthesis of Steroid derivatives of Ferrocene with two cholesteryl groups. |
1-chlorocarbonyl ferrocene (27) after reaction with diamino alkane gave 33, which after reaction with 30 yielded 3463.
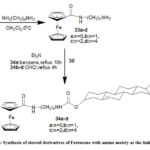 |
Scheme 9: Synthesis of steroid derivatives of Ferrocene with amino moiety as the linker group |
Conclusion
In conclusion, the synthesis and preparation of diverse ferrocene derivatives present a compelling avenue for advancing research in various fields, including materials science, catalysis, and medicinal chemistry. The versatility of ferrocene’s structural framework offers immense potential for tailoring properties to suit specific applications. Through innovative synthetic methodologies and strategic functionalization, researchers continue to expand the scope of ferrocene derivatives, unlocking novel properties and applications. As this manuscript highlights, the systematic exploration of ferrocene derivatives contributes significantly to advancing interdisciplinary research and holds promise for addressing complex challenges in diverse scientific domains. Further exploration and refinement of synthetic strategies will undoubtedly lead to the discovery of new ferrocene derivatives with enhanced properties and functionalities, driving progress in both fundamental understanding and practical applications.
Acknowledgement
The authors are grateful to the institute for the sources and facilities provided during the literature review.
Conflict of Interest
The authors do not have any conflict of interest.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
References
- Bhat F.A. Organometallic compounds of ruthenium and their anti-cancer properties. European J. Biomed. Pharm. Sci., 2014; 1(3): 600-604. https://www.ejbps.com/ejbps/abstract_id/2131
- Gasser G., Ott I. and Metzler-Nolte. Organometallic anticancer compounds. J. Med. Chem., 2011; 54(1): 3-25. https://doi.org/10.1021/jm100020w
CrossRef - Ornelas C. Application of ferrocene and its derivatives in cancer research. New J. Chem., 2011; 35(10): 1973-1985. https://doi.org/10.1039/C1NJ20172G
CrossRef - Kealy T.J. and Pauson P.L. A new type of organo-iron compound. Nature, 1951; 168(4285): 1039-1040. https://doi.org/10.1038/1681039b0
CrossRef - Miller S.A., Tebboth J.A. and Tremaine J.F. Dicyclopentadienyliron. J. Chem. Soc., 1952; 632: 632-635. https://doi.org/10.1039/JR9520000632
CrossRef - Wilkinson G., Rosenblum M., Whiting M.C. The structure of iron bis-cyclopentadienyl. J. Am. Chem. Soc., 1952; 74(8): 2125-2126. https://doi.org/10.1021/ja01128a527
CrossRef - Goermen M., Pigeon P., Top S. Synthesis, cytotoxicity, and COMPARE analysis of ferrocene and [3] ferrocenophane tetrasubstituted olefin derivatives against human cancer cells. ChemMedChem., 2010; 5(12): 2039-2050. https://doi.org/10.1002/cmdc.201000286
CrossRef - Woodward R.B., Rosenblum M. and Whiting M.C. A new aromatic system. J. Am. Chem. Soc., 1952; 74(13): 3458-3459. https://doi.org/10.1039/C1NJ20172G
CrossRef - Rosenblum M. Chemistry of the iron group metallocenes: Ferrocene, Ruthenocene, Osmocene. In: Chemistry, Geology. New York, Interscience Publishers, 1965; pp. 1.
- Han J.W., Tokunaga N., Hayashi T. Preparation of New Ferrocenylmonophosphine Ligands Containing Two Planar Chiral Ferrocenyl Moieties and Their Use for Palladium‐Catalyzed Asymmetric Hydrosilylation of 1, 3‐Dienes. Helvetica Chimica. Acta., 2002; 85(11): 3848-3854. https://doi.org/10.1002/1522-2675(200211)85:11<3848::AID-HLCA3848>3.0.CO;2-V
CrossRef - Labande A. and Astruc D. Colloids as redox sensors: Recognition of H2PO4− and HSO4− by amidoferrocenylalkylthiol–gold nanoparticles. Chem. Commun., 2000; 12: 1007-1008. https://doi.org/10.1039/B002578J
CrossRef - Labande A., Ruiz J. and Astruc D. Supramolecular gold nanoparticles for the redox recognition of oxoanions: Syntheses, titrations, stereoelectronic effects, and selectivity. J. Am. Chem. Soc., 2002; 124(8): 1782-1789. https://doi.org/10.1021/ja017015x
CrossRef - Daniel M.C., Ruiz J. and Astruc D. Supramolecular H-bonded assemblies of redox-active metallodendrimers and positive and unusual dendritic effects on the recognition of H2PO4. J. Am. Chem. Soc., 2003; 125(5): 1150-1151. https://doi.org/10.1021/ja020833k
CrossRef - Astruc D., Daniel M.C. and Ruiz J. Dendrimers and gold nanoparticles as exo-receptors sensing biologically important anions. Chem. Commun., 2004; 23: 2637-2649. https://doi.org/10.1039/B410399H
CrossRef - Armada M.P.G., Losada J., Zamora M. Electrocatalytical properties of polymethylferrocenyl dendrimers and their applications in biosensing. Bioelectrochemistry, 2006; 69(1): 65-73. https://doi.org/10.1016/j.bioelechem.2005.11.005
CrossRef - Ornelas C., Ruiz Aranzaes J., Cloutet E. Click assembly of 1, 2, 3‐triazole‐linked dendrimers, including ferrocenyl dendrimers, which sense both oxo anions and metal cations. Angew. Chem., 2007; 119(6): 890-895. https://doi.org/10.1002/ange.200602858
CrossRef - Astruc D., Ornelas C. and Ruiz Aranzaes J. Ferrocenyl-terminated dendrimers: design for applications in molecular electronics, molecular recognition and catalysis. J. Inorg. Organomet. Polym. Mater., 2008; 18: 4-17. https://doi.org/10.1007/s10904-007-9191-7
CrossRef - Ornelas C., Ruiz J. and Astruc D. Dendritic and ion-pairing effects in oxo-anion recognition by giant alkylferrocenyl dendrimers. Organometallics., 2009;28(15): 4431-4437. https://doi.org/10.1021/om900277u
CrossRef - Djeda R.., Rapakousiou A, Liang L. Click Syntheses of 1, 2, 3‐Triazolylbiferrocenyl Dendrimers and the Selective Roles of the Inner and Outer Ferrocenyl Groups in the Redox Recognition of ATP2− and Pd2+. Angew. Chem., 2010; 122(44): – 8328-8332. https://doi.org/10.1002/anie.201004756
CrossRef - Togni A. Ferrocene-Containing Charge-Transfer Complexes. Conducting and Magnetic Materials. 2008; John Wiley & Sons. http://dx.doi.org/10.1002/9783527615599.ch08
CrossRef - Wei, Vajtai R., Choi Y.Y. Structural characterizations of long single-walled carbon nanotube strands. Nano Lett., 2002; 2(10): .1105-1107. https://doi.org/10.1021/nl025719e
CrossRef - Zhang X., Cao A., Wei B. Rapid growth of well-aligned carbon nanotube arrays. Chem. Phys. Lett., 2002; 362(3-4): 285-290. https://doi.org/10.1016/S0009-2614(02)01025-4
CrossRef - Ornelas C., Salmon L., Aranzaes J.R. Catalytically efficient palladium nanoparticles stabilized by “click” ferrocenyl dendrimers. Chem. Commun., 2007; 46: 4946-4948. https://doi.org/10.1039/B710925C
CrossRef - Diallo A.K., Ornelas C., Salmon L. “Homeopathic” Catalytic Activity and Atom‐Leaching Mechanism in Miyaura–Suzuki Reactions under Ambient Conditions with Precise Dendrimer‐Stabilized Pd Nanoparticles. Angew. Chem., 2007; 119(45): 8798-8802. https://doi.org/10.1002/ange.200703067
CrossRef - Ornelas C., Aranzaes J.R., Salmon L. “Click” Dendrimers: Synthesis, Redox Sensing of Pd (OAc) 2, and Remarkable Catalytic Hydrogenation Activity of Precise Pd Nanoparticles Stabilized by 1, 2, 3‐Triazole‐Containing Dendrimers. Chem. Eur. J., 2008; 14(1): 50-64. https://doi.org/10.1002/chem.200701410
CrossRef - Daniel M.C., Sakamoto A., Ruiz J. Photoisomerization-induced Change in the Size of Ferrocenylazobenzene-attached Dendrimers. Chem. Lett., 2006; 35(1): 38-39. https://doi.org/10.1246/cl.2006.38
CrossRef - Astruc D., Ornelas C. and Ruiz J. Metallocenyl dendrimers and their applications in molecular electronics, sensing, and catalysis. Acc. Chem. Res., 2008; 41(07): 841-856. https://doi.org/10.1021/ar8000074
CrossRef - Ornelas C., Ruiz J., Astruc D. Giant dendritic molecular electrochrome batteries with ferrocenyl and pentamethylferrocenyl termini. J. Am. Chem. Soc., 2009; 131(2): 590-601. https://doi.org/10.1021/ja8062343
CrossRef - Wang A., Ornelas C., Astruc D. Electronic communication between immobilized ferrocenyl-terminated dendrimers. J. Am. Chem. Soc., 2009; 131(19): 6652-6653. https://doi.org/10.1021/ja900645j
CrossRef - Astruc D., Ornelas C. and Ruiz J. Dendritic Molecular Electrochromic Batteries Based on Redox‐Robust Metallocenes. Chem. Eur. J., 2009; 15(36): 8936-8944. https://doi.org/10.1002/chem.200901294
CrossRef - Megiatto Jr J.D., Li K., Schuster D.I. Convergent synthesis and photoinduced processes in multi-chromophoric rotaxanes. J. Phys. Chem. B., 2010; 114(45): 14408-14419. https://doi.org/10.1021/jp101154k
CrossRef - Neuse E.W. and Rosenberg H. Metallocene polymers. J. Macromol. Sci. A., 1970; 4(1): 1-145. https://doi.org/10.1080/15321797008080022
CrossRef - Neuse E.W., Woodhouse J.R., Montaudo G. An iron–organic polymeric smoke suppressant for poly (vinyl chloride). Appl. Organomet. Chem., 1988; 2(1): 53-57. https://doi.org/10.1002/aoc.590020107
CrossRef - Swarts J.C., Neuse E.W. and Lamprecht G.J. Synthesis and characterization of water-soluble polyaspartamide-ferrocene conjugates for biomedical applications. J. Inorg. Organomet. Polym. Mater., 1994; 4: 143-153. https://doi.org/10.1007/BF01036539
CrossRef - Van Staveren D.R. and Metzler-Nolte N. Bioorganometallic chemistry of ferrocene. Chem. Rev., 2004; 104(12): 5931-5986. https://doi.org/10.1021/cr0101510
CrossRef - Allardyce C.S., Dorcier A., Scolaro C. Development of organometallic (organo‐transition metal) pharmaceuticals. Appl. Organomet. Chem., 2005; 19(1): 1-10. https://doi.org/10.1002/aoc.725
CrossRef - Neuse E.W. Macromolecular ferrocene compounds as cancer drug models. J. Inorg. Organomet. Polym. Mater., 2005; 15: 3-31. https://doi.org/10.1007/s10904-004-2371-9
CrossRef - Fouda M.F., Abd‐Elzaher M.M., Abdelsamaia R.A. and et al. On the medicinal chemistry of ferrocene. Appl. Organomet. Chem., 2007; 21(8): 613-625. https://doi.org/10.1002/aoc.1202
CrossRef - Nguyen A., Vessières A., Hillard EA. Ferrocifens and ferrocifenols as new potential weapons against breast cancer. Chimia, 2007; 61(11): 716-716. https://doi.org/10.2533/chimia.2007.716
CrossRef - Hillard E.A., Vessieres A. and Jaouen G. Ferrocene functionalized endocrine modulators as anticancer agents. Medicinal Organometallic Chemistry, 2010; 81-117. https://doi.org/10.1007/978-3-642-13185-1_4
CrossRef - Biot C., François N., Maciejewski L. Synthesis and antifungal activity of a ferrocene–fluconazole analogue. Bioorganic Med. Chem. Lett., 2000; 10(8): 839-841. https://doi.org/10.1016/S0960-894X(00)00120-7
CrossRef - Zhang J. Preparation, characterization, crystal structure and bioactivity determination of ferrocenyl–thiazoleacylhydrazones. Appl. Organomet. Chem., 2008; 22(1): 6-11. https://doi.org/10.1002/aoc.1338
CrossRef - Biot C., Glorian G., Maciejewski L.A. Synthesis and antimalarial activity in vitro and in vivo of a new ferrocene− chloroquine analogue. J. Med. Chem., 1997; 40(23): 3715-3718. https://doi.org/10.1021/jm970401y
CrossRef - Delhaes L., Biot C., Berry L. Novel ferrocenic artemisinin derivatives: synthesis, in vitro antimalarial activity and affinity of binding with ferroprotoporphyrin IX. Bioorg. Med. Chem., 2000; 8(12): 2739-2745. https://doi.org/10.1016/S0968-0896(00)00206-6
CrossRef - Itoh T., Shirakami S., Ishida N. Synthesis of novel ferrocenyl sugars and their antimalarial activities. Bioorg. Med. Chem. Lett., 2000; 10(15): 1657-1659. https://doi.org/10.1016/S0960-894X(00)00313-9
CrossRef - Kondapi A.K., Satyanarayana N. and Saikrishna A.D. A study of the topoisomerase II activity in HIV-1 replication using the ferrocene derivatives as probes. Arch. Biochem. Biophys., 2006; 450(2): 123-132. https://doi.org/10.1016/j.abb.2006.04.003
CrossRef - Fiorina V.J., Dubois R.J. and Brynes S. Ferrocenyl polyamines as agents for the chemoimmunotherapy of cancer. J. Med. Chem., 1978; 21(4): 393-395. https://doi.org/10.1021/jm00202a016
CrossRef - Ge X., Chen S., Liu X. Ferrocene-Appended Iridium(III) Complexes: Configuration Regulation, Anticancer Application, and Mechanism Research. Inorg. Chem. 2019; 58(20):14175-14184. doi:10.1021/acs.inorgchem.9b02227
CrossRef - Ren S.Z., Wang Z.C., Zhu D. Design, synthesis and biological evaluation of novel ferrocene-pyrazole derivatives containing nitric oxide donors as COX-2 inhibitors for cancer therapy. Eur. J. Med. Chem. 2018; 157: 909-924. doi:10.1016/j.ejmech.2018.08.048
CrossRef - Wang R., Chen H., Yan W. Ferrocene-containing hybrids as potential anticancer agents: Current developments, mechanisms of action and structure-activity relationships. Eur. J. Med. Chem. 2020; 190: 112109. doi:10.1016/j.ejmech.2020.112109
CrossRef - Philipova I., Mihaylova R., Momekov G. Ferrocene modified analogues of imatinib and nilotinib as potent anti-cancer agents. RSC Med. Chem. 2023; 14(5): 880-889. doi:10.1039/d3md00030c
CrossRef - Tang J., Liu X.F., Zhang L.Y. A new convenient method for the synthesis of formyl ferrocene with triethyl orthoformate and A1C13. Synth. Commun., 2000; 30(9): 1657-1660. DOI: 10.1080/00397910008087203
CrossRef - Song Q.B., Li X.N., Shen T.H. Synthesis of novel chalcone analogues of ferrocene biarenes. Synth. Commun., 2003; 33(22): 3935-3941. https://doi.org/10.1081/SCC-120026317
CrossRef - Moynahan E.B., Popp F.D. and Werneke M.F. Ferrocene studies III. Platinum and palladium complexes of [(dimethylamino) methyl] ferrocene. J. Organomet. Chem., 1969; 19(1): 229-232. https://doi.org/10.1002/jhet.5570070216
CrossRef - Lewkowski J., Rzeźniczak M. and Skowroński R. α-(Ferrocenyl)-aminomethanephosphonous acids. First synthesis and preparation of their esters with cholesterol and adenosine. J. Organomet. Chem., 2004; 689(9): 1684-1690. https://doi.org/10.1016/ j.jorganchem.2004.02.025
CrossRef - Váradi M. and Skoda-Földes R. Synthetic methods towards steroid-ferrocene conjugates. ARKIVOC: Online J. Organomet. Chem., 2022; https://doi.org/10.24820/ark.5550190.p011.885
CrossRef - Medina J.C., Gay Z., Chen Z. Redox-switched molecular aggregates: the first example of vesicle formation from hydrophobic ferrocene derivatives. J. Am. Chem. Soc., 1991; 113(1): 365-366. https://doi.org/10.1021/ja00001a056
CrossRef - Cais M., Dani S., Eden Y. Metalloimmunoassay. Nature, 1977; 270(5637): 534-535. https://doi.org/10.1038/270534a0
CrossRef - Liu J., Yan J., Yuan X. A novel low-molecular-mass gelator with a redox active ferrocenyl group: Tuning gel formation by oxidation. J. Colloid Interface Sci., 2008; 318(2): 397-404. https://doi.org/10.1016/j.jcis.2007.10.005
CrossRef - Yan J., Liu J., Lei H. Ferrocene-containing thixotropic molecular gels: Creation and a novel strategy for water purification. J. Colloid Interface Sci., 2015; 448: 374-379. https://doi.org/10.1016/j.jcis.2015.02.044
CrossRef - Liu J., He P., Yan J. An organometallic super‐gelator with multiple‐stimulus responsive properties. Advanced Materials, 2008; 20(13): 2508-2511. https://doi.org/10.1002/adma.200703195
CrossRef







