Manuscript accepted on :15-03-2024
Published online on: 03-06-2024
Plagiarism Check: Yes
Reviewed by: Dr. Bhavana Gundavarapu
Second Review by: Dr. Ajit Kumar
Final Approval by: Dr. Prabhishek Singh
Pravinkumar Darji1* , Jayendrakumar Patel1
, Jayendrakumar Patel1 , Binit Patel2
, Binit Patel2  , Shalin Parikh3
, Shalin Parikh3 , Praneeth Ivan Joel Fnu1
, Praneeth Ivan Joel Fnu1 and Seshadri Nalla4
and Seshadri Nalla4
1Exemplify Biopharma Inc., Cranbury, New Jersey, USA.
2Hovione, East Windsor, New Jersey, USA.
3Shree SK Patel College of Pharmaceutical Education and Research, Ganpat University, Mehsana, Gujarat, India.
4Lamar University, S M king Jr PKWY, Beaumont, Texas, USA.
Corresponding Author E-mail:palo378@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2904
Abstract
Chronic pain occurs as a result of several diseases and ailments. The problem of improper utilization of vital opioid medication has been a topic of substantial discourse during the last two decades, in conjunction with its application for the extended-term control of persistent pain. Abuse-deterrent formulations play a crucial role in comprehensive methods to manage the risks associated with opioids. These formulations diminish the allure and narcotic properties of opioids by restricting their capacity to be assimilated by the body. This diminishes the appeal and incentives for misusing altered opioid prescriptions, and also poses challenges in extracting the opioid substance for utilization in alternative manners. This article examines various regulatory measures, projected prerequisites for the licensing of abuse-deterrent formulations, and current activities aimed at producing opioid abuse-deterrent formulations as potential remedies to combat the opioid abuse pandemic. Considering the seriousness of the global opioid problem, it is crucial for various regulatory entities to come together to safeguard society from the opioid pandemic. This involves implementing a thorough policy on prescribing opioid medications to patients, conducting evaluations to determine the likelihood of addiction, and increasing efforts to approve only opioid drugs that are specifically tailored to prevent abuse.
Keywords
Abuse; Abuse Deterrent Technology; Opioid; Overdose; Regulatory
Download this article as:| Copy the following to cite this article: Darji P, Patel J, Patel B, Parikh S, Fnu P. I. J, Nalla S. Review on Recent Development in Opioid Abuse-Deterrent Formulation Technologies and Regulatory Expectation. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Darji P, Patel J, Patel B, Parikh S, Fnu P. I. J, Nalla S. Review on Recent Development in Opioid Abuse-Deterrent Formulation Technologies and Regulatory Expectation. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/3Vf5fxE |
Introduction
Chronic pain disorders afflict a larger proportion of the global population, around 20-30%, than heart disease, cancer, and diabetes combined. Figure 1 presents a comprehensive depiction of the worldwide population’s susceptibility to chronic pain. Over the past twenty years, extensive research in pain therapy has resulted in the identification of revolutionary opioid medicines that effectively treat chronic pain and other therapeutic disorders. Prescription opioid analgesics are the mainstay of pharmacological pain control and can be administered in various dosage forms and through numerous new approaches.1,2
 |
Figure 1: Overview of Chronic Pain Impact on Global Population1 |
Chronic pain refers to a persistent medical condition that lasts for a prolonged period of time, ranging from weeks to months or even years. It is a consequence of several diseases and disorders, such as cancer, operations, and others. The issue of misusing essential opioid medicine has been a subject of extensive discussion over the past twenty years, alongside its use for long-term management of chronic pain. This discussion was initiated due to concerns regarding the potential adverse impacts of opioids, a lack of comprehensive information on their long-term consequences, and the risk of their improper use and abuse. Specifically, the expansion of the opioid market has resulted in escalating health issues and significant socio-economic challenges worldwide. Drug misuse erodes the fundamental elements of society, resulting in mortality, mistreatment of children, sexual and domestic aggression, heightened criminal activity, and a dearth of tranquilly and safety for women and children [Figure 2].
 |
Figure 2: Societal Impact of Drug Abuse Crisis on Society1 |
The Extent of the Problem
An appreciable surge in the prevalence of individuals misusing opioid prescription medications and succumbing to fatal overdoses has been noted. The list Between 1997 and 2007, the average dosage of prescription opioids consumed by persons in the United States increased by 402%, going up from 74mg to 369mg. In 2009, retail pharmacies filled 257 million opioid prescriptions, which is a 48% increase compared to the 174 million prescriptions filled in 2000. Surveys undertaken at the national level over the past decade have revealed that the misuse of prescribed opioid formulations has exceeded the misuse of heroin and cocaine. This indicates a significant rise in opioid misuse over the same period. The given text is a list containing the elements 5 and 6. Between 2002 and 2012, there was a more than fourfold increase in hospital admissions related to opioid prescriptions. Similarly, between 2000 and 2014, the number of deaths caused by overdoses of these medications climbed by about fourfold. Over ninety individuals in the United States perish everyday as a result of opioid overdoses. 1,2,3,4,5,6,7,8
Worldwide, an estimated 33 million individuals, constituting roughly 0.7% of the global adult population, engage in the misuse of opioids, either through prescribed use or without a valid prescription. In 2014, over 4.30 million individuals aged 12 years or older in the USA engaged in the nonmedical use of prescription pain drugs, accounting for roughly 1.6% of the overall population. Following marijuana, the opioid analgesic that was prescribed was the most commonly abused. While the misuse of opioid formulations obtained through a doctor’s prescription is mostly observed in the USA, it is also recognized as a significant issue in other countries of the world, such as Europe, Canada, India, Australia, and Japan. In Ontario, Canada, during the 2010-2011 school year, 15.5% of secondary school students and 6% of the adult population reported using opioid analgesics prescribed by a doctor for non-medicinal purposes. Approximately 7.7% of individuals polled in Australia acknowledged non-medical use of opioid analgesics at some point in their lives, deviating from the prescribed usage by a doctor. In addition, regulatory bodies such as the European Medicines Agency and the European Monitoring Centre on Drugs and Drug misuse specifically prioritize monitoring rates of misuse related to heroin, rather than abuse rates of prescribed opioid analgesics. It is acknowledged that there is limited evidence available regarding the misuse of prescribed opioid analgesics in the European region. However, the latest data suggest that the recreational consumption of opioid analgesics acquired with a medical prescription is increasingly worrisome in this area. According to reports, 2.4% of Japanese individuals have engaged in non-medical use of opioid analgesic prescription medications at some point in their lives . The estimated yearly societal cost of abuse, misuse, and diversion of prescribed opioid analgesics in the United States is between $55.7 billion and $72.5 billion.9,10,11,12,13,14,15,16,17,18
Hence, regulatory organizations and pharmaceutical industries face a significant task in addressing the opioid misuse issue. This article discusses several regulatory measures, expectations for the approval of abuse-deterrent formulations (ADF), and developing tactics for opioid abuse-deterrent formulations as viable solutions to address the opioid abuse issue.
Potential Approach of the Durg Abuse
Figure 3 illustrates the various mechanisms by which drugs are misused, including oral, intranasal, intravenous intake, and additional routes, such as rectal administration.
The most straightforward and prevalent way of drug addiction involves ingesting many pills simultaneously through oral administration. In order to achieve the ‘dose-dumping’ effect of the extended-release medication, individuals who abuse it typically crush and ingest the extended-release (ER) version, leading to a rapid onset of intense euphoria. This is achieved by maximizing the concentration of the opioid in the brain’s reward circuit as quickly as possible, resulting in a higher maximum concentration (Cmax) in a shorter amount of time (Tmax). Substance abuse can occur through various methods, such as crushing and consuming a larger amount than prescribed, inhaling the drug through smoking or snorting, or injecting it directly into the muscles, veins, or under the skin after extracting it from its original form. Manipulation methods include grinding or crushing the entire dosage form into minute particles or a powder, dissolving it in a solvent (such as alcohol or water), and extracting the medication by exposing it to hot or cold temperatures. According to multiple sources, the primary method of misusing prescription opioid painkillers is through oral consumption. This is followed by inhalation (smoking or snorting), ingestion through the mouth (either in its original form or after being altered by chewing, crushing, or dissolving), and finally, injection. Nevertheless, the manner in which prescription opioid analgesic formulas are abused varies significantly. For instance, the chosen manner of abuse is likely influenced by the extent to which each abuser experiences a desirable or undesirable impact from a specific opioid formulation. This can proceed in any direction. Due to the increased concentration of opioids in extended-release (ER) formulations compared to immediate-release (IR) formulations, these medications are more attractive to persons who engage in substance abuse. The method of substance usage that is most strongly correlated with elevated morbidity rates is the act of injecting and breathing the substance.19,20,21,22,23,24
 |
Figure 3: Potential Approaches of The Drug Abuse1 |
Regulatory Action
In July 2012, the US Food and Drug Administration (US FDA) implemented a new Risk Evaluation and Mitigation Strategy (REMS) for long-acting (LA) and extended-release (ER) opioid formulations. This was done because these formulations have a higher risk of being misused compared to short-acting opioid formulations (immediate release). The LA and ER formulations contain larger amounts of the drug in each dose, making them more dangerous when abused or misused compared to the shorter-acting formulations. This action was taken in response to the escalating issue of opioid misuse and abuse in the United States. It was implemented as part of a 2011 initiative by the Obama administration, which aimed to address the national crisis of prescription opioid abuse. REMS is a risk management method that focuses on monitoring beyond the typical drug prescribing information in order to address structural risks. The main objective of the program is to ensure that patients who genuinely require opioid medicine can obtain access to these opioids (extended-release and long-acting), while simultaneously providing education to healthcare providers and patients regarding the appropriate and safe utilization of opioids classified as extended-release and long-acting. Manufacturers are responsible for the creation of instructional programs and materials aimed at all prescribers registered with the DEA (Drug Enforcement Administration).27,28
The US FDA modified safety labeling as a component of its continuous endeavors. The revised labeling will incorporate the updated indication that highlights the use of LA/ER opioids exclusively in patients with sufficiently severe pain necessitating continuous, long-term opioid drug treatment, when alternative therapies are insufficient. This update will be incorporated as a component of the labeling modifications. Furthermore, the US Food and Drug Administration (US FDA) has recently issued a requirement for a new boxed warning to be included on all long-acting/extended-release opioid pain relievers. This warning is intended to inform consumers that extended use of these medicines by pregnant women can result in neonatal opioid withdrawal syndrome (NOWS).28
Abuse-Deterrent Formulation Methods
The process of creating a novel drug abuse-deterrent formulation (ADF) is akin to the development of a new opioid chemical entity. The main objectives of creating novel Abuse-Deterrent Formulations (ADF) of opioids are to produce opioid drugs that are both therapeutically safe and efficacious in treating the targeted therapeutic condition within the intended population. Moreover, it does not inflict any significant damage on any potential addict, and it is crucial for an opioid drug to be cost-effective. Generally, Abuse-Deterrent Formulations (ADFs) are modified versions of opioid drugs designed to reduce the appeal and rewarding effects of the drug. This is achieved by limiting the amount of drug that can be absorbed by the body, making it less attractive to abuse or tamper with. ADFs also make it difficult to extract the opioid drug substance, thereby preventing alternative methods of administration.30,31 ADFs diminish the allure or drug-liking characteristics of drugs, therefore restricting one or more types of drug abuse by.
Impeding the extraction of opioid substances,
Impeding administration via alternative routes,
Retard the bioavailability of the opioid, thereby reducing the euphoric effect, and
Making abuse of the manipulated opioid formulation less attractive or rewarding.
As shown in Figure 4, the ADFs product can be formulated using any of the following types of drug abuse-deterrent methods.
 |
Figure 4: Methods to Fabricate Abuse-Deterrent Formulations1 |
Implementing Physical Barrier
Integrating physical barriers inside the ADFs would effectively prevent tampering with the opioid formulation, hence prohibiting actions such as crushing, chewing, grinding, or extracting the medication. Polyethylene oxide (PEO) is the most frequently employed polymer for providing a physical barrier that effectively prevents tampering with the dosage form. The tamper-resistant characteristic is attained by subjecting the dosage form (such as a tablet) containing PEO to a high temperature, specifically above 75°C (which is higher than the polymer’s melting point), for a minimum duration of 60 minutes. Table 1 provides a concise overview of abuse-deterrent formulations that are designed to prevent misuse, focusing on those that utilize a physical barrier.[32,33,34]
Table 1: Marketed ADF Formulation Based on Physical Barrier
|
Product |
Technology |
Characteristics |
FDA Approval |
|
OxyContin® (Oxycodone HCl Extended-Release Tablets) |
Fabricated using proprietary thermal processing of high-molecular-weight polyethylene oxide (PEO): Processing Steps: Compression – Coating – Curing at 75°C for at least 60 minutes |
· It resists crushing, grinding, and chewing of the dosage form.
· When attempted to dissolve with a small amount of water, the manipulated product will form a highly viscous hydrogel that will be difficult to inject IV. |
2010 |
|
Hysingla ER® (Hydrocodone) Extended-Release Tablets |
2014 |
||
|
OPANA ER (Oxymorphone) Extended-Release Tablets |
2011 |
||
|
NUCYNTA® (Tapentadol HCl) Extended-Release Tablets |
Fabricated using a proprietary thermal manufacturing process (Hot Melt Extrusion) using high-molecular-weight polyethylene oxide (PEO): Processing Steps: Hot Melt Extrusion of a mixture of API with PEO at > 75°C – Cutting of Extrude – Shaping of extrude to form dosage form |
2011 |
|
|
Arymo ER® (Morphine Sulfate) Extended-Release Tablets |
2017 |
Implementing Chemical Barriers
By introducing chemical barriers into ADFs, the extraction of pure opioid compounds from the dosage form using commonly accessible solvents like water, ethanol, or other organic solvents and chemicals would be restricted. The primary materials commonly employed to withstand extraction are high viscosity water soluble but alcohol-insoluble polymers, such as PEO, water and alcohol-insoluble compounds like fatty acids and waxes, or chemicals with wax-like properties, as well as ion-exchange resin complexed with medicinal molecules. Table 2 provides a summary of abuse-deterrent formulations that are currently being marketed, which are designed to prevent misuse through the application of a chemical barrier.1,32,35
Table 2: Marketed ADF Formulation Based on Chemical Barrier
|
Product |
Technology |
Characteristics |
FDA Approval |
|
XTAMPZA ER® (Oxycodone HCl) Extended-Release Capsule |
Wax microsphere containing yellow beeswax, myristic acid, carnauba wax, magnesium stearate, stearoyl polyoxyl-32 glycerides |
· It limits the extraction of pure drug substances from the dosage form using conventional solvents readily available to abusers, such as water, alcohol such as ethanol, or other organic solvents and chemicals. · It also resists crushing, grinding, and chewing of the dosage form. |
2016 |
|
Remoxy® (Oxycodone HCl) Extended-Release Capsule |
A highly viscous gelatine matrix comprising fully esterified sucrose derivative sucrose acetate isobutyrate is water insoluble and highly hydrophobic. |
Not Approved |
|
|
OxyContin® (Oxycodone HCl) Extended-Release Tablets |
A tablet comprising highly viscous water soluble but alcohol insoluble polymer Polyethylene Oxide. |
2010 |
|
|
Hysingla ER® (Hydrocodone) Extended-Release Tablets |
2014 |
||
|
OPANA ER (Oxymorphone) Extended-Release Tablets |
2011 |
||
|
NUCYNTA® (Tapentadol HCl) Extended-Release Tablets |
2011 |
||
|
Arymo ER® (Morphine Sulfate) Extended-Release Tablets |
2017 |
Integration of Aversion Agent
By including aversion chemicals in the ADFs, the tampered opioid medications will cause an unpleasant reaction in individuals who abuse them, therefore decreasing the probability of abuse. For example, the presence of sodium lauryl sulfate and docusate sodium reduces the likelihood of nasal abuse by causing nasal irritation when crushed tablet particles are inhaled through snorting or sniffing. This irritation can result in symptoms such as tearing, nasal congestion, dryness, throat irritation, and excessive nasal discharge, which effectively discourages the abuse of drugs through the nasal route. Table 3 provides a summary of abuse-deterrent formulations that are marketed and rely on aversion agents.39
Table 3: Marketed ADF Formulation Based on Aversion Agent
|
Product |
Technology |
Characteristics |
FDA Approval |
|
OXAYDO® (Oxycodone HCl Immediate Release Tablet) |
Based on Aversion technology, it includes sodium lauryl sulfate and high-viscosity PEO in small concentrations. |
· It limits nasal insufflation. · It also limits syringeability using a small quantity of water as it forms a highly viscous mixture if tried to extract or add solvent. |
2012 |
Delivery System
Opioid formulations can be developed in innovative formats that discourage misuse, such as depot injectable formulations and subcutaneous implants. Manipulating these drug delivery systems can be hard because to their deliberate design for gradual release of opioids over a period of time. This unconventional technique of delivering medication is difficult to regulate once it has been delivered into the body only by medical experts. A significant benefit of this distribution system is its restricted availability to patients for home usage; it necessitates in-person deposit only by medical personnel. Currently, there is no officially approved ADF formulation that utilizes this delivery mechanism.1,31
Agonist / Antagonist Integration
Opioid agonists and opioid antagonists engage in competitive binding to the opioid receptor. Due to its high affinity for binding to the opioid receptor, the opioid antagonist will take precedence in binding to the receptor over the opioid agonist if both substances are released simultaneously. Consequently, opioid formulations could incorporate an opioid antagonist that is inactive and cannot be released, so that the antagonist only becomes clinically effective when the abuser tries to manipulate the opioid dosage form. Table 4 provides a summary of abuse-deterrent formulations that are commercialized and are based on a combination of agonists and antagonists.36,37,38
Table 4: Marketed ADF Formulation Based on Agonist / Antagonist Integration
|
Product |
Technology |
Characteristics |
FDA Approval |
|
EMBEDA® (Morphine Sulfate / Naltrexone HCl) Extended-Release Capsule |
Opioid agonist pellets are surrounded with sequestered naltrexone, which will release only upon tampering with the dosage form. |
· If the dosage form is chewed, crushed, or otherwise altered, the orally bioavailable naltrexone will be released, reducing the euphoria expected from an opioid agonist. · It limits tampering of dosage form for administration via altered routes such as parenterally. |
2009 |
|
TROXYCA® (Oxycodone HCl / Naltrexone HCl) Extended-Release Capsule |
2016 |
||
|
SUBOXONE® (Buprenorphine / Naloxone) Capsule |
Due to significant first-pass hepatic metabolism, the oral bioavailability of naloxone is extremely low, resulting in a negligible effect when taken orally as prescribed. However, it becomes active only if the dosage form is tampered with for administration via an altered route, such as parenterally. |
· It limits tampering of dosage form for administration via altered routes such as parenterally. |
2003 |
|
Targiniq® (Oxycodone / NaloxoneHCl) Extended-Release Capsule |
2014 |
Prodrug
Prodrugs are inert compounds that can undergo in vivo metabolism to generate the pharmacologically active form of the drug. Typically, this can be accomplished by hydrolyzing a group composed of either an amide or an ester. Prodrugs can be classified into two primary categories: (1) type I, where the biotransformation occurs intracellularly, and (2) type II, where it occurs extracellularly. Additional subgroups can be identified based on the specific extracellular location. For instance, the gastrointestinal (GI) tract is categorized as Type IIA. If a drug formulation is required to be in the gastrointestinal tract in order to become active, then ideally, this should decrease the incidence of misuse when the intranasal or intravenous routes are utilized. While the issue of opioid abuse through multiple doses is not explicitly discussed, the rate at which the gastrointestinal system transforms the drug will be the limiting factor. This will result in a reduced increase in the proportion of the drug that is available for absorption. This is because the enzymes in the gastrointestinal system become overwhelmed when a large dose of opioids is administered in a short period of time. As a result, the absorption of the drug will be delayed, potentially leading to a decrease in the maximum concentration of the drug in the body (Cmax) and an increase in the time it takes to reach that maximum concentration (Tmax). This may diminish the intense feeling of happiness that strengthens the activity. At present, there is no authorized ADF that utilizes a prodrug.
Ensysce Biosciences, a company headquartered in California, is currently developing prodrug technology that utilizes trypsin-activated abuse protection (TAAP). The PF614 NCE is an inactive form of oxycodone that can only be converted into its active form when taken orally. Trypsin is a proteolytic enzyme that catalyzes the hydrolysis of proteins by cleaving the peptide links between amino acids. Trypsin is synthesized in the pancreas as an inert proenzyme and subsequently released into the small intestine, where it is located and carries out its activity. Following ingestion, the TAAP-based opioid prodrug PF614 is activated and released in the gastrointestinal system through trypsin hydrolysis. In order to access the oxycodone product, PF614 must undergo metabolic transformation by trypsin in the gastrointestinal system, resulting in the formation of an intermediate prodrug. This prodrug then undergoes a self-catalyzed chemical modification process, which occurs over a specific period of time. The activation of the substance is not possible through injection, chewing, or snorting due to the absence of the initial activating enzyme (trypsin) in the bloodstream or saliva. PF614 was granted fast-track development approval by the FDA in January 2018. Ideally, this medication should possess resistance against misuse through all possible routes of administration, including chewing, crushing, injecting, and breathing. It is crucial to acknowledge that PF614, similar to oxycodone and other abuse-deterrent opioid formulations, does not release an active medication when exposed to standard or sophisticated extraction techniques. Extraction alone will not produce the intended opioid product. PF614 exhibits resistance to typical kitchen chemistry methods frequently employed to abuse prescription opioids.40,45
Implementing pH-Modulating Release Properties
To incorporate self-release retarding qualities in overdose settings, one can add a pH-elevating feature and a pH-dependent release feature to the dosage form. A pH-dependent release mechanism can be achieved by incorporating an opioid agonist into a matrix made of a pH-dependent release polymer (such as Eudragit EPO, which dissolves at pH levels below 5), or by applying a pH-dependent release coating (such as Eudragit EPO, which dissolves at pH levels below 5) around an inert core containing the opioid agonist. Incorporating pH-elevating components such as sodium bicarbonate and magnesium oxide into the dose form can introduce a pH-elevating characteristic. The dosage unit is carefully formulated to contain a specific amount of pH-elevating ingredients. This amount is adjusted to ensure that the dosage unit does not contain enough pH-elevating components to raise the pH of stomach fluid above six. This is done to facilitate the solubilization of a pH-dependent soluble polymer called eudragit EPO, which in turn enables the release of the opioid agonist present in the dosage unit. However, when a large number of dosage units (such as four or more) are taken at once in an overdose situation, the combined pH-raising substances in these dosage forms will counteract the acidity of stomach fluid. This will result in an increase in the pH level of the stomach fluid to above 5 or 6. As a consequence, the solubility of the pH-dependent soluble polymer (eudragit EPO) will be affected, leading to a delayed release of the opioid agonist contained in the dosage units.33,35
Regulatory Considerations and Expectations in Adf Approval Process
The FDA guidance document (Tablet 5) provides a comprehensive explanation of the three premarket studies that a manufacturer must carry out in order to demonstrate the abuse-deterrent properties of a formulation. It also offers recommendations on the methodologies for conducting and evaluating these studies, as well as guidance on how to accurately describe the results of the studies and their implications for labeling. Upon successful completion of the three premarket studies, the FDA will grant approval for the ADF, thereby obligating manufacturers to establish a REMS system. The Risk Evaluation and Mitigation Strategy (REMS) program, mandated by the Food and Drug Administration (FDA) Amendments Act of 2007, guarantees that the advantages of an opioid agonist surpass its hazards. In addition to the three modes of pre-market research, a post-market assessment is mandatory to evaluate the drug’s performance in real-world conditions.45
Table 5: FDA Guidance on Requirements of Premarket and Postmarket Study
|
Category 1 (Pre-market Studies) – Laboratory-based in-vivo manipulation and extraction studies |
At this stage of the evaluation process, the FDA may ask the drug product manufacturer to alter the drug formulation to the point where its abuse-deterrent properties are rendered ineffective and then compares the ADF version of the drug to non-ADF versions of the same drug. · The syringeability of the formulation, which refers to how tampered drug formulation can be quickly drawn into a syringe and injected for intravenous use, is evaluated after the integrity of the formulation has been defeated or compromised. · Grinding, crushing, cutting, or grating are some of the methods that can be used, as well as employing readily available various devices (like coffee grinders) at varying temperatures and employing readily available solvents under different conditions of temperature for variable time periods at varying pH and agitation. |
|
Category 2 (Pre-market Studies) – Pharmacokinetics studies |
At this stage of evaluation, in vivo pharmacokinetic properties of a newly developed ADF will be compared with its identical non-ADF opioid product under both intact and manipulated conditions, as well as for various routes of administration. Studies on the oral formulation are conducted with healthy volunteers who are given naltrexone HCl to block the pharmacodynamic effects of the opioids. These studies also occur under conditions where participants simultaneously consume food and alcohol. In-vivo studies on the administration of nasal drugs can be carried out on volunteers who have a history of abusing nasal drugs in the past. Ø During these studies, the main pharmacokinetic parameters to be monitored are: · Cmax · Tmax · AUC · Half-life · Adverse Event |
|
Category 3 (Pre-market Studies) – Clinical potential studies |
The likeability of a manipulated ADF is determined in this study by enrolling experienced recreational opioid abusers in randomized, double-blind, placebo-controlled, and positive-controlled crossover studies. These studies are conducted before the drug is available on the market. A comparison is made between the ADF and the non-ADF of the identical opioid drug at the same dose (and if the non-ADF does not exist, then using an opioid having similar pharmacologic properties), which is then compared with the placebo. These studies are carried out on participants who have already been prequalified to determine whether they can distinguish between the active drug and the placebo in a reliable manner. Those who have used drugs before and are familiar with their effects are in the best position to distinguish between them. The methods of substance abuse that will be investigated have been historically significant in terms of how the non-ADF has been used. These methods will almost always include inhalation through the nose and intravenous administration of the substance. The outcome measures include visual analog scales that assess how much a person likes the drug, as well as evaluations of whether or not they want to use it again. |
|
Category 4 – A post-market assessment |
A post-market assessment is obligatory in addition to the three forms of pre-market research. Studies in the fourth category, “postmarketing,” will examine how the drug performs in the real world. Studies conducted after a drug has been approved are called postmarketing studies, and their purpose is to “determine whether the marketing of a developed opioid ADF reduces the meaningful abuse potential, misuse, and also related adverse clinical outcomes, e.g., overdose, addiction and any death of abuser in the post-approval. |
Impact of ADFS on Misuse of Prescription Opioids
After the new formulation of oxycontin was approved by the FDA, a study was conducted to examine the impact of the abuse-deterrent formulation (ADF) on the use of both OxyContin and other opioids. The data indicate a significant decrease in the prevalence of oxycodone use as the main substance of abuse. Conversely, there was a substantial rise in the inclination towards other opioids such as hydrocodone, various oxycodone derivatives, hydromorphone, and fentanyl. Prior to the approval of the new OxyContin formulation, Oxycontin was among the opioids most abused for recreational purposes. However, the prevalence of heroin use more than doubled following the introduction of the new formulation. Despite 24% of patients admitting to bypassing the abuse-deterrent feature, the majority of patients transitioned to a different opioid. While there was limited evidence suggesting that the ADF effectively reduced the use of the specific drug it targeted, there was no conclusive evidence that users completely stopped using opioids for abuse after switching to ADFs. Instead, they often switched to a different substance. In comparison to traditional opioid formulations that lack measures to prevent misuse, the availability of abuse-deterrent dosage forms (ADFs) is likely to have a significantly greater impact. Recently, legislation has been introduced to tackle the opioid issue, and the FDA is currently granting approval exclusively to opioid formulations that have a lower susceptibility to abuse.48,49,50,51
Limitation of ADFS
Even opioids possessing characteristics that decrease the probability of misuse can still be subject to abuse. The federal regulators acknowledge the advancing scientific comprehension in this domain and the unresolved challenges that persist. Lately, there have been several comments on YouTube videos that provide instructions to viewers on various methods to manipulate abuse-deterrent formulations (ADFs) of opioid medicines. The modified ADFs (Abuse-Deterrent Formulations) of Opana ER (oxymorphone) extended-release tablet, which prevented nasal inhalation but still allowed for injection, were associated with an HIV outbreak in southern Indiana in 2015. The outbreak occurred in the year 2015. Despite a decline in the misuse of the opioid formulation following the introduction of the reformulated OxyContin (oxycodone) ADF, a research involving individuals who had previously misused OxyContin (oxycodone) and were enrolling in treatment programs found that 25 to 30 percent of participants persisted in using the new OxyContin ADF. This may be attributed to their discovery of a method to overcome the abuse-deterrent characteristics or their consumption of the tampered OxyContin pills orally. Moreover, abuse-deterrent compositions do not provide protection against theft or unintentional consumption by infants or children. Notably, a significant number of individuals who were dissuaded by the new ADFs disclosed that they transitioned to using non-ADFs or heroin.1
Conclusion and Author’s Perspective
The escalating fatality rates stemming from the rapidly growing opioid epidemic need the development of abuse-deterrent opioid formulations. The abuse-deterrent platform technologies used in the commercial development of abuse-deterrent opioid formulations are now being extensively studied, and several sophisticated technologies are close to receiving regulatory approval. Post-marketing statistics on currently approved Abuse-Deterrent Formulations (ADFs) show unfavorable outcomes for ADF opioid formulations. This indicates that ADFs have the potential to be a crucial element in ongoing and comprehensive initiatives aimed at reducing the hazards linked to opioid consumption.
Although the US FDA has approved several tamper-resistant opioid formulations that effectively prevent abuse through nasal and injection routes, the most prevalent method of drug abuse, known as “oral overdose” (taking multiple units of the drug at once), remains an unresolved issue in the field. The prodrug strategy has recently received significant attention in addressing the problem of opioid overdose, while its effectiveness is still being scrutinized. Oxycodegol, also known as NKTR 181, is an Oxycodone prodrug that was declined by the USFDA committee, namely the AADPAC (Anesthetic and Analgesic Drug Products Advisory Committee) and DSaRM (Drug Safety and Risk Management Advisory Committee). Despite Nektar’s claim that NKTR 181 is a specific mu-opioid agonist with prolonged pain-relieving effects and reduced risk of abuse due to its slower entry into the brain, the FDA committee voted against approving NKTR 181 due to concerns about potential drug abuse through injection or snorting.
Figure 5 illustrates the efficacy of existing abuse deterrent tactics against different forms of abuse. Every abuse deterrent strategy possesses distinct abuse-deterrent attributes and constraints when it comes to various techniques of drug abuse or different routes of administration. We suggest combining a minimum of two or more techniques to create a potent opioid abuse-deterrent formulation that targets the primary route of administration. Figure 6 demonstrates that a more realistic technique to effectively reduce opioid addiction, including overdose situations, is to combine two approaches: adding pH modifying release qualities and using an agonist-antagonist combination to create abuse-deterrent technology. Researchers must explore the potential for creating sophisticated formulations that can delay or reduce the speed at which drugs are released, depending on the dosage, by integrating qualities that modulate the pH of the environment. To effectively decrease the likelihood of overdose cases, it is not necessary to completely stop the drug from being released from the dosage form. Slowing down the release rate of the opioid from the dosage form, either by delaying it or extending the slower release, can also reduce the maximum concentration of the drug in the blood in a shorter time. This may be enough to prevent the harmful or deadly side effects of an opioid overdose, even when the same amount of the drug is consumed in its immediate release form. Incorporating non-releasable opioid antagonists with opioid agonists will additionally diminish the abuser’s inclination to manipulate the dosage form for administration through a modified pathway.
While it is true that the use of opioid ADF does not completely eliminate the risk of the opioid crisis, it is important to note that it significantly reduces the risk of abuse compared to conventional non-ADF products that lack abuse-deterrent properties. This, in turn, decreases the likelihood of misuse of opioid products. Hence, the development of safer, more diversified, cost-effective, and stronger abuse-deterrent technology is imperative to improve opioid ADFs. To address the seriousness of the global opioid crisis, it is advisable to adopt a “universal-precaution” strategy. This involves bringing together different regulatory agencies to protect society from the opioid pandemic. The goal is to establish a policy that outlines when and how opioid formulations should be prescribed to patients who genuinely require them. Additionally, efforts should be made to assess the risk of abuse and to approve only abuse-deterrent opioid medications for therapeutic purposes.
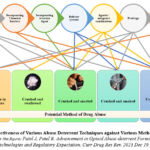 |
Figure 5: Effectiveness of Various Abuse Deterrent Techniques against Various Methods of Abuse1 |
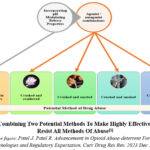 |
Figure 6: Combining Two Potential Methods To Make Highly Effective ADF That Resist All Methods Of Abuse1 |
Acknowledgment
All figures credit: Patel J, Patel R. Advancement in Opioid Abuse-deterrent Formulation Technologies and Regulatory Expectation. Curr Drug Res Rev. 2023 Dec 19.
Conflict of Interest
There is no conflict of interest.
Funding Sources
No funding was received to assist with the preparation of this manuscript.
References
- Patel J, Patel R. Advancement in Opioid Abuse-deterrent Formulation Technologies and Regulatory Expectation. Current Drug Research Reviews. 2023 Dec. DOI: 10.2174/0125899775274502231210060016. PMID: 38115616.
CrossRef - Erin K, Ahmed E, Saad M. Legislative Initiatives and Review of Abuse-Deterrent Opioid Formulations, US Pharm. 2013; 38(10):21-26.
- Manchikanti L, Fellows B, Ailinani H, et al. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010; 13:401-435.
CrossRef - Toblin R, Paulozzi L, Logan J, et al. Mental illness and psychotropic drug use among prescription drug overdose deaths: a medical examiner chart review. J Clin Psychiatry. 2010; 71:491-496.
CrossRef - Katz N. Abuse-deterrent opioid formulations: are they a pipe dream? Curr Rheumatol Rep. 2008; 10:11–8. doi: 10.1007/s11926-008-0003-z.
CrossRef - Paulozzi L, Budnitz D, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006; 15:618-627.
CrossRef - Kuehn B. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007; 297:249-251.
CrossRef - N. I. Drug Abuse (2016, August). Misuse of Prescription Drugs. Retrieved from National Institute of Drug Abuse: https://www.drugabuse.gov/publications/research-reports/ misuse-prescription-drugs/how-can-prescription-drug-misuse-be-prevented.
- United Nations Office on Drugs and Crime. World Drug Report 2015. www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf.
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: Results from the 2014 national survey on drug use and health. HHS Publication No. SMA 15-4927, NSDUH Series H-50 (2015). www.samhsa.gov/data/sites/default/files/NSDUH-FRR1–2014/NSDUH-FRR1–2014.htm.
CrossRef - Kuehn B. Prescription drug abuse rises globally. JAMA 297, 2007;1306.
CrossRef - Casati A, Sedefov R, Pfeiffer-Gerschel T. Misuse of medicines in the European Union: a systematic review of the literature. Eur. Addict. Res.18, 2012; 228–245.
CrossRef - Fischer B, Ialomiteanu A, Boak A et al. Prevalence and key covariates of non-medical prescription opioid use among the general secondary student and adult populations in Ontario, Canada. Drug Alcohol Rev. 32, 2013; 276–287.
CrossRef - Blanch B, Pearson S, Haber P. An overview of the patterns of prescription opioid use, costs and related harms in Australia. Br. J. Clin. Pharmacol. 78, 2014; 1159–1166.
CrossRef - Van Amsterdam J, van den Brink W. The misuse of prescription opioids: a threat for Europe? Curr. Drug Abuse Rev. 8, 2015; 3–14.
CrossRef - Australian Institute of Health and Welfare. National Drug Strategy Household Survey detailed report 2013 (2014). www.aihw.gov.au/ WorkArea/DownloadAsset.aspx?id=60129549848.
- Tominaga M, Kawakami N, Ono Y et al. Prevalence and correlates of illicit and non-medical use of psychotropic drugs in Japan: findings from the World Mental Health Japan Survey 2002–2004. Soc. Psychiatry Psychiatr. Epidemiol. 44, 2009; 777–783.
CrossRef - Birnbaum H, White A, Schiller M, Waldman T, Cleveland J, Roland C. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 12, 2011;657–667.
CrossRef - Katz N, Birnbaum H, Brennan M et al. Prescription opioid abuse: challenges and opportunities for payers. Am. J. Manag. Care 19, 2013;295–302.
- Farré M, Camí J. Pharmacokinetic considerations in abuse liability evaluation. Br. J. Addict. 86, 1991;1601–1606.
CrossRef - Jones J, Mogali S, Comer S. Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug Alcohol Depend. 125, 2012; 8–18.
CrossRef - Gudin J, Mogali S, Jones J, Comer S. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad. Med. 125, 2013; 115–130.
CrossRef - Katz N, Dart R, Bailey E et al. Tampering with prescription opioid: nature and extent of the problem, health consequences, and solutions. Am. J. Drug Alcohol Abuse 37, 2011; 205–217.
CrossRef - Kirsh K, Peppin J, Coleman J. Characterization of prescription opioid abuse in the United States: focus on route of administration. J. Pain Palliat. Care Pharmacother. 26, 2012;348–361.
CrossRef - Gasior M, Bond M, Malamut R. Routes of abuse of prescription opioid analgesics: a review and assessment of the potential impact of abuse-deterrent formulations. Postgrad. Med. 128, 2016;85–96.
CrossRef - Butler S, Black R, Cassidy T et al. Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm. Reduct. J. 8, 2011; 29.
CrossRef - Questions and answers: FDA approves a Risk Evaluation and Mitigation Strategy (REMS) for extended-release and long-acting opioid analgesics. July 9, 2012. www.fda.gov/Drugs/DrugSafety/ InformationbyDrugClass/ucm309742.htm.
- Extended-release (ER) and long-acting (LA) opioid analgesics Risk Evaluation and Mitigation Strategy (REMS). FDA. April 2013. www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290.pdf.
- FDA announces safety labeling changes and post market study requirements for extended-release and long-acting opioid analgesics. FDA news release. September 10, 2013. www.fda.gov/NewsEvents/ Newsroom/PressAnnouncements/ucm367726.htm.
- Katz NP, Adams EH, Chilcoat H, et al. Challenges in the development of prescription opioid abuse-deterrent formulations. Clin J Pain. 2007; 23(8):648–660.
CrossRef - Kyle S, Stacey L, Michael C, Benjamin T, Abuse-deterrent formulations: transitioning the pharmaceutical market to improve public health and safety, Therapeutics advances in Drug Safety, 2015 Apr; 6(2): 67–79.
CrossRef - Patel J, Patel R. Self-regulated Anti-Overdose Crush and Extraction-Resistant Drug Delivery System to Combat Opioid Overdose Crisis. AAPS PharmSciTech 23, 2022; 265. https://doi.org/ 10.1208/s12249-022-02423-5.
CrossRef - Patel J, Patel S. Pharmaceutical abuse deterrent composition, US Patent Application number: US20170157055A1, 2019.
- Patel J, Patel K, Patel S. Tamper Resistant Pharmaceutical Composition, US Patent Application number: US20180064817A1, 2018.
- US Patent Application number: US20210046014A1, 2021.
- Rana D, Salave S, Benival D. Emerging Trends in Abuse-Deterrent Formulations: Technological Insights and Regulatory Considerations. Curr Drug Deliv. 2022;19(8):846-859. doi: 10.2174/1567201818666211208101035.
CrossRef - Schaeffer T. Abuse-deterrent formulations, an evolving technology against the abuse and misuse of opioid analgesics. J Med Toxicol. 2012 Dec;8(4):400-7. doi: 10.1007/s13181-012-0270-y.
CrossRef - Ronald S. Litman, Olivia H. Pagán, Theodore J. Cicero; Abuse-deterrent Opioid Formulations. Anesthesiology 2018; 128:1015–1026 doi: https://doi.org/10.1097/ALN.0000000000002031.
CrossRef - Martin E, Derek M, Mary B, Maciej G, Richard M. Abuse-deterrent formulations of prescription opioid analgesics in the management of chronic noncancer pain, Pain Management 2016 6:5, 497-508.
CrossRef - Wu KM, Farrelly JG. Regulatory perspectives of Type II prodrug development and time-dependent toxicity management: nonclinical Pharm/Tox analysis and the role of comparative toxicology. Toxicology. 2007; 236:1–6. doi: 10.1016/j.tox.2007.04.005.
CrossRef - Patel B, Patel A, Ghava D, Padiya R, Darji P. Development and validation of stability indicating rp-hplc method for estimation of cyclandelate in bulk drug and capsule dosage form. Journal of medical pharmaceutical and allied sciences, 2023; V 12 – I 6, Pages – 6247 – 6253. Doi: https://doi.org/10.55522/jmpas.V12I6.5943.
CrossRef - Darji P, Patel J, Patel B, Parikh S, Joel P. Overview on osmotic drug delivery system. International journal of pharmaceutical research and applications, Jan-Feb 2024; Volume 9, Issue 1, pp: 86-100. DOI: 10.35629/7781-090186100.
- Darji P, Patel J, Patel B, Parikh S, Joel P. Comprehensive review on oral biologics. World journal of pharmaceutical research, Jan-2024; Volume 13, Issue 3, DOI: 10.20959/wjpr20243-31160.
- Darji P, Patel J, Patel B, Chudasama A, Joel P, Nalla S. Recent method to improve stability profile, pharmacokinetic and pharmacodynamic properties in anticancer drugs. World journal of pharmaceutical and life sciences, March-2024; Volume 10, Issue 3, 216-229.
- Webster LR, Bath B, Medve RA. Opioid formulations in development designed to curtail abuse: who is the target? Expert Opin Investig Drugs. 2009; 18:255–63. doi: 10.1517/13543780902751622.
CrossRef - Mastropietro DJ, Omidian H. Current approaches in tamper-resistant and abuse-deterrent formulations. Drug Dev Ind Pharm. 2012.
CrossRef - Miyazaki T, Choi IY, Rubas W, Anand NK, Ali C, Evans J, Gursahani H, Hennessy M, Kim G, McWeeney D, Pfeiffer J, Quach P, Gauvin D, Riley TA, Riggs JA, Gogas K, Zalevsky J, Doberstein SK. NKTR-181: A Novel Mu-Opioid Analgesic with Inherently Low Abuse Potential. J Pharmacol Exp Ther. 2017 Oct;363(1):104-113. doi: 10.1124/jpet.117.243030.
CrossRef - Gudin J, Fudin J. Analgesics of the Future: Low Abuse Liability Opioids – Wish List or Emerging Treatment?. Pract Pain Manag. 2022; 22(1).
- U.S. Food and Drug Administration: Abuse-deterrent opioids—evaluation and labeling. Guidance for industry, 2015. Available at: https://www.fda.gov/downloads/Drugs/Guidances/UCM334743.pdf.
- William C, David A. Abuse-Deterrent Opioid Formulations — Putting the Potential Benefits into Perspective, N Engl J Med 2017; 376:2103-2105 DOI: 10.1056/NEJMp1701553.
CrossRef - Cicero TJ, Ellis MS, Surratt HL. Effect of abuse-deterrent formulation of OxyContin. N Engl J Med. 2012; 367:187–9. doi: 10.1056/NEJMc1204141.
CrossRef







