Manuscript accepted on :21-09-2023
Published online on: 16-04-2024
Plagiarism Check: Yes
Reviewed by: Dr. Bhagyashri
Second Review by: Dr. Ankur Kumar Arya
Final Approval by: Dr. Josphert Ngui Kimatu
Sandhya A , Priya Durairaj
, Priya Durairaj and Gomathi Kannaiyaram*
and Gomathi Kannaiyaram*
Department of Biotechnology, Dr M.G.R Educational and Research Institute, Chennai, India.
Corresponding Author E-mail:sandhya.ibt@drmgrdu.ac.in
Abstract
Traditional medicines made from medicinal plants are the most abundant biosources of medications. Pharmaceutical medications, synthetic pharmaceuticals, Siddha, Ayurveda, nutraceuticals, and dietary supplements all come from plant sources. In spite of several studies using herbal plants that have shown a strong link between phytochemical, anti-diabetic, and anti-inflammatory content, Nigella sativa—a spice plant of the Ranunculaceae family—showed more significant qualities than its competitors. Numerous pharmacological properties were discovered in both the Nigella sativa essential oil and the seeds. Nigella sativa is considered to be the most powerful antioxidant and medicinal plant with known medicinal properties and therapeutic applications, and it is used as traditional medicine for respiratory, gastrointestinal, rheumatic, and inflammatory disorders. The study was conducted using Nigella sativa and compound Beta caryophyllene to determine the anti-oxidant, invitro-anti-diabetic, anti-inflammatory, and enzymatic and non-enzymatic activity of the ethanolic seed extract of Nigella sativa and compound Beta caryophyllene on Alzheimer's disease because there were no toxic effects or serious side effects observed using animal models or in clinical trials. The antioxidant activity of the compound and the extract was examined to study the qualitative and quantitative analysis of the extract and the compound beta-caryophyllene. Phytochemical screening exhibited secondary metabolites of the extract, such as phenols, flavonoids, steroids, alkaloids, and cardiac glycosides. Nigella sativa extract and its constituents such as beta-caryophyllene, possess a protective effect against toxicity, which is caused by drugs used for treating cancer as well as neurodegenerative disorders. Further, the extract prevents a decrease in hemoglobin levels as well as leukocyte counts. The purpose of the research is to demonstrate that Nigella sativa and the chemical beta-caryophyllene may be employed as medicinal agents and that beta-caryophyllene may have potential cholinesterase inhibitory effects.
Keywords
Alzheimer’s; Anti-inflammatory; Beta-caryophyllene; Cholinesterase; Nigella sativa; Pharmacological activity
| Copy the following to cite this article: Sandhya A, Durairaj P, Kannaiyaram G. Pharmacological Characterization of Nigella sativa Extract and the Compound Beta-caryophyllene. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Sandhya A, Durairaj P, Kannaiyaram G. Pharmacological Characterization of Nigella sativa Extract and the Compound Beta-caryophyllene. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/4aBRy1V |
Introduction
Medicinal plants have various forms like extracts, oils, and compounds that are identified to have anti-AD, anti-diabetic, and anti-inflammatory properties and have also undergone many clinical and pre-clinical studies. Dementia is defined as an acquired deterioration in cognitive abilities that impairs the successful performance of activities of daily living. The memory impairment in Alzheimer’s disease (AD) patients is due to the loss of cholinergic neurons, while those in non-AD dementia are due to the loss of serotonergic and glutaminergic neurons. While in the former, memory impairment is primary, in the latter, behavioral symptoms are primary, leaving memory relatively spared. The authors suggestions have led several researchers to conclude that the presence of certain types of alkaloids, saponins, and glucosides, as well as antioxidant activity, possess a neuroprotective effect against Aβ production, causing AD, which tends to be high. As medicinal plants are rich biosources of drugs from plant sources, they have been widely used in many research studies and nutraceuticals, the food industry for preparing food supplements, the drug manufacturing industry, and also as Siddha and Ayurvedic medicines. The most common and native treatment used in India is Ayurvedic medicine. The treatment and cure for diseases are through medicinal plants, which are evaluated and designed for potential cure 1.
For many health-related diseases and disorders various phytotherapies are used, which are obtained from herbal medicines. It causes a major change in the outlook for treatment around the world. According to research, herbal plants are considered to be the best remedy and have holistic therapy, which consolidates the physiological status of the patients 2 . The efficient and effective herbal medicines contain several compounds that actively interact with the in vivo environment and change the physiological status of the body through certain alterations in the biochemical processes without any side effects 3. Efficient and effective herbal medicines contain several compounds that actively interact with the in-vivo environment and change the physiological status of the body through certain alterations in the biochemical processes without any side effect. 3
Nigella sativa, a well-known spice plant (black cumin) of the Ranunculacea family, exhibited many significant therapeutic properties. The active compounds of Nigella sativa have made it to be used as a medicinal plant for thousands of years and also as medicinal ailments 4. The drugs that are formulated are from the plant’s stem, bark, flowers, leaves, roots, seeds, and fruits. The phytochemical constituents are the most important sources for designing a drug 5,6. Various essential oils and extracts, which are the richest source of phytochemicals and contain tannins, carbohydrates, alkaloids, terpenoids, phenolics, flavonoids, and steroids 7. Many studies are correlated with herbal extracts, which are a good source of phytochemical constituents, have anti-diabetic and anti-inflammatory activities, and have good potential for enzymatic assays. The generation of reactive oxygen species causes adverse effects in aerobic organisms, but the metabolism of oxygen has more beneficial effects. The macromolecules mostly undergo oxidative reactions that are mediated by reactive oxygen species. Biomedical research mainly focuses on the adverse effects of reactive oxygen species, which act upon the biological system 8. Based on the experimental studies and clinical studies, pharmacological activity occurs due to the antioxidant activity of the extract. Because it has the ability to scavenge free radicals and inhibit lipid peroxidation 9. Many studies and researches have concluded that the seed extract of Nigella sativa and its derivative products were used as a treatment for many diseases, like liver diseases, rheumatism, as well as in the treatment of inflammatory disorders and their relevant consequences 10. Further, the constituents of the extract are found to be effective, and this is evident as it decreases the nephrotoxicity induced by cisplatin in rodents and possesses high anti-tumor activity 11. Most of the therapeutic properties are due to the presence of some phenolic compounds in the seed, especially thymoquinone, beta-caryophyllene, etc which are known to be the major bioactive component. The present study was carried out using Nigella sativa extract and the compound beta-caryophyllene to phytochemically analyze the effects of the extract and the compound on Alzheimer’s disease.
Material and methods
Reagents
Amyloid peptide, Thymoquinone, beta-caryophyllene, 1,1,1,3,3,3-hexafluoro-2propanaol (HFIP) was purchased from Sigma Aldrich; 1, 1-diphenyl-2-picrylhydrazyl (DPPH), Ascorbic acid, Dimethyl Sulfoxide (DMSO), Triton X 100, and p-Nitrophenyl – α-D- glucopyranoside were obtained from AR Teck Pvt. Ltd., India. All the chemicals used for the experiments are of analytical grade.
Anti-oxidant activity of ESENS and compound beta-caryophyllene
The antioxidant activity of compounds such as 2,2 -diphenyl-1-picrylhydrazyl (DPPH), hydrogen peroxide, and nitric oxide was studied. The DPPH radical scavenging capacity was measured using the Brand-William method 12. Similarly, hydrogen peroxide and nitric oxide were measured using Ruch and Marcocci methods 13,14.
2,2 Diphenyl-1-Picrylhydrazyl (DPPH) radical scavenging assay of ESENS and the compound beta-caryophyllene
Ethanolic seed extract of Nigella sativa (ESENS) and compound beta-caryophyllene obtained through HR-LCMS were pre-dissolved using DMSO and used for the study. The compounds and the extract in various concentrations were taken and added to 100 μl of 0.1 mM DPPH, which was freshly prepared using ethanol. DPPH alone serves as a blank, and ascorbic acid is the standard. The solution mixture was further incubated for 30 minutes. The incubated mixture was studied using an absorbance of 517 nm using a UV-Vis spectrophotometer. The triplicates obtained were used to calculate the percentage of radicals that were being scavenged.
Hydrogen peroxide scavenging assay
Based on the Ruch and Marcocci method, using phosphate buffer, 40 mM of hydrogen peroxide was freshly prepared, and the pH was adjusted to 7.4. Various concentrations of the ESENS and the compound beta-caryophyllene were added to the freshly prepared hydrogen peroxide, which was further incubated for 30 minutes at RT. Phosphate buffer alone serves as a blank, and ascorbic acid is used as a standard. After the incubation period, the samples were measured at 560 nm using UV-Vis spectrophotometry. Triplicates obtained were used to calculate the percentage of inhibition that the compounds beta-caryophyllene and ESENS had undergone.
Nitric oxide radical scavenging assay
Based on modified protocol 15, the nitric oxide radical scavenging assay was performed for the various concentrations of ESENS and the compound beta-caryophyllene. Equal amounts of Griess reagent were mixed with sulphanilamide (1%), which was prepared from 2.5% phosphoric acid, and napthylethylene diamine dihydrochloride (0.1%) in 2.5% phosphoric acid. To different concentrations of Ethanolic Seed Extract of Nigella sativa (ESENS) and compound beta-caryophyllene, 0.5 ml of 10 mM sodium nitroprusside, which was prepared using phosphate buffered saline, was added. Further, the tubes containing the reaction mixture were incubated at 25°C for 180 minutes, followed by the addition of an equal amount of freshly prepared Griess reagent. For control, the reaction mixture was prepared with buffered saline without ESENS and the compound beta-caryophyllene. The samples were read at 546 nm using UV-Vis Spectrophotometers. Ascorbic acid is used as a standard. The percentage of inhibition was observed and recorded to analyze the percentage of nitrite scavenging activity of the extract, compound, and standards.
Anti-invitro studies of ESENS and the compound beta-caryophyllene
Increasing levels of proinflammatory cytokines cause metabolic disorders that lead to cell death. The ESENS and the compound beta-caryophyllene were studied to find out how they stop the proinflammatory cytokines that cause metabolic disorders.16,17
HRBC membrane stabilization assay of ESENS and the compound beta-caryophyllene
To the tube containing EDTA, 100 μl of blood sample and various concentrations of ESENS and the compound beta-caryophyllene were added. Triton x 100 serves as a positive control, while a blood sample alone serves as a negative control. The tube containing the reaction mixture was incubated at 37°C for 30 minutes. Followed by the centrifugation of the sample at 5000 rpm for 15 minutes. The supernatant is collected in a separate tube for analysis and stabilization using UV-Vis Spectrophotometer at 517 nm.
Cytotoxicity assay of ESENS and compound the beta-caryophyllene
The isolated lymphocyte cells are cultured in humidified 5% (v/v) CO2 / air at 37°C in Dulbecco’s Modified Eagle Medium (DMEM), which is supplemented with 10% fetal bovine serum and 100 U/ml of penicillin. In 96-well plates, 5×104 cells/ml were cultured. Simultaneously, amyloid beta fibrils were prepared by incubating the amyloid monomer at room temperature. The pre-formed amyloid beta fibrils with ESENS and compound beta-caryophyllene and without ESENS and compound are diluted in freshly prepared DMEM medium and added to a microtiter well plate; the wells final concentration was made to 2μm ol/L. The same volume of medium is added to the control well. The plates were then incubated at 37°C for 48 hours. Cell viability was determined by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) toxicity assay. The MTT was prepared at a concentration of 5mg/ml; added to each well, and incubated further for 3 hours at 37°C. In the medium that was removed, DMSO was added to each well. The samples in the wells were mixed well, and the samples were read using a microplate reader at 490 nm. 18,19
Enzyme linked immune-sorbent assays of ESENS and the compound beta-caryophyllene
Interleukin – 1 – beta were analyzed using lymphocyte cell culture supernatant and studied using commercially available ELISA kit protocols. The lymphocytes were collected, and 100 µl of the sample was added to each well of the 96-well microtiter plates along with an equal volume of DMEM medium. The samples were further incubated for 2 hours at 37°C. The incubated samples were again aspirated and washed 3 times. Further, 100 µl of detection reagent B was added and further incubated for 30 minutes at 37°C. The samples are washed 5 times. To achieve this, 90 µl of substrate solution was mixed and incubated for 10–20 minutes at 37 °C. To terminate the reaction, 50 µl of the stop solution was mixed, and the absorbance was measured at 450 nm immediately after mixing the stop solution.
Anti-diabetic activity of ESENS and the compound beta-caryophyllene
Anti-diabetic studies such as the α-amylase inhibitory assay and the α-glucosidase inhibitory assay were studied.
α-amylase inhibitory assay
Based on the protocol, ESENS and beta-caryophyllene of various concentrations were prepared and mixed with di-methyl sulfoxide solution (DMSO). Simultaneously, alpha-amylase was prepared by mixing it with phosphate buffer (pH 6.8). The reaction mixture prepared was incubated, after which 1% starch solution was added to all the tubes. In addition, the tubes were again incubated for another 15 minutes. The reaction was stopped by adding 1ml of di-Nitro Salicylic acid (DNS) reagent, and the tubes were boiled in a water bath for 10 minutes. The contents were then cooled, and 10 ml of distilled water was added to all the tubes. Absorbance at 540 nm was measured with acarbose as a positive control. 20,21
α-glucosidase inhibitory assay
α-glucosidase inhibitory assay was studied based on the protocol 16. 112 μl of potassium phosphate buffer at pH 6.8 was mixed with 20 μl of enzyme solution, 8 μl of the extract, and beta-caryophyllene and incubated for 15 minutes at 37°C. Following the incubation, 20 μl of NPG was added and again incubated for 15 minutes at 37°C. The reaction was then terminated using 80 μl of Na2CO3 solution. The sample absorbance was measured at 450 nm. For control, 8 μl of Dimethyl Sulfoxide (DMSO) was added to the reaction mixture instead of extract and compound. Acarbose serves as a standard.
Enzymatic and non-enzymatic assays of ESENS and the compound beta-caryophyllene
The enzymatic assays such as Acetyl cholinesterase (AchE) hydrolysis 22 and the non-enzymatic assays such as Metal chelating assay 23 were studied using the Ethanolic Seed Extract of Nigella sativa (ESENS) and beta-caryophyllene to analyze the effectiveness of the compound and the ESENS in preventing the hydrolysis of the enzyme AchE, which is a neurotransmitter.
Enzymatic assay- Acetyl cholinesterase assay
The acetylcholinesterase assay of the Ethanolic Seed Extract of Nigella sativa (ESENS) and the compounds were performed by adding 40 μl of 0.28 U/ml acetylcholine enzyme and 140 μl of 3.3 mM 5,5-dithiobis-(2-nitrobenzoic) acid, which is prepared using a 0.1 M phosphate buffered solution of pH 7.0 that also contains 6 mM of NaHCO3. The extract, the compounds of various concentrations, and 80 μl of phosphate buffered saline of pH 8.0 were added to the reaction mixture. The solution was incubated for about 20 minutes at 25°C. 40 μl of 0.5 mM acetylthiocholine iodide was further added to each well containing the solution. After the addition of substrate, the reaction mixture was measured at 412 nm using UV-Vis spectrophotometry.
Non-enzymatic assay – Metal chelating assay
To the mixture containing 168 μl of 0.1 M tris-HCL, 218 μl of 0.8% w/v sodium chloride, 150 μl of 500 μM freshly prepared FeSO4, further, various concentrations of extract and compounds were added, followed by incubation for about 20 minutes. To the incubated mixture, 3 μl of 0.25% of 1, 10 – Phenanthroline was added to read the absorbance of the mixture at 510 nm.
Results
Anti-oxidant activity of ESENS and the compound beta-caryophyllene
2,2 Diphenyl-1-Picrylhydrazyl (DPPH) radical scavenging assay:
Diphenyl-1-picrylhydrazyl (DPPH) is more stable for estimating free radicals. It is due to the ability to donate hydrogen molecules. It takes up H molecules to form a dimagnetic compound, which is more stable. The ESENS showed good activity as compared to the control ascorbic acid, whose IC50 value is 11.26±0.93μg/ml and for ascorbic acid it is 2.46±0.41μg/ml. Beta-caryophyllene exhibited good potential, and its IC50 value is 14.02±0.71μg/ml. This result suggests that ESENS and beta-caryophyllene exhibit good potential activity (Fig. 1,2).
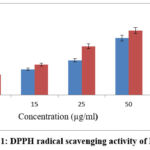 |
Figure 1: DPPH radical scavenging activity of ESENS
|
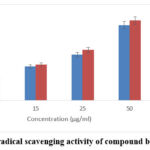 |
Figure 2: DPPH radical scavenging activity of compound beta-caryophyllene
|
Hydrogen peroxide scavenging assay
The hydrogen peroxide activity exhibited is dose dependent when compared to the control Ascorbic acid, whose IC50 values are found to be 17.50±0.11μg/ml for Ascorbic acid, 95.14±0.66μg/ml for beta-caryophyllene. This reveals that the ESENS exhibited good radical scavenging activity when compared to the control where Beta caryophyllene exhibited less radical scavenging activity (Fig. 3,4).
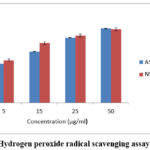 |
Figure 3: Hydrogen peroxide radical scavenging assay of ESENS
|
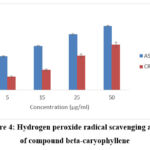 |
Figure 4: Hydrogen peroxide radical scavenging assay of compound beta-caryophyllene
|
Nitric Oxide Radical scavenging assay
ESENS and beta-caryophyllene significantly inhibit nitric oxide in a dose-dependent manner at a concentration of 122±0.11µg/ml of ESENS and 132±0.21µg/ml of beta-caryophyllene, respectively, when compared to ascorbic acid. The result suggests that the extract and the compound beta-caryophyllene are capable of inhibiting nitric oxide and proves that they can be used as drugs in the indigenous system for treating various diseases and disorders (Fig. 5,6).
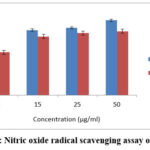 |
Figure 5: Nitric oxide radical scavenging assay of ESENS
|
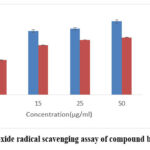 |
Figure 6: Nitric oxide radical scavenging assay of compound beta-caryophyllene
|
Anti-invitro studies of ESENS and the compound beta-caryophyllene
HRBC membrane stabilization assay
Different concentrations of ESENS and beta-caryophyllene were analyzed to study the prevention of hemolysis, which is determined by the HRBC membrane stabilization assay. Triton X 100 is a detergent that destabilizes the RBC membrane and leaks hemoglobin, and the results suggest that ESENS and beta-caryophyllene prevent the leaking of hemoglobin and prevent the integrity of the RBC; furthermore, it is proved to be non-toxic in nature and can be used in biological studies (Fig. 7,8). Increased levels of IL-1 beta cytokines are the major cause of various metabolic disorders. The compound beta-caryophyllene is found to be more potent when compared to ESENS.
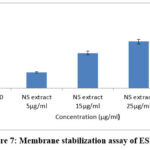 |
Figure 7: Membrane stabilization assay of ESENS.
|
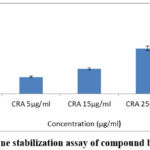 |
Figure 8: Membrane stabilization assay of compound beta-caryophyllene
|
Cytotoxicity assay
The isolated lymphocytes were treated with ESENS and beta-caryophyllene to study the cell’s toxicity. The ESENS exhibited 50% cell death, and beta-caryophyllene exhibited 40% cell death when compared to the control sample (Table 1,2). This result suggests that the extract and compound exhibited less cell proliferation (Fig 9, 10).
Table 1: Cytotoxicity assay of ESENS
|
INCUBATION TIME (HOURS) |
IC50 VALUE (MG/ML)- TRYPAN BLUE |
IC50 VALUE (MG/ML)-MTT |
|
24 |
6.83±0.71a |
8.07±0.52* |
|
48 |
4.93±0.25b |
5.20±0.10** |
|
72 |
3.70±0.10c |
4.47±0.12** |
Table 2: Cytotoxicity assay of compound Beta caryophyllene
|
INCUBATION TIME (HOURS) |
IC50 VLAUE (MG/ML)- TRYPAN BLUE |
IC50 VALUE (MG/ML)-MTT |
|
24 |
6.93±0.51a |
10.07±0.42* |
|
48 |
4.83±0.45b |
7.30±0.20** |
|
72 |
2.90±0.11c |
6.37±0.14** |
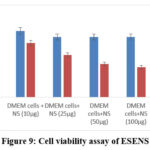 |
Figure 9: Cell viability assay of ESENS
|
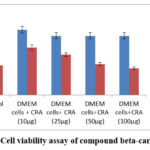 |
Figure 10: Cell viability assay of compound beta-caryophyllene
|
Enzyme linked immune-sorbent assay of ESENS and the compound beta-caryophyllene
The ESENS and the compound beta-caryophyllene in inhibition of IL-1 Beta were analyzed. The effects of ESENS and the compound beta-caryophyllene are expressed in Fig. 11. Based on the result, it is suggested that the compound has a high potency in inhibiting IL-1 Beta when compared to the extract ESENS, which showed a good potential effect. The samples are compared with the standard of IL-1 Beta and the lymphocyte cells that are treated with hydrogen peroxide. The compounds have exhibited good potential effects individually when compared to the ESENS; it is revealed that the compound beta-caryophyllene has anti-inflammatory activity.
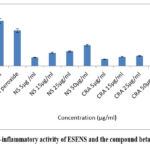 |
Figure 11: Anti-inflammatory activity of ESENS and the compound beta-caryophyllene |
Anti-diabetic activity of ESENS and the compound beta-caryophyllene
α-amylase inhibitory assay
The α-amylase inhibitory assay of the ESENS and beta-caryophyllene was used to analyze the inhibitory activity of the extract and the compound. The extract and the compound at various concentrations determined exhibited the highest inhibition rate of about 50.84% and the lowest inhibition rate of 15.49% for ESENS and beta-caryophyllene, respectively, at 55.81% and 13.04%. The extract and the compound are compared with the control acarbose. Based on the results, the compound beta-caryophyllene exhibited effective inhibitory activity when compared to ESENS, and it showed dose-dependent activity (Fig. 12).
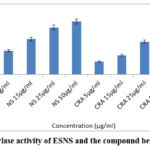 |
Figure 12: α-amylase activity of ESNS and the compound beta-caryophyllene |
α-glucosidase inhibitory assay
α-glucosidase inhibitory activity of ESENS and beta-caryophyllene was analyzed and determined using the substrate p-NPG, which was then compared with the control acarbose. ESENS and the compound beta-caryophyllene of various concentrations were studied, and the result suggests that the compound beta-caryophyllene exhibited 40% inhibition when compared to ESENS and the control. Based on the result, it is suggested that the compound beta-caryophyllene exhibited a good potential effect on the inhibitory action (Fig. 13).
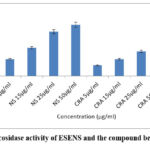 |
Figure 13: α-glucosidase activity of ESENS and the compound beta-caryophyllene |
Enzymatic and non-enzymatic assay of ESENS and the compound beta-caryophyllene
Enzymatic assay- Acetylcholinesterase assay
It is an in vitro assay that is used to study the enzyme cholinesterase, which plays a major role in neurotransmittance in Alzheimer’s disease. This reveals the role of ESENS and beta-caryophyllene, where beta-caryophyllene is 42.06±2.1μg/ml and ESENS is 84.7±4.3μg/ml. The compound exhibited higher inhibition of the enzyme cholinesterase when compared to ESENS (Fig. 14,15).
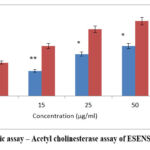 |
Figure 14: Enzymatic assay – Acetyl cholinesterase assay of ESENS VS drug Donepezil
|
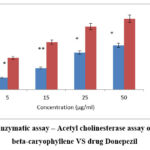 |
Figure 15: Enzymatic assay – Acetyl cholinesterase assay of compound beta-caryophyllene VS drug Donepezil
|
Non-enzymatic assay – Metal chelating assay
The results obtained from non-enzymatic assays suggest that the ESENS of various concentrations chelates the ferric ion (Fe-II) and is found to exhibit 1.75±0.17* at 50 µg/ml when compared to the compound beta-caryophyllene of about 5.67±0.53*. The results revealed that the ESENS have high scavenging activity when compared to the compounds. But Beta caryophyllene is found to have the same efficacy as beta-caryophyllene (Table 3).
Table 3: Non-Enzymatic assay – Metal chelating assay of ESENS and compound Beta Caryophyllene VS drug Donepezil
|
S.NO |
PARAMETERS ASSESSED |
NIGELLA SATIVA |
BETA CARYOPHYLLENE
|
|
1 |
5 |
4.28±0.31* |
7.11±0.93* |
|
2 |
15 |
3.12±0.22* |
6.04±0.88* |
|
3 |
25 |
2.06±0.21* |
5.89±0.67* |
|
4 |
50 |
1.75±0.17* |
5.67±0.53* |
Discussion
The tables should be marked with a number and a title. The figures must contain the captions. Figure/Photos submitted must be of high resolution. Proper reference must be given for the figures and photos.
The medicinal plants are used as the most abundant biosource for medication production. Numerous pharmacological properties of Nigella sativa have been discovered, including anti-analgesic, anti-ulcer, anti-inflammatory, anti-bacterial, anti-microbial, anti-cancer, and anti-diabetic properties. The qualitative analysis of the ESENS revealed positive relevance in both therapeutic and physiological activities, according to the current research. The isolation and identification of these bioactive substances pave the way for the creation of novel medications that may be used to treat a wide range of illnesses and ailments. Tannins have medicinal benefits for anti-diarrhoea, anti-haemostatic, and a broad spectrum of anti-microbial activity against viruses, bacteria, and fungi. Alkaloids are the largest group of compounds that have been linked to many medicinal properties for years and have a potential for cytotoxicity. Steroids have antibacterial properties, whereas flavonoids are hydroxylated phenolic compounds made from plants that have potent antioxidant and anti-cancer properties 24. Flavonoids also have a favorable response to microbial infection. Terpenoids are an example of an essential lipid that has an aromatic flavor and performs activities including controlling growth and color. Metabolites of phenols exhibit biological features, including anti-apoptosis, anti-aging, and anti-inflammatory effects. It is a compound that protects the heart and enhances endothelial health. The blood pressure is also lowered and controlled by glycosides 25.
Essential oils and other plant extracts antioxidant properties are gaining increased attention in scientific circles as well as in the food, cosmetics, and pharmaceutical sectors. By using recognized antioxidants like ascorbic acid in DPPH radical scavenging assays, hydrogen peroxide scavenging assays, and nitric oxide assays, researchers were able to assess the anti-radical or free radical scavenging capabilities of plant extracts or essential oils. Antioxidants’ ability to cause a decrease in absorbance at 517 nm was utilized to gauge their potential to lower DPPH radical generation 21. The ability to transfer hydrogen is the justification. ESENS and the substance beta-caryophyllene demonstrated effective nitric oxide scavenging abilities. The substance showed promise for scavenging free radicals in the DPPH test as well. According to research 26, beta-caryophyllene exhibited reduced activity, and hydrogen peroxide was shown to be dosage-dependent.
When compared to the crude extract of Nigella sativa, the in vitro investigations showed that the compound beta-caryophyllene has a large number of phytochemical elements that are medicinally relevant. The isolated component beta-caryophyllene and the crude extract of Nigella sativa both exhibit anti-inflammatory and anti-diabetic properties, which were shown by the IL-1 beta test, alpha amylase, and alpha glucosidase inhibitory assays. While the crude extract of Nigella sativa and the compound beta-caryophyllene (CRA) showed less effectiveness in hydrogen peroxide scavenging activity, the molecule beta-caryophyllene is more efficient than ESENS in DPPH radical scavenging, the nitric oxide test, the acetyl cholinesterase assay, and the metal chelating assay.
Conclusion
The in vitro studies revealed that the ESENS have many phytochemical properties that have been found to be medically significant. The compound beta-caryophyllene tends to have reduced hydrogen peroxide activity when compared to ESENS, and the compound beta-caryophyllene has potential anti-inflammatory, anti-diabetic, AchE, and metal chelating properties. Since the compound is more effective in vitro, pharmacological studies have shown it to be successful and promising for the treatment of Alzheimer’s disease, which needs an effective therapy to control brain functions and other complications. Due to these qualities, the compound beta-caryophyllene is a prospective candidate for the treatment of Alzheimer’s disease. The future prospective of the current study is to conduct biophysical, kinetic characterization and animal studies of the ESENS and the compound beta-caryophyllene.
Acknowledgement
I feel thankful to acknowledge that Dr. M.G.R Educational and Research Institute (Deemed to be University) for providing me the workspace for my research. I thank Dr. K. Gomathi, Professor and Deputy Head, Department of Biotechnology, Dr. M.G.R Educational and Research Institute, Chennai for supporting my work and for guiding me.
Conflict of Interest
The authors have no conflict of interest to declare. The co-author has seen and agreed with the contents of the manuscript and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication.
Funding Source
There is no funding Sources
Reference
- Ramadan M. F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): an overview.International Journal of Food Science and Technology., 2007; 42(10): 1208–1218. https://doi.org/10.1111/j.1365-2621.2006.01417.x
CrossRef - Azra Kamal. Phytochemical screening of Syzygium Cumini Seeds. Indian Journal of Plant Sciences., 2014; 3(4): 1-4. 10.3329/ jbs.v24i0.37483
- Wu C and Shea J. E. Structural similarities and differences between amyloidogenic and non-amyloidogenic islet amyloid polypeptide (IAPP) sequences and implications for the dual physiological and pathological activities of these peptides. PLoS Computational Biology., 2013; 9(8): 1-12. https://doi.org/10.1371/journal.pcbi.1003211
CrossRef - Toncer O and Kizil S. Effect of seed rate on agronomic and technologic characters of Nigella sativa L. International Journal of Agriculture & Biology., 2004; 6(3): 529-532.
- Mojab F, Kamalinejad M, Ghaderi N and Vahidipour H. R. Phytochemical screening of some species of Iranian plants. Iranian Journal of Pharmaceutical Research., 2003; 2(2): 77-82.
- Parekh J and Chanda S. Antibacterial and phytochemical studies on twelve species of Indian medicinal plants. African Journal of Biomedical Research., 2007; 10(2): 175-181. 10.4314/ajbr.v10i2.50624
CrossRef - Abbas M, Mehmood T, Bashir A, Zafar M and Afzal A. Economics of Lallemantia royleana (tukham-ebalangoo) production in the low intensity cropping zone of the Punjab, Pakistan. Pakistan Journal of Agricultural Research., 2012; 25(2): 110-119.
- Uslu C, Taysi S and Bakan N. Lipid peroxidation and antioxidant enzyme activities in experimental maxillary sinusitis. Annals of Clinical and Laboratory Science., 2003; 33(1):18-22.
- Gupta M, Mazumder U.K, Kumar T.S, Gomathi P and Kumar R.S. Antioxidant and hepatoprotective effects of Bauhinia racemosa against paraetamol and carbon tetrachloride induced liver damage in rats. Iranian Journal of Pharmacology and Therpeutics., 2004; 3(1): 12-20.
- Al-Khalaf M.I and Ramadan K.S. Antimicrobial and anti-cancer activity of Nigella sativa oil- A Review. Australian Journal of Basic Applied Science., 2013;7(1): 505-514.
- Badary O.A, Nagi M.N, Al-Shabanah O.A, Al-Sawaf H.A, Al-Sohaibani M.O and Al-Bekairi A.M. Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Canadian Journal of Physiological and Pharmacology., 1997; 75(12): 1356-1361.
CrossRef - Brand – Williams W, Cuvelier M. E and Berset CLWT. Use of free radical method to evaluate antioxidant activity. LWT- Food Science and Technology., 1995; 28(1): 25-30.
CrossRef - Ruch R. J, Cheng S. J and Klaunig J. E. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis., 1989; 10(6): 1003-1008. https://doi.org/10.1093/carcin/10.6.1003
CrossRef - Marcocci L, Marguire J. J, Droy-Lefaiz M. T and Packer L. The nitric oxide scavenging properties of Gingko biloba extract. Biochemical and Biophysical Research Communications., 1994; 201(2): 748-755. https://doi.org/10.1006/bbrc.1994.1764
CrossRef - Panda S. K, Thatoi H. N and Dutta S. K. Antibacterial activity and phytochemical screening of leaf and bark extracts of Vitex negundo I. from similipal biosphere reserve, Orissa. Journal of Medicinal Plants Research., 2009; 3(4): 294-300.
- Hamburger M and Hostettmann K. Bioactivity in plants: the link between phytochemsitry and medicine. Phytochemistry., 1991; 30(12): 3864-3874. https://doi.org/10.1016/0031-9422(91)83425-K
CrossRef - James O, Nnacheta O. P, Wana H. S and Aliyu U. R. In vitro and invivo studies on the antioxidative activities, membrane stabilisation and cytotoxicity of water spinach from ipogi ponds (Nigeria). International Journal of PharmTech Research., 2009; 1(3): 474-482.
- Khan M. S, Siddiqui S. P and Tarannum N. A systematic review on the synthesis and biological activity of hydrazide derivatives. Hygeia Journal for Drugs and Medicine., 2017; 9(1): 61-79. DOI:10.15254/H.J.D.Med.9.2017.165
CrossRef - Zhang L, Li J, Hogan S, Chung H, Welbaum G. E and Zhou K. Inhibitory effect of raspberries on starch digestive enzyme and their antioxidant properties and phenolic composition. Food Chemistry., 2010; 119(2): 592-599. https://doi.org/10.1016/j.foodchem.2009.06.063
CrossRef - Tamil I. G, Dineshkumar B, Nandhakumar M, Senthilkumar M and Mitra A. Invitro study on α-amylase inhibitory activity of an Indian medicinal plant, Phyllanthus amarus. Indian Journal of Pharmacology., 2010; 42(5): 280-282. doi: 10.4103/0253-7613.70107
CrossRef - Ting L, Zhang X. D, Song Y. W and Liu J. W. A microplate-based screening method for alpha-glucosidase inhibitors. Chinese Journal of Clinical Pharmacology and Therapeutics., 2005; 1(10): 1128-1134.
- Adam Kostelnik, Alexander Cegan and Miroslav Pohanka. Acetylcholinesterase inhibitors assay using colorimetric pH sensitive strips and image analysis by a smartphone. International Journal of Analytical Chemistry., 2017; Vol. 2017. Article ID: 3712384: 1-8. https://doi.org/10.1155/2017/3712384
CrossRef - Rasleen Sudan, Madhulika Bhagat, Sahil Gupta, Jasvinder Singh and Anupurna Koul. Iron chelation, Ferric reducing, antioxidant power and Immune modulating potential of Arisaema jacquemontii (Himalayan Cobra Lily). Biomedical Research International., 2014; vol. 2014, Article ID: 179865:1-7. https://doi.org/10.1155/2014/179865
CrossRef - Daba M. H and Abdel-Rehman M. S. Hepatoprotective activity of thymoquinone in isolated rat hepatocytes. Toxicology Letters., 1998; 95(1): 23-29. doi: 10.1016/s0378-4274(98)00012-5
CrossRef - Ngonda F. In-vitro antioxidant activity and free radical scavenging potential of roots of Malawian Trichodesma Zeylanicumm (burm.f.). Asian Journal of Biomedical and Pharmaceutical Sciences., 2013; 3(20): 21-25.
- Singleton L. V, Orthofer R and Lamuela-Raventos R. M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology., 1999; 299: 152-178. http://dx.doi.org/10.1016/S0076-6879(99)99017-1
CrossRef







