Manuscript accepted on :07-04-2023
Published online on: 10-01-2024
Plagiarism Check: Yes
Reviewed by: Dr. Fatma Çavuş Yonar
Second Review by: Dr. Niharika Kondepudi
Final Approval by: Dr Jihan Seid Hussain
Oyedeji K. O.* and Oyakhilome O. J.
Department of Physiology, College of Medicine and Health Sciences, Afe Babalola University, Ado-Ekiti, Nigeria.
Corresponding Author E-mail: sinaoyedeji@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2839
Abstract
This study was designed to investigate ovarian gene transcriptional responses to selected anticonvulsant drugs (diazepam and phenytoin) in female rats. Fifteen female rats (120 – 140 g) were used for this study. Diazepam (0.14 mg/kg) and phenytoin (2.8 mg/kg) were given to the rodents orally for 50 days. The method of RT-PCR involving isolation of RNA, conversion of cDNA and electrophoresis was employed to investigate the expression of FSH-R, aromatase and GPX-1genes. Graphics were generated as mean +/- SEM using Graph-pad Prism version 8.0. The expression of GPX-1 was significantly (p<0.05) up-regulated, while the FSH-R and aromatase expressions were significantly (p<0.05) and insignificantly (p>0.05) down-regulated respectively in the diazepam treated rats when compared with their respective controls. The FSH-R and aromatase expressions were significantly (p<0.05) up-regulated, while the GPX-1 expression was insignificantly (p>0.05) up-regulated in the phenytoin treated rats relative to their respective controls. Conclusively, it can be suggested that diazepam: inhibited follicular growth through the down-regulation of FSH-R expression, reduced estrogen level through the down-regulation of aromatase expression, inhibited the production of reactive oxygen species and oxidative stress through the up-regulation of GPX-1 expression. In addition, it can be suggested that phenytoin: induced follicular growth through the up-regulation of FSH-R expression, increased estrogen level through the up-regulation of aromatase expression, inhibited the production of reactive oxygen species and oxidative stress through the up-regulation of GPX-1 expression.
Keywords
Diazepam; FSHR; Phenytoin; RT-PCR; Rats
Download this article as:| Copy the following to cite this article: Oyedeji K. O, Oyakhilome O. J. Ovarian Gene Transcriptional Responses to Anticonvulsant Drugs (Diazepam and Phenytoin) in Female Wistar Rats. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Oyedeji K. O, Oyakhilome O. J. Ovarian Gene Transcriptional Responses to Anticonvulsant Drugs (Diazepam and Phenytoin) in Female Wistar Rats. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/3S9KGCi |
Introduction
Anticonvulsants are a group of drugs used to treat epilepsy 1. They can be found to be useful in curing bipolar disorder 2 and personality disorder because many of them appear to behave like mood stabilizers and analgesics 3. Anticonvulsants inhibit excess neuronal excitation during epilepsy as well as inhibiting epilepsy spread in the brain 4. Anticonvulsant drugs may act as sodium channel blockers or potentiate GABA function with many anticonvulsant drugs having numerous or unknown mechanisms of action; their targets include GABA receptors, calcium channels, SV2A e.t.c. 5 By obliterating the sodium or calcium channels, anticonvulsant drugs inhibit the release of glutamate which is responsible for excitation 6.
The effects of anticonvulsant drugs on: pharmacokinetic activity in rats 7, short-term cypermetrin poisoning in rodents 8, behavioural effect in rats 9, prenatal and early post natal exposure 10, rats’ teratogenicity 11 as well as on pregnant rats renal corpuscle 12 have been reported.
But, as a result of limited information obtained from literature concerning the effects of anticonvulsant agents (diazepam and phenytoin) on ovarian gene expression in female rats, hence, this research intends to bridge this gap.
Materials and Methods
Experimental Animals
Fifteen female rodents of weight range 120 – 140 g raised in the Animal Holding of ABUAD were used in the current study. These rodents were accommodated in a conducive laboratory atmosphere with unlimited supply of feed and water; the acclimatization period was for two weeks prior to starting the experiments. All animal experiments were done in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Drugs
Diazepam (Juhel Pharm, Nigeria Ltd.) and phenytoin (Merit Pharm, Ltd., India) were purchased from Danax Pharmacy, Ibadan, Nigeria. Among these, diazepam (5 mg) and Phenytoin (100 mg) were liquefied in l0 ml of distilled water to produce concentrations of 0.5 mg/ml and 10 mg/ml respectively. The dosages of the anticonvulsant agents considered in this study were in accordance with that reported by the manufacturing industries.
Experimental Design
Fifteen matured female rats (five per group) used for this study received the following oral doses of the anticonvulsant drugs and distilled water (control) for 50 days as follows:
Group I rodents (control group) were given 0.5 ml/100 g of distilled water.
Group II rodents were given 0.14 mg/kg of diazepam.
Group III rodents were given 2.8 mg/kg of phenytoin.
On the next day after the last treatment (day 51), the rodents were euthanized by overdosing with diethyl ether (Standard Reagents Ltd.); ovaries were harvested with the fatty tissue removed and transferred quickly into TRIzol reagent (ThermoFisher Scientific) for isolation of RNA.
Isolation of RNA
Total RNA was isolated from whole tissues following a method described by 13. Briefly, tissues were homogenized in cold (4 °C) TRI reagent (Zymo Research, USA, Cat:R2050-1-50, Lot: ZRC186885). Total RNA was partitioned in chloroform (BDH Analytical Chemicals, Poole, England Cat: 10076-6B) following centrifugation at 15,000 rpm/15 min (Abbott Laboratories, Model: 3531, Lake Bluff, Illinois, United States). RNA from the clear supernatant was precipitated using equal volume of isopropanol (Burgoyne Urbidges & Co, India, Cat: 67-63-0). RNA pellet was rinsed twice in 70% ethanol (70 ml absolute ethanol (BDH Analytical Chemicals, Poole, England Cat: 10107-7Y) in 30 ml of nuclease-free water (Inqaba Biotec, West Africa, Lot no: 0596C320, code: E476-500ML). The pellets were air-dried for 5 min and dissolved in RNA buffer (1 mM sodium citrate, pH 6.4).
Conversion of cDNA
Spectrophotometer (ThermoFisher Scientific Ltd.) was used to determine the purity and quantity of total RNA at an absorbance of A260/A280 as described by 13.
Polymerase chain reaction (PCR)/Electrophoresis
FSH-R, aromatase and GPX-1 genes were amplified by PCR targeting primers highlighted in the table below. A software called Primer3 was used to design the primers. The PCR amplification process was carried out as described by 13.
Amplification products were electrophoresed in agarose gel (1.5%) using 0.5X TBE (Tris-borate EDTA, JHD chemicals, China) containing ethidium bromide at 100V for 60 minutes. The gel was visualized with UV light with the aid of a photo documentation system (ThermoFisher Scientific Ltd.) fitted with a camera (ThermoFisher Scientific Ltd.). Gel images were analyzed using keynote platform as previously described by 14 and Image J software (National Institute of Health) was used to quantify it. Graph-pad prism version 8.0 was used to plot the graphs in the form of average +/- S.E.M.
Table 1: List of primers
|
Primer |
Sequence |
Product length |
Annealing temperature |
|
FSH-R
Aromatase |
F:ATTCTTGGGCACGGGATCTG R:TGGTGAGCACAAACCTCAGTT F:GCTTCTCATCGCAGAGTATCCGG R:CAAGGGTAAATTCATTGGGCTTGG |
140
192 |
55.09 °C 60.00 °C |
|
GPX-1 |
F:ATCAGTTCGGACATCAGGAGA R:TCACCATTCACCTCGCACTT |
124 |
53.93 °C |
Results
From figure 1, it was reported that FSH-R expression was down-regulated significantly (p<0.05) in the diazepam treated rats when compared with the control. Furthermore, from the results presented in figure 2, it was reported that aromatase was insignificantly (p>0.05) down-regulated in the diazepam treated rats as compared to the control. Similarly, figure 3 also suggested that GPX-1 expression was up-regulated significantly (p<0.05) in the diazepam treated rats relative to the control.
In addition, results presented in figures 1 and 2 revealed that the expressions of FSH-R and aromatase were significantly (p<0.05) up-regulated in the phenytoin treated rats relative to their controls; while the GPX-1 expression was insignificantly (p>0.05) up-regulated in the phenytoin treated rats relative to the control.
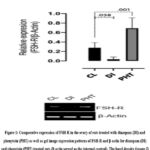 |
Figure 1: Comparative expression of FSH-R in the ovary of rats treated with diazepam (DI) and phenytoin (PHT) as well as gel image expression patterns of FSH-R and β-actin for diazepam (DI) and phenytoin (PHT) treated rats (β-actin served as the internal control). |
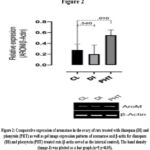 |
Figure 2: Comparative expression of aromatase in the ovary of rats treated with diazepam (DI) and phenytoin (PHT) as well as gel image expression patterns of aromatase and β-actin for diazepam (DI) and phenytoin (PHT) treated rats (β-actin served as the internal control). |
 |
Figure 3: Comparative expression of GPX-1 in the ovary of rats treated with diazepam (DI) and phenytoin (PHT) as well as gel image expression patterns of GPX-1 and β-actin for diazepam (DI) and phenytoin (PHT) treated rats (β-actin served as the internal control). |
Discussion
The FSH-R expression was significantly down-regulated in the diazepam treated rats which suggests that diazepam inhibited follicular growth. Similar result was reported by 15 in Pacific Oyster (Crassostrea gigas) treated rats, in which treatment with Pacific oyster (Crassostrea gigas) markedly decreased the expression level of FSH-R in the ovarian tissues of rats exposed to bisphenol. Contrarily, FSH-R expression was significantly up-regulated in the phenytoin treated rats which probably indicates that phenytoin induced follicular growth. Similar result was reported by 16 in Dandelion T-1 extract treated mice, in which FSH-R expression was significantly up-regulated in mice treated with Dandelion T-1 extract. These results were corroborated by the assertions of 17, 18.
The aromatase expression was insignificantly down-regulated in the diazepam treated rats which suggests that diazepam reduced estrogen level. Similar result was reported by 19 in Ginkgo biloba extract flavonoids treated JEG-3 cells, in which three flavonoids (kaempferol, quercetin, and isorhamnetin) from Ginkgo biloba extract synergistically inhibit estrogen biosynthesis through aromatase inhibition using JEG-3 cells. In contrast, aromatase expression was significantly up-regulated in the phenytoin treated rats which could mean that phenytoin increased estrogen level. Similar result was reported by 20 in PELP1 treated mice, in which PELP1 (proline, glutamic acid, leucine rich Protein 1) which is a novel estrogen receptor co-regulator up-regulated aromatase expression via activation of aromatase promoter in transgenic mouse model. These results were validated by the assertions of 21.
The GPX-1 expression was significantly and insignificantly respectively up-regulated in the diazepam and phenytoin treated rats which suggests that diazepam and phenytoin inhibited or decreased the production of reactive oxygen species and oxidative stress. Similar result was reported by 22 in young and aged bilaterally ovariectomized mice mitochondria oxidative stress investigation, in which there was in increased GPX-1 expression in bilaterally ovariectomized mice tissues in conditioned ovarian media. These results were corroborated by the assertion of 23.
Conclusion
Conclusively, it can be suggested that diazepam: inhibited follicular growth through the down-regulation of FSH-R expression, reduced estrogen level through the down-regulation of aromatase expression, inhibited the production of reactive oxygen species and oxidative stress through the up-regulation of GPX-1 expression. In addition, it can be suggested that phenytoin: induced follicular growth through the up-regulation of FSH-R expression, increased estrogen level through the up-regulation of aromatase expression, inhibited the production of reactive oxygen species and oxidative stress through the up-regulation of GPX-1 expression.
Limitation of the Sudy
Scantiness of prior research studies on the topic: There were limited information obtained from literature pertaining studies on ovarian gene transcriptional responses to anticonvulsant drugs (diazepam and phenytoin) in female rats prior to the commencement of this study.
Contribution to Future Studies
The mRNA (FSH-R, aromatase and GPX-1) expressed in this study should serve as precedence for future studies on their protein expressions.
Acknowledgement
The authors would like to acknowledge the Management of Afe Babalola University, Ado-Ekiti for supporting this work.
Conflict of Interest
There is absence of conflicting interests in this research work.
Funding Source
There is no funding sources
References
- Al-Otaibi F. An overview of structurally diversified anticonvulsant agents. Acta Pharmac. 2019; 69 (3): 321–344.
CrossRef - Joshi A., Bow M. Agius, Pharmacological therapies in bipolar disorder: a review of current treatment options. Psychiatr. Danubina. 2019; 31 (3): 595–603.
- Rogawski M.A., Löscher W. The neurobiology of antiepileptic drugs. Natur. Rev. Neurosci. 2004;5 (7): 553–64.
CrossRef - Harden C.L. New antiepileptic drugs. Neurol. 1994; 44 (5): 787–95.
CrossRef - Meldrum B.S., Rogawski M.A. Molecular targets for antiepileptic drug development. Neurotherapeut. 2007; 4 (1): 18–61.
CrossRef - Kammerer M., Rassner M.P., Freiman T.M. and Feuerstein T.J. Effects of antiepileptic drugs on GABA release from rat and human neocortical synaptosomes. Naunyn-Schmiedeberg’s Archiv. Pharmacol. 2011; 384 (1): 47–57.
CrossRef - Wolfgang L. The pharmacokinetics of antiepileptic drugs in rats: consequences for maintaining effective drug levels during prolonged drug administration in rat models of epilepsy. Epilepsia. 2007; 48: 1245–1258.
CrossRef - Salawu O.A., Iyaniwura T.T., Adaudi A.O. Effect of anticonvulsants on acute cypermetrin poisoning in mice and rats. Vet. Hum. Toxicol. 2000; 42:303-305
- Eva Z, Jan J, Iva K, Julius S. and Jana M. Behavioral effects of antiepileptic drugs in rats: are the effects on mood and behavior detectable in open-field test? Neurolog. 2017; 44 (5): 787–95.
- Kimono F, Takayuki M. and Takao T. Adverse effects of prenatal and early postnatal exposure to antiepileptic drugs: validation from clinical and basic researches. Brain Dev. 2017; 39 (8): 635-643.
CrossRef - Nely S., Aragão M., Reinaldo A. and Waldir A. Teratogenic effects of Lamotrigine on rat fetal brain, Arq. Neuropsiquiatr. 2001; 59 (2-B): 362-4.
CrossRef - Ayfer A., Yusuf N., Murat A. and Yasemin N. The effects of valproic acid on renal corpuscle of pregnant rats and protective role of folic acid and vitamin E. Afri. J. Biotech. 2010; 9 (34): 5605-5610.
- Omotuyi O.I., Nash O., Inyang O.K. and Ogidigo J. Flavonoid-rich extract of Chromolaena odorata modulate circulating glp-1 in wistar rats: Computational evaluation of tgr5 involvement. 3 Biotech. 2018; 8 (2): 124.
CrossRef - Omotuyi O.I., Nash O., Enejoh O.A. and Oribamise E.I. Chromolaena odorata flavonoids attenuate experimental nephropathy: Involvement of pro-inflammatory genes downregulation. Toxicol. Rep. 2020;7: 1421-1427.
CrossRef - Jue Z, Fan Q., Yue J. and Dong-Xia Y. The extracts of Pacific Oyster (Crassostrea Gigas) alleviate ovarian functional disorders of female rats with exposure to bisphenol a through decreasing FSHR expression in ovarian tissues. Afr. J. Tradit. Complement. Altern. Med. 2014; 11 (5): 1–7.
CrossRef - Xu Z, Ken-Ichi H., Koji O., Takuya M., et al. Dandelion T-1 extract up-regulates reproductive hormone receptor expression in mice. Interant. J. Molecul. Med. 2007; 20 (3): 287-292.
- Dewailly D., Robin G., Peigne M., Decanter C., Pigny P. and Catteau-Jonard S. Interactions between androgens, FSH, anti-Mullerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum Reprod Update. 2016; 22: 709–724.
CrossRef - Jamnongjit M., Hammes S.R. Ovarian steroids: the good, the bad, and the signals that raise them. Cell Cycl. 2006; 5: 1178–1183.
CrossRef - Yong J.P., Wun H.C., Ha R.K., Kyu H.C., et al. Inhibitory aromatase effects of flavonoids from Ginkgo biloba extracts on estrogen biosynthesis. Asian Pac. J. Cancer Prev. 2015; 16: 6317-6325.
CrossRef - Ratna K.V., Rajib R., Dimple C., Binoj C.N., et al. Regulation of aromatase induction by nuclear receptor coregulator PELP1. J Steroid Biochem. Mol. Biol. 2010; 118 (4-5): 211-8.
CrossRef - Carlos S. Aromatase expression in the ovary: Hormonal and molecular regulation. Steroids. 2008;73 (5): 473-487.
CrossRef - Valencia A.P., Schappal A.E., Morris E.M., Thyfault J.P., et al. The presence of the ovary prevents hepatic mitochondrial oxidative stress in young and aged female mice through glutathione peroxidase 1. Exp Gerontol. 2016; 73:14-22.
CrossRef - Burk R.F., Hill K.E. Regulation of selenium metabolism and transport. Annu. Rev. Nutr. 35 (2015) 109-34.
CrossRef







