Manuscript accepted on :08-05-2023
Published online on: 25-01-2024
Plagiarism Check: Yes
Reviewed by: Dr. Audrey Jacob
Second Review by: Dr. Hany Akeel
Final Approval by: Dr. Ian James Martin
Shaik Aminabee1* , K. Ravi Shankar2
, K. Ravi Shankar2 , KNV Chenchu Lakshmi2
, KNV Chenchu Lakshmi2 , K. Saritha2, R. Kavya3
, K. Saritha2, R. Kavya3 , K. Chaitanya Babu2
, K. Chaitanya Babu2 and Santhi Krupa Dasari4
and Santhi Krupa Dasari4
1Department of Pharmacology, V. V. Institute of Pharmaceutical Sciences, Gudlavalleru-521356, Krishna District, A.P., India.
2KVSR Siddhartha College of Pharmaceutical Sciences, Vijayawada, Krishna District, A.P., India.
3QIS College of Pharmacy, Ongole, Prakasam District, A.P., India.
4Krishna University, College of Pharmaceutical Sciences and Research, Machilipatnam, Krishna District, A.P., India.
Corresponding Author E-mail: aminaammi786@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2864
Abstract
This study depicts the selected dose of Allium sativum (104 mg/kg body weight) on the hypoglycemic activity of preferred dose of gliclazide which are studied in normal wistar rats. Materials required for the study are procured from Sai Chemicals, Visakhapatnam, India. Mature wistar rats of both the sex were used for the study. Prior to the trial the rats were confined for 18 hr with access to water ad libitum. During the study water was removed. Orally gliclazide was administered at 0.5 mg/kg, 1 mg/kg, 2 mg/kg body weight to I, II and III groups respectively. Blood samples are taken by retro-orbital puncture at intervals of 0, 1, 2, 3, 4, 6, 8, 10, 12 & 16 hr and blood glucose levels were determined by GOD/POD method. The acute dose of Allium sativum when given along with gliclazide increased the hypoglycemic activity of gliclazide at 2nd, 6th and 8th hr intervals but it was significant at 2nd hr only and the effect was shown to be reduced at all other ie., 1st, 3rd, 10th and 12th hr time intervals. The aqueous extract of Allium sativum is influencing the absorption pattern of gliclazide since it was reported to have an increasing motility of the gastro intestinal tract.
Keywords
Allium sativum; Drug Interaction; Gliclazide; Hypoglycemic Activity
Download this article as:| Copy the following to cite this article: Aminabee S, Shankar K. R, Lakshmi K. N. V. C, Saritha K, Kavya R, Babu K. C, Dasari S. K. Influence of Allium Sativum on the Hypoglycaemic Activity of Gliclazide in Normal Rats: A Possible Approach to Herb-Drug Interaction. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Aminabee S, Shankar K. R, Lakshmi K. N. V. C, Saritha K, Kavya R, Babu K. C, Dasari S. K. Influence of Allium Sativum on the Hypoglycaemic Activity of Gliclazide in Normal Rats: A Possible Approach to Herb-Drug Interaction. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/3Sv1eVn |
Introduction
The mechanisms behind the majority of reported herb-drug interactions are unclear. However, these interactions are significantly influenced by changed medication metabolism, absorption, and clearance caused by combination herbs. Numerous herbs can change medication concentration and clearance by inducing or inhibiting cytochrome P450s and other liver and intestine drug metabolizing enzymes. According to clinical investigations, cyclosporine, digoxin, amitriptyline, nevirapine, indinavir, theophylline, oral contraceptives, and simvastatin plasma concentrations were all decreased by St. John’s wort. Prednisolone, a synthetic steroid utilized in a variety of compounds as an anti-inflammatory, anti-allergic medication and immune suppressive. Licorice raises the plasma concentration of prednisolone1.The primary cause of these observed herb-drug interactions is thought to be the stimulation or inhibition of cytochrome P450s.
On the other hand, some components of herbs may interact with drugs in a synergistic or antagonistic manner by acting on the identical drug target molecules (such as receptors or enzymes). While antagonistic interactions can lead to decreased efficacy & therapeutic failure, combined outcomes may cause adverse effects & intricate the dose schedule of long-term medications2.
So monitoring drug therapy and study of food-drug interactions has become important to get a clear data about the food-drug interactions. It is very essential to study the food-drug interactions in tracking of pharmacotherapy along with other drugs in case of some disorders like diabetes and hypertension. Since a slight decrease or increase in the plasma concentration of the drug may lead to either decrease in the effect or may lead to toxic effect. Depending upon the data obtained modification in the dosage regimen (if at all needed) is done for the better therapeutic benefit with maximal safety.
Type-II diabetes is more common than type-I (juvenile) diabetes. Most preferable drug in the treatment of diabetes are sulphonylureas. Amongst them gliclazide is extensively used medication because of its high potency, long duration of action, lower incidence of side effects and antioxidant property. In these modern days the consumption of fast foods is increasing. Fast foods contain large amount of spices and many of these spices reported to have many biological properties. Allium sativum (garlic) is a most commonly used spices in many types of Indian, Arabic as well as in western diets3. Garlic is reported to lower blood glucosein animal models. Hence in the current research it is planned to find its influence on blood glucose in addition to gliclazide pharmacodynamic activity in normal rat models.
Materials and Methods
Inbred mature albino wistar rats of both the sex were purchased from Ghosh Enterprises, Kolkata, India. The prior authorization for the study was acquired from our Institutional Animal Ethics Committee (IAEC). Gliclazide (5 gm) sample was obtained from Wock Hardt Pharmaceuticals, Ourangabad, India. Blood glucose kits (Auto span) manufactured by Span diagnostics Limited, Surat, India are purchased from a local supplier. Standard animal pellet dietary supplements for animals, designed by Rayan Biotechnologies Private Limited, Hyderabad, India was taken. Gliclazide was dissolved in a few drops of 0.1 N Sodium Hydroxide and then diluted with distilled water to the desired volume. Allium sativum aqueous suspension was prepared by dissolving it in distilled water4.
In the study, albino wistar rats of both the sex ranging from 180-200 gm are used. Rats were split into three groups, each group with 6 rats. Rats were kept in a standard environment with a 12 hr /12hr light and dark cycle. They were kept in cages made of polypropylene. Rats were given a normal animal pellet diet along with water5.
Prior to the trial, the rats were fasted for 18 hr with access to water and ad libitum. Additionally water was withdrawn during the experiment. Gliclazide was administered orally at 0.5 mg/kg, 1 mg/kg, 2 mg/kg body weight to group-I, group-II and group-III respectively. By retro-orbital puncture, blood samples are withdrawn at 0, 1, 2, 3, 4, 6, 8, 10, 12 & 16 hr intervals to analyze blood glucose by GOD/POD method6.
Results
With gliclazide
A dose of 1 mg/kg body weight of gliclazide produced hypoglycaemia with about 43.78 (1 hr) and 41.51 (12 hr) mean percent reduction in blood glucose.The same dose was taken for interaction study. Dose dependent response was observed with the three doses tried (0.5, 1 and 2 mg/kg). The optimal blood glucose reduction, which is the STD of gliclazide was chosen based on ideal blood glucose reduction which is about 30-40%. 0.5 mg/kg of gliclazide (oral) produced 17.43 (1 hr) and 19.8 (4 hr) mean percent reduction in blood glucose and 2 mg/kg of gliclazide produced 57.75 (2 hr) and 56.4 (10 hr) mean percent reduction in blood glucose.The blood glucose levels observed with 0.5 mg/kg, 1 mg/kg, 2 mg/kg rat body weight of gliclazide were shown in table 1, 2 and 3 respectively. The percent blood glucose reduction with 0.5 mg/kg,1 mg/kg, 2 mg/kg rat body weight of gliclazide was shown in table 1, 2 and 3 respectively and the graphical representation was done in figure 1.
Table 1: Blood glucose levels (mg/dL) with gliclazide (0.5 mg/kg body weight) in normal rats.
|
Time (hr) |
Rats |
Mean ±SEM |
|||||
|
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
||
|
0 |
67 |
96 |
109 |
88 |
76 |
67 |
83.8±1.29 |
|
1 |
62 (7.4) |
80 (16.6) |
69 (36.9) |
49 (44.2) |
71 (6.58) |
73 (-7.0) |
70.5±3.03 |
|
2 |
65 (2.9) |
98 (-2.1) |
93 (14.86) |
56 (36.36) |
72 (5.45) |
41 (38.0) |
70.8±3.45 |
|
3 |
50 (25.37) |
69 (28.12) |
103 (5.4) |
48 (45.45) |
72 (5.26) |
74 (-10.4) |
69.6±2.49 |
|
4 |
45 (32.4) |
90 (6.24) |
65 (40.6) |
79 (10.23) |
79 (-3.4) |
47 (29.8) |
67.5±1.05 |
|
6 |
53 (20.89) |
95 (1.04) |
126 (-15.6) |
91 (-4.5) |
55 (27.63) |
47 (29.8) |
77.8±1.32 |
|
8 |
70 (-4.7) |
80 (-1.04) |
79 (27.5) |
79 (-3.4) |
74 (2.6) |
76 (14.9) |
77.6±1.71 |
|
10 |
59 (19.4) |
97 (1.04) |
134 (-22.4) |
87 (1.36) |
78 (-2.6) |
57 (3.0) |
88.16±1.75 |
|
12 |
74 (-10.4) |
89 (7.7) |
131 (-20) |
74 (15.4) |
67 (11.8) |
65 (16.83) |
85.3±2.22 |
Table 2: Blood glucose levels (mg/dL) with gliclazide (1 mg/kg body weight) in normal rats.
|
Time (hr) |
Rats |
Mean ±SEM |
|||||
|
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
||
|
0 |
101 |
89 |
83 |
103 |
112 |
115 |
100.5±2.8916 |
|
1 |
64 (36.63) |
54 (39.33) |
44 (46.99) |
58 (43.69) |
57 (49.11) |
61 (46.96) |
56.3±3.8044 |
|
2 |
57 (43.56) |
50 (43.82) |
66 (20.48) |
70 (32.04) |
90 (19.64) |
59 (48.69) |
65.3±3.7193 |
|
3 |
48 (52.47) |
62 (30.34) |
51 (38.55) |
59 (42.72) |
80 (28.57) |
71 (38.37) |
61.83±3.6395 |
|
4 |
54 (46.53) |
52 (41.57) |
45 (45.78) |
73 (29.13) |
62 (44.64) |
86 (25.72) |
62±5.2915 |
|
6 |
81 (19.8) |
40 (55.1) |
40 (51.81) |
82 (20.38) |
104 (7.14) |
86 (25.22) |
72.16±4.1081 |
|
8 |
88 (12.87) |
50 (43.82) |
55 (33.73) |
55 (46.6) |
64 (42.86) |
77 (33.04) |
64.83±2.2535 |
|
10 |
93 (7.92) |
50 (43.82) |
67 (19.27) |
56 (45.63) |
66 (41.06) |
76 (33.91) |
68±2.8156 |
|
12 |
91 (9.9) |
52 (41.57) |
58 (30.12) |
43 (58.25) |
52 (53.57) |
51 (55.65) |
57.83±3.0048 |
Table 3: Blood glucose levels (mg/dL) with gliclazide (2 mg/kg body weight) in normal rats
|
Time (hr) |
Rats |
Mean ±SEM |
|||||
|
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
||
|
0 |
103 |
114 |
76 |
104 |
107 |
78 |
97±1.16 |
|
1 |
27 (73.77) |
35 (69.29) |
32 (57.89) |
47 (71.1) |
30 (54.81) |
82 (-5.13) |
42.17±2.43 |
|
2 |
21 (79.61) |
25 (78.67) |
24 (68.42) |
28 (69.16) |
33 (73.67) |
95 (-21.79) |
37.67±2.42 |
|
3 |
40 (61.16) |
63 (44.74) |
61 (19.74) |
39 (44.86) |
59 (62.7) |
111 (-42.31) |
62.17±2.89 |
|
4 |
44 (57.28) |
76 (33.33) |
31 (59.21) |
27 (42.05) |
62 (74.04) |
104 (-33.33) |
57.33±2.02 |
|
6 |
42 (59.22) |
40 (64.91) |
29 (61.84) |
28 (83.24) |
18 (73.07) |
100 (-28.2) |
42.83±2.62 |
|
8 |
38 (63.11) |
40 (64.91) |
24 (68.42) |
22 (82.2) |
19 (78.85) |
104 (-33.3) |
41.17±1.37 |
|
10 |
40 (61.16) |
31 (72.18) |
33 (56.58) |
23 (73.83) |
28 (77.88) |
81 (-3.84) |
39.33±4.39 |
|
12 |
46 (55.34) |
41 (64.03) |
26 (65.79) |
30 (71.96) |
30 (71.15) |
112 (-43.58) |
47.5±3.57 |
 |
Figure 1: Percent reduction of blood glucose with different doses of gliclazide in normal rats (n=6). |
With Allium sativum
Allium sativum has shown effect on the blood glucose levels when administered alone. The blood glucose levels observed with 1 mg/kg of gliclazide prior to and following treatment of Allium sativum (104 mg/kg) were shown in table 2 and 5 respectively. The mean percent blood glucose reductions observed with 1 mg/kg of gliclazide before and after Allium sativum (104 mg/kg) treatment were shown in table 2 and 5 respectively and graphically represented in figure 2.
The blood glucose levels and percent blood glucose reduction with Allium sativum (104 mg/kg) were shown in table 4.
The combination of Allium sativum (104 mg/kg) and gliclazide (1 mg/kg) produced a mean percent blood glucose reduction 53.8 (2 hr) and 41.3 (8 hr) (table 5), while gliclazide matching control produced 43.78 (1 hr) and 35.48 (8 hr) (table 2).
The student’s paired t-test was applied to the data to find out the statistical significance between combination (Allium sativum + gliclazide) group and gliclazide matching control group. The enhancement of gliclazide induced hypoglycaemic effect by Allium sativum was statistically significant at time intervals of the study i.e. at 2nd hr intervals.
Table 4: Blood glucose levels (mg/dL) with Allium sativum (104 mg/kg body weight) in normal rats
|
Time (hr) |
Rats |
Mean ±SEM |
|||||
|
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
||
|
0 |
75 |
110 |
74 |
69 |
62 |
70 |
76.6±6.9 |
|
1 |
80 (-6.6) |
76 (-8.8) |
87 (-17.56) |
67 (2.9) |
72 (-16.9) |
75 (-7.1) |
76.2±2.7 |
|
4 |
82 (-9.3) |
79 (55.4) |
91 (-22.9) |
71 (-2.89) |
76 (-22.6) |
74 (-5.7) |
79±2.8 |
|
8 |
56 (25.3) |
52 (52.72) |
72 (2.7) |
59 (14.49) |
44 (29.0) |
60 (14.3) |
57.1±3.7 |
|
10 |
57 (24.0) |
58 (47.27) |
80 (-8.1) |
71 (-2.89) |
38 (38.7) |
79 (-12.8) |
63.8±6.5 |
|
12 |
50 (33.3) |
48 (56.36) |
81 (-9.4) |
57 (25.0) |
45 (27.41) |
58 (17.1) |
56.4±5.3 |
|
16 |
41 (45.3) |
48 (56.36) |
67 (9.45) |
58 (22.4) |
72 (-16.12) |
58 (17.1) |
57.2±4.7 |
|
20 |
55 (26.6) |
47 (56.0) |
69 (6.75) |
58 (7.2) |
72 (2.67) |
58 (37.0) |
55.8±3.7 |
|
24 |
62 (17.3) |
55 (50.0) |
71 (4.05) |
64 (7.2) |
62 (0.00) |
55 (21.4) |
61.5±4.6 |
Table 5: Blood glucose levels (mg/dL) with gliclazide (1mg/kg body weight) in combination with Allium sativum (104 mg/kg body weight) in normal rats
|
Time (hr) |
Rats |
Mean ±SEM |
|||||
|
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
||
|
0 |
70 |
59 |
71 |
65 |
67 |
66 |
66.4 ± 1.7 |
|
1 |
55 (21.4) |
28 (52.5) |
45 (35.7) |
37 (43.1) |
67 (0.00) |
37 (43.9) |
44 ± 5.7 |
|
2 |
35 (50.0) |
30 (53.8) |
44 (38.0) |
42 (35.4) |
34 (49.3) |
29 (56.1) |
30.7 ± 2.5 |
|
3 |
53 (24.2) |
38 (35.6) |
52 (26.8) |
60 (7.7) |
42 (37.3) |
52 (21.2) |
49.5 ± 3.3 |
|
4 |
56 (-20.0) |
24 (59.3) |
54 (23.9) |
43 (36.1) |
40 (40.3) |
38 (42.4) |
42.4 ± 4.7 |
|
6 |
40 (42.8) |
33 (44.1) |
47 (33.8) |
45 (30.1) |
40 (40.3) |
37 (37.9) |
41 ± 2.1 |
|
8 |
37 (47.1) |
29 (50.8) |
48 (32.3) |
47 (27.7) |
36 (46.3) |
37 (43.9) |
39 ± 3 |
|
10 |
49 (30.0) |
41 (30.5) |
61 (14.1) |
49 (24.6) |
37 (44.8) |
42 (36.4) |
46.5 ± 3.5 |
|
12 |
48 (-31.4) |
40 (32.2) |
51 (28.2) |
44 (32.3) |
36 (46.3) |
39 (40.1) |
43 ± 2.4 |
*Significant at P < 0.05 compared to gliclazide (1mg/kg) matching control.
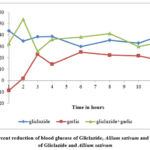 |
Figure 2: Percent reduction of blood glucose of Gliclazide, Allium sativum and combination of Gliclazide and Allium sativum |
Discussion
A biphasic blood glucose level drop was caused by gliclazide. It is known that sulphonylureas stimulate insulin secretion and improve glucose uptake at cellular level. It is well established that insulin cause secretion and improve tissue utilization of glucose at cellular levels7-9. The biphasic effect might be due to its secretion and reabsorption through bile into the duodenum by Entero-hepatic cycling as reported earlier. Allium sativum alone produced a slight reduction in blood glucose level, this could be because of the presence of S-allyl cysteine and other proposed mechanism of actions was insulin secretagogue through pancreatic secretion/release of bound insulin10-12.
The acute dose of Allium sativum when administered in combination with gliclazide enhanced the hypoglycemic activity of gliclazide at 2nd, 6th and 8th hr intervals but it was significant at 2nd hr only and the effect was shown to be reduced at all other ie.,1st, 3rd,10th and 12th hr time intervals. This shows that the aqueous extract of Allium sativum is influencing the absorption pattern of gliclazide since it was reported to have an increasing motility of the gastro intestinal tract13-14.
Conclusion
The dose related hypoglycaemic effect was observed for 0.5 mg/kg, 1 mg/kg, 2 mg/kg rat body weight of gliclazide with in normal rats. Allium sativum alter the blood glucose level, but when given in combination with gliclazide, enhanced the hypoglycaemic impact of gliclazide in normal rats demonstrating the existence of interaction.
Acknowledgement
Authors are thankful to V. V. Institute of Pharmaceutical Sciences, Gudlavalleru, India for providing necessary facilities of research work.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There are no funding sources.
References
- Eswar Kumar K, Ramesh A, Yadav RS, Satyanarayana S. Determination of gliclazide in rabbit serum by RP-HPLC. Acta Ciencia Indica. Chemistry. 2007;33(3):273-278.
- Trinder P. Determination of blood glucose using an oxidaseperoxidase system with a non-carcinogenic chromogen. Journal of Clinical Pathology. 1969;22(2):158-161.
CrossRef - Beatrice I. Research article:Effects of Garlic on Cytochromes P450 2C9and 3A4-Mediated Drug Metabolism in Human Hepatocytes.Sceintia. 2010;78:473-481.
CrossRef - Chang-Kai Yan, Fan-Dian Zeng. Pharmacokinetics and Tissue Distribution of S-allylcysteine in Rats.Asian Journal of Drug Metabolism and Pharmacokinetics. 2005;5(1):61-69.
- Sanjay K, Banerjee, Subir K Maulik. Effect of garlic on cardiovascular disorder: A review.Nutritional journal. 2002;1:4.
CrossRef - Augusti KT, Sheela CG. Antiperoxide effect of S-allyl cysteine sulfoxide, A insulin secretagoguein diabetic rats. Experientia. 1996;52:115-120.
CrossRef - Mathew PT, Augusti KT. Studies on the effect of allicin (diallyl disulphide-oxide) on alloxan diabetes I. Hypoglycaemic action and enhancement of serum insulin effect and glycogen synthesis. Indian J BiochemBiophys. 1973;10:209-212.
- Sheela CG, Augusti KT. Antidiabetic effects of S-allyl cysteine sulphoxide isolated from garlic Allium Sativum Linn. Indian J Exp Biol. 1992;30:523-526.
- H. Miyazaki T, FujiiK, Yoshida S. Arakawa, Furukawa H. Disposition and metabolism of [3H] gliclazide in rats. European Journal of Drug Metabolism and Pharmacokinetics. 1983;2(4):117-131.
CrossRef - Cerveny JC, Leder ,Weart CW. Issues surrounding tight glycemic control in people with type 2 diabetes mellitus. Ann Pharmacother. 1998;32(9):869-905.
CrossRef - Fetrow CW, Avila JR. Professionsals Handbook of Complementary and Alternative Medicine. Springhouse Corporation.1999.
- Gribble FM, Tucker SJ, Seino S, Ashcroft FM. Tissuue specificity of sulfonylureas: studies on cloned cardiac and beta-cell K(ATP) channels. Diabetes. 1998;47(9):1412-1418.
CrossRef - Schernthaner G. Gliclazide modified release: A critical review of pharmacodynamic, metabolic and vasoprotective effects. Metabolism. 2003;52(8):29-34.
CrossRef - Zeigler O, Drouin P. Hemobiological properties of gliclazide. J diabetes complications. 1994;8(4):235-239.
CrossRef







