Manuscript accepted on :11-03-2024
Published online on: 19-03-2024
Plagiarism Check: Yes
Reviewed by: Dr. Hind Shakir and Dr. Ramlah Kadir
Second Review by: Dr. Tetty A
Final Approval by: Dr. Luis Jesús Villarreal-Gómez
Ashraf Askar 1 , Adel El-Sayed 1
, Adel El-Sayed 1 , Lyla Yosef 1, Osama Abdelaal 1
, Lyla Yosef 1, Osama Abdelaal 1 , Eman Sabet 2
, Eman Sabet 2 , Ahmed Sadek 3
, Ahmed Sadek 3 , Wafaa Wafy 4, Mina Wassef Girgiss 5
, Wafaa Wafy 4, Mina Wassef Girgiss 5 , Moushira Zaki 6
, Moushira Zaki 6 and Eman R. Youness 7*
and Eman R. Youness 7*
1Department of Internal Medicine, Faculty of Medicine, Sohag University.
2Clinical Pathology Department, Faculty of Medicine, Sohag University.
3Department of Hepatology and Gastroenterology, Theodor Bilharz Research Institute, Imbaba Giza, Egypt.
4Public Health Department, Theodor Bilharz Research Institute, Imbaba Giza, Egypt.
5Medical Department, Medical Researches and Clinical Studies Institute, National Research Centre Cairo, Egypt.
6Department of Biological Anthropology, Medical Researches and Clinical Studies Institute, National Research Centre Cairo, Egypt.
7Department of Medical Biochemistry, Medical Researches and Clinical Studies Institute, National Research Centre Cairo, Egypt.
Corresponding Author E-mail: hoctober2000@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2871
Abstract
Microalbuminuria is defined as an elevated urine albumin to creatinine ratio of 30-300 g/mg. It has been recognized as a strong indicator of the onset of diabetic nephropathy. Damage to vascular endothelium and systemic inflammation can result from H. Pylori infection. Thus, it stands to reason that the resulting glomerular damage might raise the excretion of albumin in the urine. Our goal was to find out if H. pylori and microalbuminuria are related in individuals with diabetes mellitus (DM). This is a case – control observational study conducted in 6 months. Random blood glucose, Urinary Albumin Creatinine Ratio (UACR) and H. Pylori Antigen (H. Pylori antigen) in stool were measured. To minimize possible confounding factors, selecting various matching variables was put consideration when choosing the control group. Therefore, most of the matching variables of the study were comparable in both groups makes the results of studying of H. pylori as a possible risk factor for microalbuminuria greatly accurate. we concluded that H. pylori infection is highly suggested to be an independent risk factor for the development of microalbuminuria in diabetic patients. As proven by the present work; a highly significant statistical relationship between H. pylori infection and the presence of microalbuminuria in patients with diabetes.
Keywords
Diabetic patients; Helicobacter Pylori seropositivity; low grade inflammation; UACR microalbuminuria
Download this article as:| Copy the following to cite this article: Askar A, El-Sayed A, Yosef L, Abdelaal O, Sabet E, Sadek A, Wafy W, Girgiss M. W, Zaki M, Youness E. R. Helicobacter Pylori Infection and Microalbuminuria in Diabetic Patients. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Askar A, El-Sayed A, Yosef L, Abdelaal O, Sabet E, Sadek A, Wafy W, Girgiss M. W, Zaki M, Youness E. R. Helicobacter Pylori Infection and Microalbuminuria in Diabetic Patients. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/43nO8x0 |
Background
The bacteria Helicobacter pylori (H. pylori) causes persistent gastric irritation 1. it is the most frequent and widespread illness in the world2. It is primarily found in the stomach and duodenum and is brought on by gram-negative, spiral-shaped acidophilic bacteria. According to estimates, 40% of Americans and more than half of the global population are infected with HP3. In developing nations, the incidence is higher. HP infection risk factors include contaminated food and water, poor hygiene, low socioeconomic level, crowded living conditions, smoking, and contact with infected individuals 4. H. pylori seropositivity was found to be associated with coronary artery calcium scores 5. A meta-analysis of cross-sectional studies revealed that the presence of H. pylori infection was connected to low levels of High-density lipoprotein cholesterol (HDL-C), Low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), or high levels of triglycerides (TG) or 6,7,8. Additionally, a prospective single-center trial revealed that eliminating H. pylori improved lipid abnormalities, low-grade inflammation, and insulin resistance (IR) 8. All of these data imply that patients with metabolic syndrome, including diabetes, may also have H. pylori infection. A higher urine albumin to creatinine ratio (UACR) of 30–300 g/mg is known as microalbuminuria 9. Although microalbuminuria is used as a standard screening tool since it is an early indicator of diabetic nephropathy (DN), renal damage may be occurring even in the absence of this symptom. Several signficant kidney diseases and damage biomarkers aid in the early identification of DN 10. Microalbuminuria has also been shown to increase the risk of cardiovascular disease in both non-diabetic and diabetic populations 11. Although the exact cause of the association between microalbuminuria and cardiovascular morbidity is unknown, one theory is that the increased urine albumin leakage is a sign of vascular damage, such as endothelial dysfunction or low-grade chronic inflammation 12.
This work aims to investigate the association between microalbuminuria and H. pylori infection in diabetic patients.
Methods
The present work is a case – control observational study carried out atTheodor Bilharz Research institute (TBRI) from 1 August 2021 to 1 Septemper 2022 . The study included all diabetic patients who agreed to share in the study, signed an informed written consent, and fulfilled the inclusion criteria; the case group included 60 diabetic patients with microalbuminuria, and the control group had[ 50 diabetics without microalbuminuria. Personal data including name, age, gender, number of years with diabetes, and smoking (those who had quit smoking for more than six months were considered nonsmokers).After measuring height in meters and weight in kilograms, the body mass index (BMI) was computed as follows: weight in kilograms divided by height in meters2. Blood pressure was measured using a mercurial sphygmomanometer, and hypertension was diagnosed in cases of increased blood pressure in patients who were not previously recognized to have it (Blood pressure readings less than 140/90 mmHg were considered normal and high blood pressure was considered if blood pressure was >= 140/90 mmHg).
Laboratory investigations
Sample collection
Blood sample
Both the case and control groups had 5 mL of venous blood obtained under aseptic circumstances. The samples were split into two groups
Using EDTA (Ethylene Diamine Tetra Acetic acid), 2ml of blood is used to measure Hb A1C.
Serum lipid profile (total serum cholesterol, HDL, LDL, and TG) are determined using 3 cc of blood in a sterile, simple vacationer.
Stool sample for H. Pylori Ag:
Fresh or frozen faeces samples were used for the test. The sample was kept at a temperature of -20°C or below if the test could not be wholly completed in a single day. 100 mg of faeces was added to 1 ml of the sample dilution buffer (SAMPLEBUF), which was then completely homogenized using a vortex mixer. The suspension was centrifuged at 3000 rpm for 15 minutes. The commercially available (ELISA) enzyme linked immunosorbent assay, given by Pishtaz Teb Diagnostics, was used to screen for H. Pylori Antigen (H. Pylori Ag) in faeces. The ELISA test was conducted in accordance with the manufacturer’s recommendations. ELISA reader from Thermo was used to analyze the data. Cut- off values for the detection of Helicobacter pylori Ag in faeces are 0,150 at 450/620 nm and 0,190 at 450 nm. Samples are deemed positive if their absorbance is greater than 0,020 (450/620 nm measurement) or 0,025 (450 nm measurement) above the cutoff threshold. As borderline samples, those with absorbance’s 0,020 (0,025) below or over the cutoff value underwent a second analysis. A new sample was analyzed if the result of the repeated measurement fell once again inside the borderline area. Samples classed as unfavourable have an absorbance below the cutoff threshold.
Urine sample
An autoanalyzer Roche HITACHI Cobas C 311 system was used to collect, centrifuge, and analyze a fasting single-void fresh morning urine sample in order to calculate the Urinary Albumin Creatinine Ratio (UACR) and assess microalbuminuria (g/mg).
Inclusion criteria
All diabetic mellitus patients who had consented to participate in the trial were incorporated.
Exclusion criteria
This study excludeed patients with macroalbuminuria (UACR > 300 ug/mg). Prior to collecting the data, patients were split into two groups based on whether they had microalbuminuria or not. Control group: included 50 diabetics without microalbuminuria. The case group included 60 diabetics with microalbuminuria.
Ethical approval
The study protocol was granted TBRI Ethical Committee approval number (FWA 00010609).
Statistics
The Statistical Package for Social Science (SPSS), 20th edition, was used with Windows 7 to gather and analyze the information. Body mass index (BMI), blood pressure reading, age, gender, length of diabetes, smoking status, Hb A1c level, and serum lipids statistics were performed in both groups to demonstrate the degree of similarity. The connection between microalbuminuria and H. Pylori stool Ag and consequently, current H. Pylori infection in both groups was investigated using the Pearson Chi-Square Test. If the P value was more than 0.05, it was deemed to be inconsequential. If it was lower than 0.05, it was deemed to be significant. Logistic regression analysis was used to detect difference in risk variables between diabetic cases with the H. Pylori and those without using the odds ratios (ORs).
Results
This case group included 60 diabetic patients with microalbuminuria and the control group without microalbuminuria. The mean age of the cases was 57.93 ± 9.174 years, while for the control group it was 55.74 ± 10.596 years. Concerning gender, 61.67% of the cases group was male while in controls, males represented 54% of the group (Table 1). Of the cases group, 56.67 % were smokers, and in the control group, smokers represented 54% of the group . Concerning the duration of diabetes, the mean duration was 8.47 ± 3.039 years for case group, while for the econtrol group, it was 9.54 ± 2.83 years. The mean body mass index of the case group was (25.3 ± 2.9), and in control group, it was (24.3 ± 3.1). The blood pressure of 40% of cases group was normal, while 42% of control group had normal blood pressure . The mean HbA1c level of the case group was 7.93 ± 1.09 and 28.3% of this group had controlled HbA1c level while, in the control group, the mean HbA1c level was 8.1 ±1.29 and 26% of the group had controlled HbA1c level . The mean total serum cholesterol of the case group was (166.93 ± 53.759), while in control group it was (178.58 ± 48.382). The mean serum HDL in the case group was (29.18 ± 8.056), while in control group, it was (30.88 ± 14.988). The mean serum LDL in the case group was (119.7 ± 48.61), while in control group it was (113.46 ± 47.903), The mean serum Triglycerides was (126.82 ± 66.116), while in the control group, it was (120 ± 68.36). There were no statistical difference between the two groups (Table 2). Concerning H. Pylori stool Ag, 83.3% of the case group had positive results while 52% of the control group had positive results (Pearson Chi- Square Test was used to examine the association between the microalbuminuria as the dependent variable and the H. Pylori infection (indicated by positive H. Pylori stool Ag) as the independent variable and the results were as follows: Pearson Chi-Square value was 12.538 and P value was <0.01 indicating that there is a highly significant statistical relationship between microalbuminuria in diabetic patients and positivity of H. pylori stool antigen which means current H. Pylori infection. Univariate logistic regression statistical model was used to assess the association of H. Pylori with the studied variables (Table 3) by comparing between H. pylori-infected individuals with the H. pylori-uninfected individuals. The OR of microalbuminuria in cases with H. pylori-infected diabetic cases was 2.88 in comparsion to uninfected (p < 0.01). The other studied biomarkers were not statistically significant. Figure 1 shows the relationship between microalbuminuria and H. Pylori stool Ag).
Table 1: Gender, smoking and H. Pylori stool Ag of both study groups
|
Study Group |
Gender |
Number of patients |
Percent % |
|
Diabetic patients |
Male Female |
37 23 |
61.67 38.33 |
|
Total |
60 |
100 |
|
|
Control
|
Male Female |
27 23 |
54 46 |
|
Total |
50 |
100 |
|
|
Diabetic patients |
Smoking |
Number of patients |
Percent % |
|
Yes No |
34 26 |
56.67 43.33 |
|
|
Total |
60 |
100 |
|
|
Control |
Yes No |
27 23 |
54 46 |
|
Total |
50 |
100 |
|
|
Diabetic patients |
H. Pylori stool Ag |
Number of patients |
% |
|
Positive Negative |
50 10 |
83.3 16.7 |
|
|
Total |
60 |
100 |
|
|
Positive Negative |
26 24 |
52 48 |
|
|
Total |
50 |
100 |
|
|
Control |
Positive Negative |
26 24 |
52 48 |
|
Total |
50 |
100 |
Table 2: Clinical and laboratory characteristics of patients and control group
|
Characteristic |
Diabetic patients Mean ± SD |
Control Mean ± SD |
p |
|
HbA1c (%) |
8.1 ±1.29 |
6.9 ±1.0 |
0.77 |
|
Systolic blood pressure (mm Hg) |
140.10±10.56 |
130±8.33 |
0.67 |
|
Diastolic blood pressure (mm Hg) |
86.52±.12.9 |
79.98±13.78 |
0.65 |
|
BMI(kg/m2 ) |
25.3 ± 2.9 |
24.3 ± 3.1 |
0.84 |
|
Cholesterol (mg/dL) |
166.93 ± 53.759 |
178.58 ± 48.38 |
0.55 |
|
HDL (mg/dL) |
29.18 ± 8.056 |
30.88 ± 14.988 |
0.86 |
|
LDL (mg/dL) |
119.7 ± 48.61 |
113.46 ± 47.903 |
0.46 |
|
Triglycerides (mg/dL) |
126.82 ± 66.116 |
120 ± 68.36 |
0.72 |
Table 3: Univariate logistic regression analyses for determining the association of H. pylori infection with the risk parameters in diabetic patients.
|
Variables |
OR |
p-value |
|
Increased HbA1c |
0.629 |
0.54 |
|
Increased Systolic blood pressure (mm Hg) |
0.63 |
0.56 |
|
Increased Diastolic blood pressure (mm Hg |
0.83 |
0.66 |
|
BMI (kg/m2 ) ≥ 30 |
0.99 |
0.45 |
|
Smoking |
0.89 |
0.75 |
|
Increased LDL-C (mg/dL) |
0.78 |
0.67 |
|
Decreased HDL-C (mg/dL) |
0.67 |
0.95 |
|
Increased Triglycerides (mg/dL) |
0.88 |
0.85 |
|
Microalbuminuria |
2.88 |
< 0.01 |
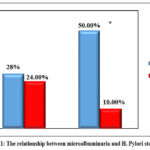 |
Figure 1: The relationship between microalbuminuria and H. Pylori stool Ag |
Discussion
This case-control study investigated the relationship between diabetic individuals’ microalbuminuria and H. pylori infection. In order to minimize possible confounding factors, we have chosen various matching variables to be put into consideration when choosing the control group. These factors were age, where the mean age of the case group was 57.93 ± 9.174 years, while for the control group, it was 55.74 ± 10.596 years. These means are markedly comparable. Concerning gender, 61.7% of the case group were males, while in controls, males represented 55% of the group, but gender is not known to affect microalbuminuria and it was only estimated as it is a part of socio-demographic data. Duration of diabetes: the mean duration was similar in both groups. It was 8.47 ± 3.039 years for case group, and 9.54 ± 2.83 years for the control group. Smoking: 56.67% of case group were smokers, and 54% of the control group were smokers and Percent is nearly the same in both groups. The mean BMI of the case group was (25.3 ± 2.9), while in control group, it was (24.3 ± 3.1) and these numbers are also similar in both groups. Blood pressure: 40% of case group and 42% of control group had normal blood pressure. Percent is nearly the same in both groups. The mean HbA1c level of the case group was 7.93 ± 1.09 and 28.3% of this group had controlled HbA1c level, while, in control group, the mean HbA1c level was 8.1 ±1.29 and 26% of the group had a controlled HbA1c level and these levels are greatly comparable. Regarding, blood lipids all levels of HDL, LDL and TG are comparable. Thus, most of the matching variables of the study were comparable in both group which makes the results of studying H. pylori as a possible risk factor for microalbuminuria are remakably accurate. On examining the relationship between microalbuminuria as the dependent variable and the H. pylori infection (indicated by positive H. Pylori stool Ag) as the independent variable, results showed that there was a highly significant statistical association between H. pylori infection, as indicated by positive H. Pylori Ag in stool, and microalbuminuria in diabetic patients1314. This indicates that microalbuminuria in diabetic patients is independently correlated with H. Pylori infection15. According to these results, H. infection via pylori might have an influence on microvascular damage and might even play a role in the etiology of early atherosclerosis in diabetics. Although the causes of augmented urine albumin secretion are unknown, endothelial cell dysfunction seems to be a significant pathogenic factor 16. Furthermore, microalbuminuria was related with atherogenic risk factors, as hyperinsulinemia, central obesity, hyperglycemia and hypertension 6. The capacity of H. pylori to colonize gastric epithelial cells through the direct action of soluble bacterial components or adhesions that facilitate interaction of bacterial cells with epithelial cell receptors is the basis for the unique method of H. pylori invasion and survival in the organism 17. H. pylori seropositivity was linked to soluble intercellular adhesion molecule-1 and higher levels of C-reactive protein (CRP) , according to previous research suggesting association between the infection and endothelial dysfunction 18. This suggests that the pathophysiology of atherosclerosis may involve a persistent H. pylori infection. The association between IR and H. pylori infection suggests a putative pathway connecting H. pylori infection and endothelial dysfunction. A homeostatic model for determining insulin resistance and H. pylori infection were found to be positively correlated 19. A large Japanese population showed a substantial link between metabolic syndrome and H. pylori infection. Eliminating H. pylori had a positive impact on IR low-grade inflammation and blood lipids, according to a prospective trial. The impact of H. pylori on the pathogenic process of insulin resistance is yet unclear. The disruption of pro-inflammatory and vasoactive compounds, as interlukin-6, and C-reactive protein , tumor necrosis factor- α, may play a role in the etiology of IR] 2021. Additionally, it is impacted by the reactive oxygen species brought on through an H. pylori infection 2223. Increased amounts of urine albumin are independently correlated with cigarette smoking. Type2 diabetic patients who were active smokers showed a higher annual incidence of microalbuminuria compared to non-smokers 2425. Numerous studies have shown that microalbuminuria in diabetic people is related to high blood pressure, inadequate glycemic control, ageing, and insulin resistance 26. Due to recognized impairments in cellular and humoral immunity in diabetes patients, direct bacterial invasion of the artery wall may occur in these patients more frequently than in non-diabetic individuals 12. Some earlier research have discovered that diabetes patients had a greater incidence of H. pylori infection 27. However, other research did not find a connection between diabetes and H. pylori infection 28. Additionally, the disparate methodologies utilized by the researches and the irregular H. pylori infection epidemiological distribution may be to blame for the inconsistent findings. There was only one study that examine the connection between H. pylori and IR in a cross sectional observational study, reporting that in a sizable asymptomatic population, H. Pylori infection was a major and independent factor in the promotion of insulin resistance 29. Yet, that study had some boundaries, first, it was a cross sectional one and it is well known that this type of studies is weaker than a case control one. Second, it used H. pylori serum antibody and in fact that does not necessarily indicate current infection but it may refer to a past infection. On the other hand, this study has several advantages. First, it is a case control one and this type of study is the ideal one for the initial examination of a putative risk factor for a prevalent ailment. Second, this study invesigates H. pylori stool Ag which indicates current H. pylori infection and did not use the serum antibody which might indicate past infection and thus we avoided possibility of false negative or false positive subjects. Third, to the best our knowledge, this is the first case control study investigating the relationship between microalbuminuria and H. pylori infection. Last, this study has chosen control group with similar matching variables with the case group which minimizes the profounding factors and makes the results are highly accurate.
Conclusion
The present study showed a highly relation between H. pylori infection and microalbuminuria in diabetic patients. Thus, H. pylori infection is highly suggested to be an independent risk factor for development of microalbuminuria in diabetic patients. More researches are needed to confirm our results and those researches should consider studying H. pylori-induced inflammatory and virulence factors specially cag A gene.
Acknowledgments
Many thanks to all the participants
Conflicts of interest
None
Funding source
No funding
Reference
- Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Off J Am Coll Gastroenterol ACG. 2017;112(2):212-239.
CrossRef - Tshibangu-Kabamba E, Yamaoka Y. Helicobacter pylori infection and antibiotic resistance—from biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2021;18(9):613-629.
CrossRef - Milivojevic V, Milosavljevic T. Burden of gastroduodenal diseases from the global perspective. Curr Treat Options Gastroenterol. 2020;18:148-157.
CrossRef - Nazmi A, Diez-Roux A V, Jenny NS, Tsai MY, Szklo M, Aiello AE. The influence of persistent pathogens on circulating levels of inflammatory markers: a cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis. BMC Public Health. 2010;10(1):1-8.
CrossRef - Park MJ, Choi SH, Kim D, et al. Association between Helicobacter pylori seropositivity and the coronary artery calcium score in a screening population. Gut Liver. 2011;5(3):321.
CrossRef - Kim YI, Kim CH, Choi CS, et al. Microalbuminuria is associated with the insulin resistance syndrome independent of hypertension and type 2 diabetes in the Korean population. Diabetes Res Clin Pract. 2001;52(2):145-152.
CrossRef - Scheithauer TPM, Rampanelli E, Nieuwdorp M, et al. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front Immunol. 2020;11:571731.
CrossRef - Shimamoto T, Yamamichi N, Gondo K, et al. The association of Helicobacter pylori infection with serum lipid profiles: An evaluation based on a combination of meta-analysis and a propensity score-based observational approach. PLoS One. 2020;15(6):e0234433.
CrossRef - Résimont G, Cavalier E, Radermecker R, Delanaye P. Albuminuria in diabetic patients: how to measure it?-a narrative review. J Lab Precis Med. 2022;7.
CrossRef - Thipsawat S. Early detection of diabetic nephropathy in patient with type 2 diabetes mellitus: A review of the literature. Diabetes Vasc Dis Res. 2021;18(6):14791641211058856.
CrossRef - Romundstad S, Holmen J, Hallan H, Kvenild K, Krüger Ø, Midthjell K. Microalbuminuria, cardiovascular disease and risk factors in a nondiabetic/nonhypertensive population. The Nord‐Trøndelag Health Study (HUNT, 1995–97), Norway. J Intern Med. 2002;252(2):164-172.
CrossRef - Chung GE, Heo NJ, Park MJ, Chung SJ, Kang HY, Kang SJ. Helicobacter pylori seropositivity in diabetic patients is associated with microalbuminuria. 2013;19(1):97-102. doi:10.3748/wjg.v19.i1.97
CrossRef - Oruç M, Köroğlu E. Helicobacter Pylori Infection and Kidney Damage: Is There An Association? Published online 2020.
- Alzahrani AM, Al Zaidi AA, Alzahrani SM, Binmahfouz SA, Farahat FM. Association between type 2 diabetes mellitus and Helicobacter pylori infection among Saudi patients attending National Guard Primary Health Care Centers in the Western Region, 2018. J Family Community Med. 2020;27(1):8.
CrossRef - Sahoo OS, Mitra R, Bhattacharjee A, Kar S, Mukherjee O. Is Diabetes Mellitus a Predisposing Factor for Helicobacter pylori Infections? Curr Diab Rep. Published online 2023:1-11.
CrossRef - Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep. 2010;12:364-368.
CrossRef - Krupa A, Gonciarz W, Rusek-Wala P, et al. Helicobacter pylori infection acts synergistically with a high-fat diet in the development of a proinflammatory and potentially proatherogenic endothelial cell environment in an experimental model. Int J Mol Sci. 2021;22(7):3394.
CrossRef - Watanabe J, Kotani K. The effect of Helicobacter pylori eradication on C-reactive protein: results from a meta-analysis. Arch Med Sci AMS. 2022;18(4):958.
CrossRef - Polyzos SA, Kountouras J, Zavos C, Deretzi G. The association between Helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter. 2011;16(2):79-88.
CrossRef - Basso D, Plebani M, Kusters JG. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2010;15:14-20.
CrossRef - Megha KB, Joseph X, Akhil V, Mohanan P V. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine. 2021;91:153712.
CrossRef - Aslan M, Horoz M, Nazligul Y, et al. Insulin resistance in H pylori infection and its association with oxidative stress. World J Gastroenterol WJG. 2006;12(42):6865.
CrossRef - Kebapcilar L, Sari I, Renkal AH, et al. The influence of Helicobacter pylori eradication on leptin, soluble CD40 ligand, oxidative stress and body composition in patients with peptic ulcer disease. Intern Med. 2009;48(24):2055-2059.
CrossRef - Kar D, Gillies C, Nath M, Khunti K, Davies MJ, Seidu S. Association of smoking and cardiometabolic parameters with albuminuria in people with type 2 diabetes mellitus: a systematic review and meta-analysis. Acta Diabetol. 2019;56:839-850.
CrossRef - Kar D, El-Wazir A, Nath M, et al. Relationship of cardiorenal risk factors with albuminuria based on age, smoking, glycaemic status and BMI: a retrospective cohort study of the UK Biobank data. BMJ Public Heal. 2023;1(1).
CrossRef - De Cosmo S, Minenna A, Ludovico O, et al. Increased urinary albumin excretion, insulin resistance, and related cardiovascular risk factors in patients with type 2 diabetes: evidence of a sex-specific association. Diabetes Care. 2005;28(4):910-915.
CrossRef - Hamed SA, Amine NF, Galal GM, et al. Vascular risks and complications in diabetes mellitus: the role of Helicobacter pylori infection. J Stroke Cerebrovasc Dis. 2008;17(2):86-94.
CrossRef - Demir M, Gokturk HS, Ozturk NA, Kulaksizoglu M, Serin E, Yilmaz U. Helicobacter pylori prevalence in diabetes mellitus patients with dyspeptic symptoms and its relationship to glycemic control and late complications. Dig Dis Sci. 2008;53:2646-2649.
CrossRef - Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M. Helicobacter pylori Infection Significantly Increases Insulin Resistance in the Asymptomatic Japanese Population. Published online 2009:496-502.
CrossRef







