Manuscript accepted on :13-10-2023
Published online on: 06-02-2024
Plagiarism Check: Yes
Reviewed by: Dr. Santoshi Naik
Second Review by: Dr. Noor Al-Huda Akram Yahya Al-Zarqi
Final Approval by: Dr. Patorn Promchai
Hansraj Kumar* , Subodh Kumar
, Subodh Kumar , Sumit Kumar Mahato
, Sumit Kumar Mahato and Harminder Singh
and Harminder Singh
Department of Pharmacology, AIIMS Deoghar, India
Corresponding Author E-mail:hansraj.pharmacology@aiimsdeoghar.edu.in
DOI : https://dx.doi.org/10.13005/bpj/2888
Abstract
Lepra Reactions (LR) are inflammatory conditions with immune mediators that are highly morbid. Patients with the lepromatous end of the leprosy spectrum (BL-LL) are the only ones that develop type-2 LR. 90% of the time, it happens during or right after treatment, usually within two years. Here, we present a type-2 LR case. The sixty-year-old woman who was diagnosed with lepromatous leprosy (LL) after developing a hypopigmented skin lesion six months prior. She was treated twice at our hospital over the course of the previous six months after developing type-2 LR, which manifested as recurrent fever episodes, polyarthritis, erythema nodosum, and mononeuritis multiplex.
Keywords
Erythema Nodosum Leprosum; Hansen's disease; Naranjo Scaling; Type 2 Reaction
Download this article as:| Copy the following to cite this article: Kumar H, Kumar S, Mahato S. K, Singh H. Erythema nodosum leprosum (Type 2 Reaction) in a Patient with Hansen's Disease from a Tertiary care hospital in Jharkhand: A case report. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Kumar H, Kumar S, Mahato S. K, Singh H. Erythema nodosum leprosum (Type 2 Reaction) in a Patient with Hansen's Disease from a Tertiary care hospital in Jharkhand: A case report. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/494OZ7T |
Introduction
The bacterium Mycobacterium leprae, which is responsible for leprosy also called Hansen’s disease (HD), is named after the medical professional Gerhard Armauer Hansen 1. Clinically, it primarily affects the skin, peripheral nervous system, upper respiratory tract mucosa, eyes, and testes 2. For more than 4,000 years, leprosy has been a problem for people and was well-known in ancient China, Egypt, and India. The World Health Organisation (WHO) estimated that 1.5 million people had leprosy-related irreversible disabilities in 2005. There are two different forms of LR (lepra Reactions): Type 1 Reaction or Reversal Reaction and Erythema Nodosum Leprosum (ENL) or Type 2 Reaction, Patients who have leprosy bacilli in large concentrations, such as those with multi-bacillary or infiltrative leprosy, experience type 2 reaction. When a high number of leprosy bacilli are destroyed and their antigens are released, this causes an allergic reaction of the arthus type (Coombs and Gell type III hypersensitivity), which results in an antigen antibody immune complex reaction when the complement system is present. The tissues (skin, eyes, joints, lymph nodes, kidneys, liver, spleen, bone marrow, endothelium, and testes) and the circulation both precipitate immune complexes 3.
Case History
A 60-year-old woman was admitted to our hospital with polyarthritis affecting both major and small joints for six months, repeated episodes of erythema nodosum, an erythematous skin lesion, and peripheral nerve loss (Fig. 1). Two months prior, the patient experienced a symmetrical hypopigmented anaesthetic skin patch without any indication of a nerve lesion. After breakfast, one dose of Tab. Prednisolone at 1 mg/kg body weight per day was given, coupled with 150 mg of Tab. Ranitidine. Prednisolone was reduced by 10 mg and subsequently by 5 mg/day till cessation after 24 weeks once the reaction/inflammation was under control. According to Naranjo Scale, this is a case of probable ADR (Adverse Drug Reaction) with a score of 6 (Table 1).
Table 1: Causality analysis using Naranjo Scale
|
Question |
Yes |
No |
Do Not Know |
Score |
|
1. Are there previous conclusive reports on this reaction? |
+1 |
0 |
0 |
+1 |
|
2. Did the adverse event appear after the suspected drug was administered? |
+2 |
-1 |
0 |
+2 |
|
3. Did the adverse event improve when the drug was discontinued or a specific antagonist was administered? |
+1 |
0 |
0 |
+1 |
|
4. Did the adverse event reappear when the drug was readministered? |
+2 |
-1 |
0 |
0 |
|
5. Are there alternative causes that could on their own have caused the reaction? |
-1 |
+2 |
0 |
0 |
|
6. Did the reaction reappear when a placebo was given? |
-1 |
+1 |
0 |
0 |
|
7. Was the drug detected in blood or other fluids in concentrations known to be toxic? |
+1 |
0 |
0 |
0 |
|
8. Was the reaction more severe when the dose was increased or less severe when the dose was decreased? |
+1 |
0 |
0 |
0 |
|
9. Did the patient have a similar reaction to the same or similar drugs in any previous exposure? |
+1 |
0 |
0 |
+1 |
|
10. Was the adverse event confirmed by any objective evidence? |
+1 |
0 |
0 |
+1 |
|
Total Score |
|
|
|
6 |
Total score:
► >9 – definite
►5 to 8 – probable
►1 to 4 – possible
► ≤0 – doubtful
Discussion
Leprosy primarily affects those from low socioeconomic backgrounds, and its peak incidence occurs in the second and third decades. Males are twice as likely as females to have the lepromatous version of the disease. According to Indian research, the prevalence of LR (Lepra Reactions) is 9% in cases of BL (Borderline leprosy) and approximately 50% in cases of LL (Lepromatous leprosy), and the mean time to presentation with Erythema Nodosum Leprosum is 3.7 months following the initiation of multi-drug therapy (MDT) 4. The majority of patients improve while receiving MDT. LR manifested in our patient six months after the end of the treatment. The type-2 reaction, which often lasts for a few weeks to many months, may be the initial symptom of the illness. The distinctive skin nodules first show up before or simultaneously with general symptoms as fever, headache, and body pain 5. Lesion show variable-sized plaques and the erythema nodosum symptoms that are typical of type 2 reactions. Nodules often have several, bilaterally and symmetrically distributed nodules that blanch upon pressure. They are more frequently present on the face and limbs’ outer surfaces, with less frequency on the trunk, where the skin is cooler. They often avoid the warmer bodily areas, such as the perineum, axilla, groin, and scalp with hair 6. The similar characteristic was also present in our patient. This case has a probable ADR (Adverse Drug Reaction), score of 6 according to Naranjo Scaling. The scale consists of ten questions that evaluate various aspects of the ADR, such as the temporal relationship between drug administration and the appearance of the reaction, the presence of alternative explanations, and the response to rechallenge with the drug. Each question is assigned a score and the scores are then totalled to determine the overall probability of the drug causing the ADR. Based on this Naranjo algorithm, ten questions on the ADR Probability Scale can be responded with the words “Yes,” “No,” or “Do not know.” Each response is given a different point value (-1, 0, +1, or +2). The overall score can be anywhere between -4 and +13; the reaction is considered to be definite if total score is 9 or above, probable when it ranges from 5 and 8, possible if it is between 1 and 4, and doubtful if it is less than 0 7.
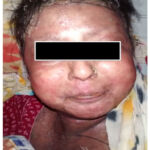 |
Figure 1: Showing erythematous skin lesions with characteristic nodules. |
Conclusion
Despite being a treatable illness, leprosy continues to be a major contributor to human impairment. This case report highlights the occurrence of Erythema nodosum leprosum (ENL), also known as Type 2 Reaction, in a patient with Hansen’s disease. ENL is an immune-mediated complication that can occur in individuals with leprosy, characterized by painful nodules, systemic symptoms, and organ involvement. The case underscores the importance of recognizing and managing ENL promptly to prevent complications and improve patient outcomes.
Acknowledgement
None
Conflicts of interest
There is no conflict of interest.
Funding Sources
There are no funding source.
References
- Sasaki, S., Takeshita, F., Okuda, K., & Ishii, N. (2001). Mycobacterium leprae and leprosy: a compendium. Microbiology and immunology, 45(11), 729–736. https://doi.org/10.1111/j.1348-0421.2001.tb01308.x
CrossRef - Gelber RH. Leprosy (Hansens disease). In: Kasper DL, Braunwald E, Fauci AS, Longo DL, Jamison LJ, editors. Harrisons Principles of Internal Medicine.17th ed. New York: Mc Graw Hill;2008.p.1021-26.
- University of Texas M. D. Anderson Cancer Center. (2008, November 28). New Leprosy Bacterium: Scientists Use Genetic Fingerprint To Nail ‘Killing Organism’. ScienceDaily. Retrieved June 6, 2023 from www.sciencedaily.com/releases/2008/11/081124141047.htm
- Pocaterra, L., Jain, S., Reddy, R., Muzaffarullah, S., Torres, O., Suneetha, S., & Lockwood, D. N. (2006). Clinical course of erythema nodosum leprosum: an 11-year cohort study in Hyderabad, India. The American journal of tropical medicine and hygiene, 74(5), 868–879.
CrossRef - Sethuraman, G., Jeevan, D., Srinivas, C. R., & Ramu, G. (2002). Bullous erythema nodosum leprosum (bullous type 2 reaction). International journal of dermatology, 41(6), 362–364.
CrossRef - Ali, M. Y., Mahmood, R., Barman, R. C., & Islam, M. M. S. U. (2013). A Case Report on Recurrent Lepra Reaction-Type 2. Faridpur Medical College Journal, 7(2), 93–97. https://doi.org/10.3329/fmcj.v7i2.13524.
CrossRef - LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Adverse Drug Reaction Probability Scale (Naranjo) in Drug Induced Liver Injury. [Updated 2019 May 4]. Available from: https://www.ncbi.nlm.nih.gov/books/ NBK548069/







