Manuscript accepted on :19-02-2024
Published online on: 14-03-2024
Plagiarism Check: Yes
Reviewed by: Dr. Joel Omage and Dr. Ravishankar Patil
Second Review by: Dr. Zena Sideeq tawfeek
Final Approval by: Dr. Luis Jesús Villarreal-Gómez
SkenderTelaku1 , Arber Veliu2
, Arber Veliu2  , Aida Polloshka2
, Aida Polloshka2 , Remzi Berisha2
, Remzi Berisha2 , Fatos Haxhosaj3
, Fatos Haxhosaj3  , Mimoza Telaku4
, Mimoza Telaku4  and Fitim Alidema1
and Fitim Alidema1
1University for Business and Technology, Prishtina, Kosovo
2 University Clinical Centre of Kosovo, Prishtina, Kosovo
3 Regional Hospital of Gjakova, Kosovo
4 University of Tirana, Faculty of Medicine, Tirana, Albania.
Corresponding Author E-mail: fitim.alidema@ubt-uni.net
DOI : https://dx.doi.org/10.13005/bpj/2887
Abstract
Pyoderma gangrenosum (PG) is a rare non-infectious neutrophilic dermatosis and its diagnosis is often challenging. Its pathogenesis remains unclear but in almost half of the cases, there is an underlying disease. The incidence of PG in patients with inflammatory bowel disease (IBD) in individual studies ranged from 0.4 to 2.6%. Several case reports and studies suggest infliximab's effectiveness in treating PG. This is the first case report from Kosovo of a successful treatment of PG in ulcerative colitis (UC) using infliximab. Infliximab is a chimeric monoclonal antibody to tumour necrosis factor-alpha (TNF-α) used to treat moderate to severe IBD. Our patient, a 28-year-old female diagnosed with UC developed PG one year later. Due to technical obstacles, skin biopsy was not collected at presentation therefore the diagnosis was made based on clinical presentation after six months. The lesions were typical, ulcerative with localization in the lower part of the right leg as well as in the left hand. Due to the failure of other therapies, we transitioned to infliximab and in the second cycle we saw improvements. This case shows that infliximab can be used to manage UC and its complications. PG should always be suspected in patients with UC with skin changes. A delay in diagnosis causes the appearance of scars, which affect the overall quality of life.
Keywords
Infliximab; Pyoderma Gangrenosum; Ulcerative Colitis
Download this article as:| Copy the following to cite this article: Telaku S, Veliu A, Polloshka A, Berisha R, Haxhosaj F, Telaku M, Alidema F. Effectively Using Infliximab to Treat Pyoderma Gangrenosum in a Woman With Ulcerative Colitis - Case Report. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Telaku S, Veliu A, Polloshka A, Berisha R, Haxhosaj F, Telaku M, Alidema F. Effectively Using Infliximab to Treat Pyoderma Gangrenosum in a Woman With Ulcerative Colitis - Case Report. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/3PkRqva |
Introduction
Pyoderma gangrenosum (PG) is considered a reactive inflammatory dermatosis and part of the spectrum of neutrophilic dermatosis. The disease was first described by Brocq in 1916 as a “phagedenisme geometrique”. 1 It is usually related to inflammatory bowel disease (IBD), myeloproliferative disorders or various arthropathies such as spondylitis and rheumatoid arthritis, but it can be idiopathic in about 50% of cases. 2,3, 4,5 Skin lesions could follow, precede or appear at the same time as the disease they follow. Its diagnosis is usually a diagnosis of exclusion and it is made based on Delphi criteria – including major and minor criteria. 6
The incidence of PG in patients with inflammatory bowel disease (IBD) in individual studies ranged from 0.4 to 2.6%.7 Infliximab is a chimeric monoclonal antibody to tumour necrosis factor-alpha (TNF-α) used to treat moderate to severe IBD.
Using TNF-α antagonists in IBD patients has been successful both in treating intestinal changes and extra-intestinal manifestations, including PG. 4,8
Here we will present the case of a patient with ulcerative colitis (UC) complicated by PG involving the left hand and right lower leg, successfully treated with infliximab. This is the first case report from Kosovo of a successful treatment of PG in UC using infliximab.
Case Report
Our patient is a 28-year-old female with a history of UC who was under treatment with sulfasalazine and prednisone when she developed chronic bloody diarrhoea, ulcerative lesions on her left wrist, right foot (Figure 1,2) and pain in both knees that began one month after the diarrhoea. She was diagnosed with UC 2 years ago.
The patient denied any family history of IBD in the family. She does not smoke or use alcohol. No significant past surgical history.
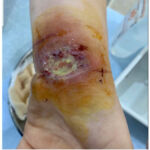 |
Figure 1: L hand ulceration of pyoderma gangrenosum.
|
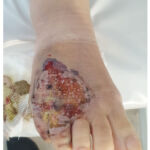 |
Figure 2: R foot ulceration of pyoderma gangrenosum
|
Laboratory studies were significant for 100 mm/hour erythrocyte sedimentation, C-reactive protein 79.5 mg/L, red blood cells 3,08 10 6/µL, haemoglobin 96 g/L, MCV 96 fL, platelets 500,000 x 109/L, leukocytes 13, 6 x 109/L, iron 6.5 μmol/L, ferritin 1178 µg/L, vitamin D 19.7 ng/mL. Faecal calprotectin was 1426.1 µg/mg.
Antibiotic therapy was initiated, and cultures were collected from the wounds. E. coli and Staphylococcus spp. sensitive-based antibiotic therapy was initiated without any significant improvement in the subsequent visits. No skin biopsy was collected on the initial visit.
Plastic surgeons were consulted and recommendations for daily dressings were followed by the patient.
She was treated with corticosteroids, azathioprine, sulfasalazine tablets, mesalazine suppositories and antibiotics according to the antibiogram but there was only a small improvement in the general condition and skin changes.
Diarrhoea workup for Clostridium difficile toxins A and B were negative. Further Infectious disease workup for PPD, and QuantiFERON-TB Gold, HLA-B27 were all negative.
Since there were no improvements with sulfasalazine and prednisone, we decided to start with infliximab 5 mg/kg at weeks 0, 2, and 6 and every 8 weeks subsequently. At the same time, azathioprine 2,5 mg/kg/day was continued. The patient also received mesalazine suppositories of 1 g before sleeping.
After the fifth cycle with infliximab, the changes in the skin were withdrawn, but the scars remained (Figure 3). At the same time, intestinal symptoms such as bloody diarrhoea and abdominal pain significantly improved.
The patient now regularly follows up and is currently on a maintenance dose of infliximab, azathioprine, and mesalazine.
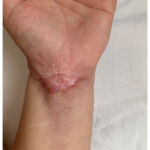 |
Figure 3: Healed ulceration of pyoderma gangrenosum |
Discussion
There are no definitive guidelines for the treatment of PG. Oral corticosteroids, cyclosporine, and biological agents such as infliximab and adalimumab have traditionally been used for months, even years. 2,6
Late diagnosis results in the appearance of rough scars causing a significantly poor quality of life. Usually, the ulcerative variant is seen on the legs, whereas atypical pyoderma gangrenosum which is a more superficial form, is more likely to appear on hands. Our patient had changes in the left hand and the right foot.
In some IBD patients, treating the bowel disease leads to treatment of PG, however, this is not often the case. Fortunately, in our case, controlling the underlying disease led to the withdrawal of skin changes. To our knowledge, this is the first case report from Kosovo with a successful treatment of PG-associated UC with infliximab.
The diagnosis of PG in our patient was a diagnosis of exclusion and was made with Delhi criteria.6 Treatment of PG remains a challenging subject. TNF-α, a proinflammatory cytokine, stimulates the synthesis and release of other cytokines, promoting the recruitment of inflammatory cells in the skin through increased expression of adhesion molecules. Inhibiting TNF-α may mitigate the inflammatory response. In 2005, the Food and Drug Administration (FDA) approved the utilisation of infliximab for adult patients with moderate to severe active UC who have shown an inadequate response to conventional therapy. 9 As we said above, there is no FDA-approved therapy for PG. At our department, we use infliximab only for IBD. After the second cycle of infliximab treatment, there was a significant improvement in stool frequency and bleeding and the skin changes started to improve. At the same time, we continued treatment with azathioprine 150 mg/day and a mesalazine suppository 1000 mg every night before going to bed. This is consistent with other cases in the literature, where improvements were noticed in the induction phase. Regueiro’s study assessed the reaction of medically intractable PG to infliximab. 10 The study of Brooklyn with collaborators demonstrated that infliximab is more effective in the treatment of PG than placebo. 11 There is more and more evidence for the use of TNF-α inhibitors as first-line therapy, mainly infliximab and adalimumab. 12,13,14 Agarwal et al. in their systematic review of 49 publications have identified 60 patients with PG-associated IBD. More than half of them (36 patients) received an anti-TNF-α. Thirty-four patients received infliximab, four received adalimumab and two patients received both. Thirty-three (92%) responded to one or the other. 15
A semi-systematic review of Ben Abdallah et al. evaluated and compared the clinical effect of TNF-α inhibitors in adults with PG-associated IBD. There was no significant statistical difference in response rate and complete response rate between infliximab, adalimumab, and etanercept. 16 Typically, patients with PG can expect a favourable prognosis, however, recurrences may happen and residual scarring is possible. In our case, infliximab has been observed to be effective in the treatment of PG caused by UC. However, some cases in the literature show that infliximab alone was not sufficient. In two cases reported, ustekinumab was successful in treating patients refractory to anti-TNF-a. 17,18
Conclusion
PG is a rare condition in Kosovo, therefore it is prone to misdiagnosis and it might take time to diagnose. This case being the first one reported from Kosovo, confirms that the usage of infliximab in the treatment of PG associated with UC is effective in suppressing symptoms and stimulating clinical remission of skin lesions. Most lesions improve leaving visible scars on the affected sites. For this reason, early recognition and treatment of PG is very important to prevent secondary infections and poor quality of life. A multidisciplinary approach is essential in treating PG.
Acknowledgements
We thank, Dr. Loran Rakovica , for the English language editing of the paper.
Conflict of interest
The authors declare no conflict of interest.
Funding Sources
None
Authors’ Contribution
ST, FA, AV, AP (Conceptualization, formal analysis, data curation, supervision, writing original draft, review, and editing). FH, RB, ST, FA (Investigation, patient administration, software, visualization, validation, review, and editing). All authors declared that they contributed to this article and that they have read and approved the final manuscript.
Data Availability
The authors confirm that the data supporting the findings of this case report are available within the article.
Statement of Ethics
Informed consent was obtained from the patient for the publication of this case report and any accompanying images.
References
- Brocq L. A new contribution to the study of geometric phagedenism. Ann Dermatol Syphiligr., 1916;9:1–39.
- Wollina U. Clinical management of pyoderma gangrenosum. Am J Clin Dermatol., 2002;3:149-158.
CrossRef - Cantisani C, Naqeshbandi AF, Goldust M, Lampitelli S, Cantoresi F, Alsorori E. Type I leucocyte adhesion deficiency in Yemenian family managed with appropriate treatment: A case series. Dermatol Ther. 2019;32(3):e12864.
CrossRef - Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13(3):191-211.
CrossRef - Pereira N, Brites MM, Gonçalo M, Tellechea O, Figueiredo A. Pyoderma gangrenosum–a review of 24 cases observed over 10 years. Int J Dermatol. 2013;52(8):938-945
CrossRef - Maverakis E, Ma C, Shinkai K, et al. Diagnostic Criteria of Ulcerative Pyoderma Gangrenosum: A Delphi Consensus of International Experts. JAMA Dermatol. 2018;154(4):461-466.
CrossRef - States V, O’Brien S, Rai JP, et al. Pyoderma Gangrenosum in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2020;65(9):2675-2685.
CrossRef - Martinelli VF, Martinelli Barbosa P, Dantas de Oliveira LS, de Melo LALV, Casa Nova JM, de Brito CAA. Atypical Forms of Pyoderma Gangrenosum in Inflammatory Bowel Disease: Report of Four Cases and Literature Review. Int Med Case Rep J. 2022;15:449-456.
CrossRef - https://www.drugs.com/history/remicade.html
- egueiro M, Valentine J, Plevy S, Fleisher MR, Lichtenstein GR. Infliximab for treatment of pyoderma gangrenosum associated with inflammatory bowel disease. Am J Gastroenterol. 2003;98(8):1821-1826.
CrossRef - Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double-blind, placebo-controlled trial. Gut. 2006;55(4):505-509.
CrossRef - Dini V, Romanelli M, Bertone M, Talarico S, Bombardieri S, Barachini P. Improvement of idiopathic pyoderma gangrenosum during treatment with anti-tumor necrosis factor alfa monoclonal antibody. Int J Low Extrem Wounds. 2007;6(2):108-113.
CrossRef - Poritz LS, Lebo MA, Bobb AD, Ardell CM, Koltun WA. Management of peristomal pyoderma gangrenosum. J Am Coll Surg. 2008;206(2):311-315.
CrossRef - Marzano AV, Tourlaki A, Alessi E, Caputo R. Widespread idiopathic pyoderma gangrenosum evolved from ulcerative to vegetative type: a 10-year history with a recent response to infliximab. Clin Exp Dermatol. 2008;33(2):156-159.
CrossRef - Agarwal A, Andrews JM. Systematic review: IBD-associated pyoderma gangrenosum in the biologic era, the response to therapy. Aliment Pharmacol Ther. 2013;38(6):563-572.
CrossRef - Ben Abdallah H, Fogh K, Vestergaard C, Bech R. Pyoderma Gangrenosum and Interleukin Inhibitors: A Semi-Systematic Review. Dermatology. 2022;238(4):785-792.
CrossRef - Piqueras-García J, Sahuquillo-Torralba AJ, Torres-Navarro I, Botella-Estrada R. Pyoderma Gangrenosum With Ulcerative Colitis Successfully Treated With Ustekinumab. Pioderma gangrenoso asociado a colitis ulcerosa con buena respuesta a ustekinumab. Actas Dermosifiliogr. 2019;110(9):776-778.
CrossRef - López González J, Lázaro Sáez M, Moreno Moraleda I, Hernández Martínez Á. Pyoderma gangrenosum solved by ustekinumab therapy. Pioderma gangrenoso resuelto mediante terapia con ustekinumab. Gastroenterol Hepatol. 2021;44(4):299-300.
CrossRef







