Manuscript accepted on :29-02-2024
Published online on: 19-03-2024
Plagiarism Check: Yes
Reviewed by: Dr. Elisha Paikray
Second Review by: Dr. Niharika Kondepudi
Final Approval by: Dr. Najam A Siddiqi
Olena Aliyeva1* , Igor Belenichev2
, Igor Belenichev2 , Nina Bukhtiyarova3
, Nina Bukhtiyarova3 , Denis Semenov4
, Denis Semenov4 and Sergiy Voloshchuk5
and Sergiy Voloshchuk5
1Department of Histology, Cytology, and Embryology, Zaporizhzhia State Medical and Pharmaceutical University, Zaporizhzhia, Ukraine.
2Department of Pharmacology and Medical Formulation with Course of Normal Physiology, Zaporizhzhia State Medical and Pharmaceutical University, Zaporizhzhia, Ukraine.
3Department of Clinical Laboratory Diagnostics, Zaporizhzhia State Medical and Pharmaceutical University, Zaporizhzhia, Ukraine.
4Department of Surgical and Propaedeutic Dentistry, Zaporizhzhia State Medical and Pharmaceutical University, Zaporizhzhia, Ukraine.
5Department of Neurology and neurosurgery of postgraduate education faculty, National Pirogov Memorial Medical University, Vinnytsya, Ukraine.
Corresponding Author E-mail: aliyeva1eg@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2850
Abstract
Prenatal hypoxia (PH) poses a significant threat to fetal development and may be responsible for neonatal mortality or neurodevelopmental abnormalities. The proteins HSP70 and HIF-1, which hold a distinct significance in the cellular reaction to PH, can be regarded as potential targets for pharmaceutical interventions aimed at mitigating the repercussions of chronic PH. This study aimed to identify a possible correlation between offspring survival and stages of expression of endogenous neuroprotective factors (HSP70 and HIF-1) after chronic prenatal hypoxia with course administration of potential HSP70 modulators (angiolin, piracetam, thiotriazoline, nicomex, cerebrocurin, tamoxifen, L-arginine, glutoredoxin, HSF-1, and mildronate). In the rat offspring after PH we determined the plasma concentrations of HSP70 and HIF-1 by solid-phase ELISA immunoassay, and the expression of HIF-1 mRNA and HSP70 mRNA by real-time PCR. For the first time, we found a positive correlation between offspring survival after PH and the expression of HIF-1 and HSP70, both in groups without experimental therapy and in groups receiving pharmacological agents. The course administration of HSP70/HIF-1α modulators, especially angiolin (50 mg/kg), cerebrocurin (150 mg/kg), and HSF-1 (50 mg/kg), to rats that underwent PH reduces postnatal lethality, increases blood plasma concentrations of HSP70 and HIF-1α, and positively affects the expression level of HIF-1α mRNA in the rat brain. These drugs can be considered as the most promising drug candidates for new therapeutic strategies of pharmacological correction of the consequences of chronic PH.
Keywords
Central nervous system; HIF-1; HSP70; neuroprotection; pharmacological correction; prenatal hypoxia
Download this article as:| Copy the following to cite this article: Aliyeva O, Belenichev I, Bukhtiyarova N, Semenov D, Voloshchuk S. Comparative Assessment of the Effectiveness of HSP70 / HIF-1α System Modulators after Prenatal Hypoxia. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Aliyeva O, Belenichev I, Bukhtiyarova N, Semenov D, Voloshchuk S. Comparative Assessment of the Effectiveness of HSP70 / HIF-1α System Modulators after Prenatal Hypoxia. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/4an8jxA |
Introduction
Prenatal hypoxia (PH), characterized by insufficient oxygen supply to the developing fetus, poses a significant threat to fetal development and, depending on the timing and level of exposure, may be the cause of neonatal mortality or the development of pathological neuropsychiatric changes manifested after birth at early and later ages 1, 2, 3, 4. Under conditions of chronic PH, the main negative changes occur in the brain, which is the most aerobic organ of the body. Oxygen deficiency induces oxidative and nitrosative stresses, resulting in an elevation of ROS levels to excitotoxicity, mitochondrial and endothelial dysfunction, and damage to developing neurons 5, 6, 7, 8. It is known that endogenous neuroprotective mechanisms – thiol-disulfide system, heat shock proteins and HIF-1 – are activated in neurons in response to hypoxic damage. There are works devoted to the role of the complementary HSP70/HIF-1 system in the implementation of mechanisms in response to acute hypoxia and ischemia 9, 10, 11. The cellular response to hypoxic stress results in the activation of stress-reactive proteins, among which a special role is attributed to heat shock proteins (HSPs), namely HSP 70, which are molecular chaperones 9, 12, . They participate in protein folding, prevent aggregation of denatured proteins and promote cell survival under stress conditions; therefore, they can be considered as potential therapeutic targets for pharmaceutical correction of the effects of PH. It is also known that HSP 70 prolongs the lifespan of HIF-1 and, through it, influences the activation and regulation of compensatory energy shunts under hypoxia 9, 11, 12, 13. Currently, the neuroprotective effects of HSP 70 modulators – tamoxifen, HSF-1, glutaredoxin, L-lysine 3-methyl-1,2,4-triazolyl-5-thioacetate (angiolin) – are known 11, 14, 15, 16. The above provides theoretical support for considering HSP 70 modulators as neuroprotective agents and requires conducting preclinical studies.
Aim: To identify a possible correlation between offspring survival and stages of expression of endogenous neuroprotective factors (HSP70 and HIF-1) after chronic prenatal hypoxia with course administration of potential HSP70 modulators (angiolin, piracetam, thiotriazoline, nicomex, cerebrocurin, tamoxifen, L-arginine, glutoredoxin, HSF-1, and mildronate).
Material and methods
The experiment was conducted on white females rats which were obtained from the nursery of the Institute of Pharmacology and Toxicology of the Academy of Medical Sciences of Ukraine (n=50, weight 220-240 g, age 4.5 months). In our research, we were guided by the the national “General Ethical Principles of Animal Experiments” (Ukraine, 2001) and Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes (EU, 2010). The protocols of experimental studies and their results were approved by the decision of the Commission on Bioethics of ZSMU (Minutes No. 33 of June 26, 2019). The animals were housed in standard vivarium conditions. A model of chronic PH of medium severity induced by sodium nitrite was selected for the experiment. PH was induced by administering a daily intraperitoneal injection of sodium nitrite solution (50 mg/kg) to pregnant female rats from the 16th to the 21st day of pregnancy 17. This model causes histological, neurochemical, metabolic disorders of the CNS and cognitive-mnestic deficits in offspring 18, 19. In the intact group, pregnant females received saline solution in the same manner. To study the effect of drugs, the offspring were divided into groups (Table 1).
Table 1: Experimental groups
|
Group No |
Prenatal conditions |
Administered drugs |
Method of administra-tion |
Manufacturer |
Dosage |
|
1 |
Normal (Intact) |
– Physiological solution |
intra-peritoneally |
– |
5 mL/g |
|
2 |
PH (control) |
– Physiological solution |
intra-peritoneally |
– |
5 mL/g |
|
3 |
PH |
Thiotriazolin (tiazotic acid) (2.5% solution for injection) |
intra-peritoneally |
Arterium, Ukraine |
50 mg/kg |
|
4 |
PH |
Tamoxifen* |
intranasally |
Zdorovye, Ukraine |
0.1 mg/kg |
|
5 |
PH |
Angiolin ((S)-2,6-diaminohexanoic acid 3-methyl-1,2,4-triazolyl-5-thioacetate) |
intra-peritoneally |
Farmatron, Ukraine |
50 mg/kg |
|
6 |
PH |
Glutaredoxin |
intra-peritoneally |
Sigma-Aldrich, USA |
200 µg/kg |
|
7 |
PH |
Cerebrocurin |
intra-peritoneally |
NIR, Ukraine |
150 µl/kg |
|
8 |
PH |
L-arginine (42% solution for injection) |
intra-peritoneally |
Yuria-Pharm, Ukraine |
200 mg/kg |
|
9 |
PH |
Nikomex (2-ethyl-6-methyl-3-hydroxypyridine succinate, solution for injection 50 mg/mL) |
intra-peritoneally |
Nikopharm, Ukraine |
100 mg/kg |
|
10 |
PH |
HSF-1 (recombinant) |
intra-peritoneally |
Sigma-Aldrich, USA |
50 mg/kg |
|
11 |
PH |
Mildronate ((2-(2-carboxyethyl)-1,1,1-trimethylhydrazinium, 10% solution for injection) |
intra-peritoneally |
Grindex, Latvia |
50 mg/kg |
|
12 |
PH |
Piracetam (20% solution for injection) |
intra-peritoneally |
Farmak, Ukraine |
500 mg/kg |
* – intranasal gel with active substance content of 1 mg/1 mL, manufactured on the basis of tablets 20 mg ex temporae at the Department of Drug Technology of ZSMFU.
Rat pups were administered drugs from day 1 to day 30 of life. For dosage calculation, the instructions for the drugs and data from previous studies were used 9, 14, 15.
Animals (10 from each group) were removed from the experiment on the 30th and 60th days of life using thiopental anesthesia (40 mg/kg).
Preparation of Biological Material
Blood was obtained from the abdominal artery by syringe, plasma was separated by centrifugation with Ependorff 5804R centrifuge at +4°C at 1500 rpm for 20 min. Blood was obtained from the abdominal artery by syringe, plasma was separated by centrifugation with Ependorff 5804R centrifuge at +4°C at 1500 rpm for 20 min. The brains were fixed in Bouin’s fixative for 24 h and processed according to standard histological techniques, then embedded in Paraplast (MkCormick, Hunt Valley, MD, USA). For real-time PCR, serial histologic brain sections 5 μm thin were sectioned on a Microm-325 rotary microtome (Microm Corp., Munich, Germany) and processed with o-xylene and ethanol.
Enzyme-Linked Immunoassay
HSP70 and HIF-1 protein concentrations in plasma were measured by solid-phase sandwich immunosorbent assay using ELISA Kit test sistems: AMP’DR HSP70 high-sensitivity ELISA kit # ENZ-KIT-101-0001, Enzo (Solna, Sweden) for determination of HSP70 level; AMP'D HSP70 high-sensitivity (Enzo, Sweden) for determination of HIF-1 concentration. HSP70 and HIF-1 concentrations were expressed in ng/mL.
Polymerase Chain Reaction in Real-Time
Quantitative real-time PCR was employed to assess HSP70 mRNA expression using Maxima SYBR Green/ROX qPCR Master Mix (2x) (ThermoScientific, Waltham, MA, USA) with gene-specific primers. The analysis was conducted on a CFX96™ Real-Time PCR Detection System by Bio-Rad Laboratories, Inc., USA. Fluorescence intensity was automatically registered through the automatic SybrGreen channel at the end of elongation stage of each cycle. Beta-actin (Actb) served as the reference gene for determining the relative change in the expression level of the target gene. Quantification was performed by the comparative delta Ct method.
Statistical Analysis
Statistical analysis of the experimental data was performed with “STATISTICA 13.0 TIBCO Software Inc.” and “Microsoft Office Excel 2016”. The normality of the results was checked by the Shapiro-Wilk and Kolmogorov-Smirnov tests. For comparative analysis, we used the parametric Student’s t-test for normal distributions and the Mann-Whitney U-test for non-normal distributions. ANOVA dispersion analysis was used for normal distributions and Kruskal-Wallis test for non-normal distributions to compare independent variables in more than two selections. Correlation analysis based on Pearson’s or Spearman’s correlation coefficient was used to analyze correlations between parameters. For all analyses, differences were considered significant at p<0.05 (95%).
Results
According to the results of the conducted experiment it was established that in the blood plasma of healthy animals proteins HSP70 and HIF-1 are present in relatively constant concentrations without significant age differences. Chronic PH resulted in a 5.6-fold decrease in HSP70 level and a 10.6-fold decrease in HIF-1 plasma concentration on the day 30 of life, and on the day 60 these parameters remained significantly lower than the values of the intact group (Figure 1a), demonstrating the long-term after effects of prenatal hypoxia in postnatal development.
Course administration of the studied drugs, except thiotriazoline and piracetam, increased the blood level of HSP70 with the most significant values in the groups of animals receiving angiolin (2-fold), glutaredoxin (by 95%), cerebrocurin (2.6-fold), L-arginine (2.25-fold), nikomex (2.37-fold), HSF-1 (2.36-fold).
Long-term results of pharmacological correction of PH consequences showed positive effects in the groups of animals administered thiotriazolin, mildronate, glutaredoxin with maximum indicators compared to the control in the groups after administration of angiolin, glutaredoxin and cerebrocurin.
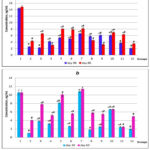 |
Figure 1: Concentration of HSP70 (a) and HIF-1α (b) in the blood plasma of rats after PH and course of drug administration on the day 30 and day 60 of life (M ± m, n = 10); *, |
In all groups, an increase in plasma HIF-1 concentration was observed during the first month, with maximum values in the groups of animals receiving cerebrocurin (10.8-fold), angiolin (8.1-fold), and HSF-1 (6.6-fold). At the end of the second month, the level of HIF-1 increased in all experimental groups except those receiving HSF-1and mildronate. In animals treated with angiolin and cerebrocurin, this parameter remained maximum, corresponding to the intact parameters. (Figure 1b). A correlation relationship between HIF-1 and HSP70 concentrations levels was established (Pearson correlation coefficient R = 0.70 for day 30 of life and R = 0.68 for day 60 of life).
PH reduces the level of HIF-1α mRNA expression by 37%, and when corrected by the studied drugs, this parameter increases manifold, exceeding intact values, with maximum results in the groups after treatment with cerebrocurin, thiotriazoline, angiolin and HSF-1 (Table 2). The results of HSP70 mRNA expression generally correlate with HIF-1α mRNA expression levels (R = 0.71) with maximum results in the groups after treatment with angiolin, cerebrocurin, HSF-1, glutaredoxin and mildronate.
Table 2: Expression levels of HIF-1α mRNA and HSP70 mRNA in the brain of rats after PH on day 60
|
Groups |
mRNA HIF1α levels |
mRNA HSP70 levels |
|
|
1 |
Intact |
1.00±0.002 |
1.00±0.003 |
|
2 |
Control |
0.331±0.002 |
0.409±0.008 |
|
3 |
Thiotriazolin |
2.06±0.001*1 |
2.13±0.006*1 |
|
4 |
Tamoxifen |
1.63±0.004*1 |
1.98±0.001*1 |
|
5 |
Angiolin |
4.61±0.004*1 |
5.21±0.004*1 |
|
6 |
Glutaredoxin |
2.80±0.002*1 |
3.88±0.001*1 |
|
7 |
Cerebrocurin |
5.24±0.002*1 |
7.11±0.008*1 |
|
8 |
L-arginine |
0.37±0.0011 |
0.568±0.003* |
|
9 |
Nikomex |
0.77±0.006* |
0.68±0.004* |
|
10 |
HSF-1 |
4.70±0.730*1 |
4.37±0.051*1 |
|
11 |
Mildronate |
1.30±0.022*1 |
3.73±0.140*1 |
|
12 |
Piracetam |
0.603±0.003* |
0.563±0.005* |
*, significantly different from control group, p<0.05; 1, significantly different from intact group, p<0.05.
We have established a correlation between the levels of HIF-1 and HSP70 protein concentration in plasma and survival of animals after PH. Modeling of chronic PH increased offspring mortality 10.5-fold compared to offspring obtained after normal physiological pregnancy (69.56% and 6.67%, respectively) (Figure 2). The majority of lethal cases were registered in newborns and at the age of 9-12 days. PH negatively affects the processes of neural tissue formation, disrupting proliferation and migration of neuroblasts, which leads to pathological changes in the morphofunctional organization of the brain, the consequences of which are manifested after birth.
 |
Figure 2: Survival rates (%) of offspring after PH and its pharmacological correction (a) and correlation analyses of offspring survival rates and HSP70 concentration in blood plasma on the day 30 (b) and day 60 (c) of life, |
Course administration from the first day of life of pharmacological agents with potential and proven neuroprotective effect reduced the mortality of offspring after PH action. The highest offspring survival rates were recorded in the groups of rats that were given mildronate (76.97%, n=39), angiolin (64.29%, n=42), thiotriazoline (63.41%, n=41), HSF-1 (62.96%, n=40) and cerebrocurin (62.86%, n=35). The results of the correlation analysis of offspring survival rates and HIF-1 and HSP70 concentrations for the first month of life showed a high positive correlation (R=0.73) for HIF-1 and a lower one with HSP70 plasma concentration (R=0.63). On the 60th day, on the contrary, the correlation coefficient between rat survival and HSP70 level was R=0.80 and for HIF-1 it decreased to R=0.51 (Figure 2). Offspring survival after PH depends on the efficiency of the complex action of endogenous mechanisms of neuroprotection, and HIF-1/HSP70 dependent mechanisms play a key role in these processes.
Discussion
The use of sodium nitrite in the PH model causes maternal hemic hypoxia due to methemoglobin formation and tissue hypoxia through dissociation of products of oxidation and phosphorylation processes. This leads to disturbances in the uteroplacental circulation system, resulting in the formation of persistent oxygen deficiency in the fetus, which leads to hypoxic organ damage 18, 20. It is known that oxygen deficiency leads to abnormal development of the fetal brain 2, 3. PH leads to postnatal individual physical and intellectual disorders, early mortality and disability. PH causes persistent disorders of transcriptional processes, protein synthesis, mitochondrial function, and transmitter autocoidosis 5, 7. In response to PH, endogenous defense mechanisms are activated in the cells. Important links of these mechanisms are the HIF-1 factor and HSP70 proteins 9, 21, 22, 23, 24. It is known that hypoxia-induced factors (HIFs) play the role of transcription factors and regulate the expression of genes encoding the synthesis of proteins involved in the physiological response to hypoxia/ischemia. Thus, HIF-1 stimulates erythropoiesis, activates additional ATP synthesis shunts, and increases the concentration of reduced glutathione 25, 26. HIF-1 is able to enhance HSP70 expression indirectly through HSF-1 activation to maintain cellular redox homeostasis. In turn, by possessing chaperone activity, HSP70 is able to stabilize HIF-1α and prolong its activity under hypoxic and post-hypoxic conditions 9, 27, 28. HSP70 proteins are molecular chaperones actively involved in the mechanisms of cellular response to ischemia, stress, hypoxia, temperature increase 9, 12, 29. The regulation of HSP70 expression occurs with the participation of heat shock factor (HSF-1) 24, 30. Under hypoxic conditions, HSP70 proteins perform cytoprotective functions by preventing the aggregation of misfolded proteins, promoting protein refolding and protein translocation across cell membranes. The chaperone activity of HSP70 also extends to stabilization of cell membranes, preserving cellular integrity 9, 12, 29, 31. In addition, HSP70 inhibits apoptosis by interaction with key regulators of the apototic pathway, caspases 21, 32. HSP70 in acute ischemia conditions are able to influence the activity of compensatory energy shunts by preserving HIF-1 38. We found that chronic PH causes a decrease in HIF-1 mRNA expression in rat target organs and inhibition of HIF-1-dependent protective and compensatory-adaptive mechanisms 15, 19. There is information about multidirectional changes in HIF-1 expression in different forms of hypoxia/ischemia. In intensive hypoxia against the background of oxidative stress activation and energy deficit, HIF-1 decreases due to its ubiquitylation and lower synthesis 35, 38. It is also known that subtotal cerebral ischemia causes suppression of HSP70 expression. The correlation between HSP70 deficiency in the brain and the severity of neurological disorders in cerebral ischemia was found. In this study, we also found a correlation between HSP70 deficiency and early postnatal lethality of newborn rats after PH. All this leads us to consider HSP70 as an important component of endogenous neuroprotection, the level of which determines the fate of the fetus and the prospects of health and social development of the neonate. Therefore, we have proposed the modulation of HSP70 expression by pharmacological agents as a promising approach to neuroprotection after PH.
As a result of this study, it was found that the most significant effects on the expression of HSP70 and HIF-1 and the reduction of offspring lethality after PH were demonstrated by angiolin, cerebrocurin and HSF-1. The results obtained can be explained by the available data on the mechanisms of action of these pharmacological agents.
The effect of Angiolin on cell protective mechanisms under PH conditions that we have identified is not contradicted by other studies 33, 34. It was demonstrated that Angiolin exhibits antioxidative, cardioprotective, neuroprotective and endothelioprotective properties in conditions of myocardial and cerebral ischemia. The mechanism of its neuroprotective action is based on regulation of HSP70 expression, especially in mitochondria, mitoprotective action and improvement of cell energy metabolism due to activation of mitochondrial-cytosolic compensatory shunts. Angiolin increases NO bioavailability, regulates eNOS and iNOS expression, and inhibits the formation of cytotoxic forms of NO9, 15, 35. These effects are based on the peculiarities of the molecular structure of Angiolin, which provides opportunities to form complexes with NO, as well as to regulate SH/SS equilibrium and influence GSH-dependent neuroprotective mechanisms. Angiolin increases the level of glutathione reduced in the cytosol and mitochondria of neurons during cerebral ischemia. Through the regulation of glutathione, Angiolin regulates the synthesis and stability of HSP70 9, 35. Our in vitro experiments show that GSH depletion of neurons leads to a decrease in HSP70 levels 35.
Cerebrocurin contains regulatory neuropeptides and can activate subtotal transcription factors and influence the expression of genes encoding growth factors, chaperone proteins and antioxidant enzymes. Cerebrocurin is known to have antioxidant, neuroprotective, nootropic and mitoprotective activities. 14, 36, 37 The neuroprotective effect of Cerebrocurin is attributed to its ability to significantly increase the expression of HSP70 and intramitochondrial Mn-SOD 35, 38, 39.
HSF-1 is the main transcriptional regulator that initiates the cellular response to stresses of different nature. Stress factor leads to increase of ROS in the cell, which triggers HSF-1 activation, and it binds to promoters of HSE genes, activating first of all HSP protein genes. HSF-1 targets are also genes responsible for metabolic processes; it takes part in the regulation of apoptosis processes, intracellular transport and signaling 25, 40.
Understanding the protective role of HIF-1 and HSP70 in hypoxia opens pathways for potential therapeutic interventions (Figure 3). Strategies aimed at enhancing the expression or activity of HIF-1 and HSP70 may represent novel approaches to pharmacological correction of the effects of PH.
We have experimentally substantiated the expediency of using HSP70 modulators as neuroprotective agents. We also showed the most promising ways of pharmacological modulation of HSP70 – through the increase of SH-dependent mechanisms (Angiolin) and through activation of transcription factors (Cerebrocurin, HSF-1).
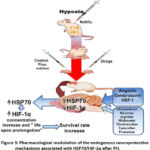 |
Figure 3: Pharmacological modulation of the endogenous neuroprotective mechanisms associated with HSP70/HIF-1α after PH. |
Conclusion
PH modeling leads to increased lethality of offspring during 2 months of life against the background of decreased concentrations of HSP70 and HIF-1α in blood plasma and decreased brain HIF-1α mRNA expression levels in experimental animals. Offspring survival after PH was positively correlated with HIF-1 and HSP70 expression levels. The course administration of HSP70/HIF-1α modulators to rats that underwent PH reduces postnatal lethality, increases blood plasma concentrations of HSP70 and HIF-1α, and positively affects the expression level of HIF-1α mRNA in the rat brain.
The most significant effect of modulation of the HSP70/HIF-1α related endogenous neuroprotective mechanisms after PH was obtained after treatment with angiolin (50 mg/kg), cerebrocurin (150 mg/kg) and HSF-1 (50 mg/kg). Therefore, they can be considered as the most promising drugs for new therapeutic strategies of pharmacological correction of the consequences of chronic PH.
Acknowledgement
The authors would like to acknowledge the Zaporizhzhia State Medical and Pharmaceutical University for providing some facilities in carrying out the research.
Conflict of Interest
The authors declare no conflict of interest.
Funding Source
This research received no external funding.
References
- Wang B., Zeng H., Liu J., Sun M. Effects of Prenatal Hypoxia on Nervous System Development and Related Diseases. Front. Neurosci., 2021; 15:755554. doi: 10.3389/fnins.2021.755554.
CrossRef - Millar L.J., Shi L., Hoerder-Suabedissen A., Molnár Z. Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges. Front. Cell. Neurosci., 2017; 11: 78. doi: 10.3389/fncel.2017.00078.
CrossRef - Piesova M., Mach M. Impact of perinatal hypoxia on the developing brain. Physiol. Res., 2020; 69: 199–213. doi: 10.33549/physiolres.934198
CrossRef - Belenichev I.F.and Aliyeva E.G. New targets for pharmacological correction of cognitive disorders in prenatal hypoxia action. Pharmacology and Drug Toxicology, 2019; 13 (4): 235–248. doi: 10.33250.13.04.235.
CrossRef - Silvestro S., Calcaterra V., Pelizzo G., Bramanti P. and Mazzon E. Prenatal Hypoxia and Placental Oxidative Stress: Insights from Animal Models to Clinical Evidences. Antioxidants (Basel), 2020; 9 (5): 414. doi: 10.3390/antiox9050414.
CrossRef - Giannopoulou I., Pagida M.A., Briana D.D. and Panayotacopoulou M.T. Perinatal hypoxia as a risk factor for psychopathology later in life: the role of dopamine and neurotrophins. Hormones, 2018; 17: 25–32. doi:10.1007/s42000-018-0007-7.
CrossRef - Wood C.E., Keller-Wood M. Current paradigms and new perspectives on fetal hypoxia: implications for fetal brain development in late gestation. Am J Physiol. Regul. Integr. Comp. Physiol., 2019; 317(1): R1-R13. doi: 10.1152/ajpregu.00008.2019.
CrossRef - Wilson E.N., Mabry S., Bradshaw J.L., Gardner J.J., Rybalchenko N., Engelland R., Fadeyibi O., Osikoya O., Cushen S.C., Goulopoulou S. and Cunningham R.L. Gestational hypoxia in late pregnancy differentially programs subcortical brain maturation in male and female rat offspring. Biol. Sex. Differ., 2022; 13(1): 54. doi: 10.1186/s13293-022-00463-x.
CrossRef - Belenichev I.F., Aliyeva O.G., Popazova O.O. and Bukhtiyarova N.V. Involvement of heat shock proteins HSP70 in the mechanisms of endogenous neuroprotection: the prospect of using HSP70 modulators. Front. Cell. Neurosci., 2023; 17: 1131683. doi: 10.3389/fncel.2023.1131683.
CrossRef - Zhao M., Zhu P., Fujino M., Zhuang J., Guo H., Sheikh I., Zhao L. and Li X.K. Oxidative Stress in Hypoxic-Ischemic Encephalopathy: Molecular Mechanisms and Therapeutic Strategies. Int. J. Mol. Sci., 2016; 17(12): 2078. doi: 10.3390/ijms17122078.
CrossRef - Orzeł A., Unrug-Bielawska K., Filipecka-Tyczka D., Berbeka K., Zeber-Lubecka N., Zielińska M. and Kajdy A. Molecular Pathways of Altered Brain Development in Fetuses Exposed to Hypoxia. Int. J. Mol. Sci., 2023; 24: 10401. doi:10.3390/ijms241210401.
CrossRef - Kim J. Y., Barua S., Huang M. Y., Park J., Yenari M. A., and Lee J. E. Heat Shock Protein 70 (HSP70) Induction: Chaperonotherapy for Neuroprotection after Brain Injury. Cells, 2020; 9: 2020. doi: 10.3390/cells9092020.
CrossRef - Bochenek L.M.S., Parisotto E.B., Salomão E.A., Maldonado M.J.M., Silva I.S. Characterization of oxidative stress in animal model of neonatal hypoxia. Acta Cir Bras., 2021; 36(11): e361108. doi: 10.1590/ACB361108.
CrossRef - Chekman I.S., Belenichev I.F., Demchenko A.V., Bobrova V.I., Kucherenko L.I., Gorchakova N.A. and Bukhtiyarova N.V. Nootropics in complex therapy of chronic brain ischemia. Sci. Innovat., 2014; 10 (4): 61-75.
CrossRef - Belenichev I.F., Aliyeva O.G., Bukhtiyarova N.V., Popazova O.O. and Ryzhenko V.P. Positive Pharmacological Modulation of Hsp70 in Recovery of Brain Energy Metabolism in Various Models of Cerebral Ischemia. Biol. Life Sci. Forum, 2022; 20(1): 24. doi: 10.3390/IECBM2022-13511.
CrossRef - Zou W., Fang C., Ji X., Liang X., Liu Y., Han C., Huang L., Zhang Q., Li H., Zhang Y., Liu J. and Liu J. Estrogen Receptor (ER)-α36 Is Involved in Estrogen- and Tamoxifen-Induced Neuroprotective Effects in Ischemic Stroke Models. PLoS One, 2015; 10(10): e0140660. doi: 10.1371/journal.pone.0140660.
CrossRef - Petruk N.S. Influence of chronic prenatal hypoxia on the specialized contact apparatus of rat heart ventricles during ontogeny. Pathologia, 2014; 2(31): 30-33. doi: 10.14739/2310-1237.2014.2.28550.
CrossRef - Popazova O., Belenichev I., Yadlovskyi O., Oksenych V. and Kamyshnyi A. Altered Blood Molecular Markers of Cardiovascular Function in Rats after Intrauterine Hypoxia and Drug Therapy. Curr. Issues Mol. Biol., 2023; 45(11): 8704-8715. https://doi.org/10.3390/cimb45110547.
CrossRef - Aliyeva O., Belenichev I. and Popazova O. Modulation of Hsp70 in the Pharmacological Correction of Nervous System Disorders after Prenatal Hypoxia. Med. Sci. Forum, 2023; 21(1): 39. https://doi.org/10.3390/ECB2023-14091.
CrossRef - Evidence on Developmental and Reproductive Toxicity of Sodium Nitrite. Reproductive and Cancer Hazard Assessment Section (RCHAS) Office of Environmental Health Hazard Assessment (OEHHA) California Environmental Protection Agency (CAL/EPA) DRAFT. USA. 3 March 2000. Available online: https://oehha.ca.gov/proposition-65/crnr/draft-hid-available-sodium-nitrite (accessed on 27 October 2023).
- Luo S.Y., Wang J.Q., Liu C., Gao X.M., Zhang Y.B., Ding J., Hou C.C., Zhu J.Q., Lou B., Shen W.L., Wu X.F., Zhang C.D. and Tang D.J. Hif-1α/Hsf1/Hsp70 signaling pathway regulates redox homeostasis and apoptosis in large yellow croaker ( Larimichthys crocea) under environmental hypoxia. Zool. Res., 2021; 42(6): 746-760. doi: 10.24272/j.issn.2095-8137.2021.224.
CrossRef - Zeng H., Wei B., Liu J., Lu L., Li L., Wang B. and Sun M. Hypoxia-inducible Factor Regulates Ten-eleven Translocated Methylcytosine Dioxygenase 1-c-Myc Binding Involved in Depression-like Behavior in Prenatal Hypoxia Offspring. Neuroscience, 2022; 502: 41-51. doi: 10.1016/j.neuroscience.2022.08.014.
CrossRef - Zhang Z., Yan J., Chang Y., ShiDu Yan S. and Shi H. Hypoxia inducible factor-1 as a target for neurodegenerative diseases. Curr. Med. Chem., 2011; 18(28):4335-43. doi: 10.2174/092986711797200426.
CrossRef - Semenza G.L. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J. Applied Physiology, 2000; 88(4): 1474-1480. doi: 10.1152/jappl.2000.88.4.1474.
CrossRef - Luo Z., Tian M., Yang G., Tan Q., Chen Y., Li G., Zhang Q., Li Y., Wan P. and Wu J. Hypoxia signaling in human health and diseases: implications and prospects for therapeutics. Signal. Transduct. Target Ther., 2022; 7(1): 218. doi: 10.1038/s41392-022-01080-1.
CrossRef - Chen H., Ma D., Yue F., Qi Y., Dou M., Cui L. and Xing Y. The Potential Role of Hypoxia-Inducible Factor-1 in the Progression and Therapy of Central Nervous System Diseases. Curr. Neuropharmacol., 2022; 20(9): 1651-1666. doi: 10.2174/1570159X19666210729123137.
CrossRef - Kletkiewicz H., Hyjek M., Jaworski K., Nowakowska A. and Rogalska J. Activation of hypoxia-inducible factor-1α in rat brain after perinatal anoxia: role of body temperature. Int. J. Hyperthermia, 2018; 34(6): 824-833. doi: 10.1080/02656736.2017.1385860.
CrossRef - Majmundar A.J., Wong W.J. and Simon M.C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell., 2010; 40(2): 294-309. doi: 10.1016/j.molcel.2010.09.022.
CrossRef - Kaur P. and Asea A. The Chaperokine Activity of Heat Shock Proteins. In Chaperokine activity of heat shock proteins. Heat shock proteins. eds A. Asea and P. Kaur (Cham: Springer), 2019; 16: 3-22. doi: 10.1007/978-3-030-02254-9_1.
CrossRef - Gomez-Pastor R., Burchfiel E.T. and Thiele D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol., 2018; 19(1): 4-19. doi: 10.1038/nrm.2017.73.
CrossRef - Liu T., Juan Z., Xia B., Ren G., Xi Z., Hao J. and Sun Z. HSP70 pro-tects H9C2 cells from hypoxia and reoxygenation injury through STIM1/IP3R. Cell Stress Chaperones, 2022; 27(5): 535-544. doi: 10.1007/s12192-022-01290-0.
CrossRef - Cao J., Yang L., Wang L., Zhao Q., Wu D., Li M. and Mu Y. Heat shock protein 70 attenuates hypoxia‑induced apoptosis of pulmonary microvascular endothelial cells isolated from neonatal rats. Mol. Med. Rep., 2021; 24(4): 690. doi: 10.3892/mmr.2021.12327.
CrossRef - Pivtorak K.V., Mazur I.A., Voloshyn M.A. The ultrastructure of hepatic endothelial cells in the correction of steatosis with new biologically active compound Angiolin. Pathologia, 2015; 35: 49–52. doi: 10.14739/2310-1237.2015.3.55587.
CrossRef - Meloni B.P., Milani D., Edwards A.B., Anderton R.S., O’Hare Doig R.L., Fitzgerald M., Palmer T.N. & Knuckey N.W., Neuroprotective peptides fused to arginine-rich cell penetrating peptides: Neuroprotective mechanism likely mediated by peptide endocytic properties. Pharmacol. Ther., 2015; 153: 36-54. doi: 10.1016/j.pharmthera.2015.06.00.
CrossRef - Belenichev I. F., Cherniy V. I., Nagornaya E. A., Pavlov S. V., Cherniy T. V. Neuroprotection and neuroplasticity. Kyiv: Logos, 2015; 512.
- Morguntsova S. A. Neuroprotection neurotrophic cerebroprotector Сerebroсurin in terms of modeling acute stroke. Zaporozhye Med. J., 2014; 5(86): 36-40. doi: 10.14739/2310-1210.2014.5.28772.
CrossRef - Panchenko N.V., Gonchar E.N., Arustamova G.S., Pereiaslova A.S., Prikhod’ko D.O., Friantseva M.V. Influence of the fetal neuropeptide complex on changes in retinal light sensitivity over time in patients with primary open-angle glaucoma. J.ophthalmol.(Ukraine).2017;6:16-19. doi: 10.31288/oftalmolzh201761619.
CrossRef - Gorbacheva S. V. Nitrosative stress restriction in vitro conditions using modulators of thiol-disul-fide system. Bull. Prob. Boil. Med., 2015 4(125):133-135.
- Yevtushenko O., Yanovskaya N., Yevtushenko S., Kutyakova Y., Omelyanenko A., Dubina S., Sokhan D., Poroshina Y., Fomichova E. and Sachinova I. 15-Year Experience of Cerebrocurin Use in Combined Therapy in Children With Organic Diseases of the Nervous System. INJ, 2014; 3.65: 13-19. doi: 10.22141/2224-0713.3.65.2014.81082.
CrossRef - Baird N.A., Turnbull D.W. and Johnson E.A. Induction of the heat shock pathway during hypoxia requires regulation of heat shock factor by hypoxia-inducible factor-1. J. Biol. Chem., 2006; 281(50): 38675-81. doi: 10.1074/jbc.M608013200.
CrossRef







