Manuscript accepted on :08-01-2024
Published online on: 09-02-2024
Plagiarism Check: Yes
Reviewed by: Dr. Sharath BS and Dr. Ayan Chatterjee
Second Review by: Dr. Tamer Addissouky
Final Approval by: Dr. Anton R Keslav
Enas R. Abdelhamid1 , Alyaa H. Kamhawy1
, Alyaa H. Kamhawy1 , Lobna S. Sherif1
, Lobna S. Sherif1 , Hanaa H. Ahmed2*
, Hanaa H. Ahmed2* , Maysa T. Saleh1
, Maysa T. Saleh1 , Sondos Salem 3
, Sondos Salem 3 and Manal A. Gad2
and Manal A. Gad2
1Child Health Department, Medical Research and Clinical Studies Institute, National Research Centre, Dokki, Giza, Egypt.
2Hormones Department, Medical Research and Clinical Studies Institute, National Research Centre, Dokki, Giza, Egypt.
3 Reproductive Health and Family planning Research Department,Medical Research and Clinical Studies Institute, National Research Centre, Dokki, Giza, Egypt.
Corresponding Author E-mail: hanaaomr@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2845
Abstract
Background: Placental growth factor (PlGF) contributes to fetoplacental circulatory system development, whichever revealed to have an effect on the fetal size and growth. Objectives: To explore the inference between umbilical cord blood [fetal] PlGF, fetal doppler parameters, gestational age and neonatal growth parameters particularly birth weight, birth length, head circumference and mid arm circumference. Research protocol: This cross-sectional investigation was implemented on 50 pregnant women in their third trimester, aged 18-35years and their full term newborns. Full history taking, gestational age, general and obstetric examination and ultrasound investigation, fetal biometrics and doppler ultrasound were carried out. Umbilical cord blood PlGF was quantified using ELISA. All the enrolled neonates were submitted to full clinical examination by pediatrician and their anthropometric parameters were measured before breast feeding started. Results: Umbilical artery pulsatility index (UAPI) revealed significant negative correlation with neonatal weight and PlGF level. Meanwhile, gestational age (GA) showed significant positive correlation with the neonatal anthropometric parameters including mid arm circumference (MAC), head circumference, weight, length, weight Z score, length Z score, head Z score weight to length (W/L) and weight to length Z score (W/L Z Score). Also, significant positive correlation between PlGF and gestational age as well as the neonatal anthropometric measurements was registered except head Z Score. Multiple linear regression analysis for PlGF with UA PI and neonatal weight indicated that UAPI and neonatal weight were significant predictors for fetal PlGF. Conclusions: The current data illuminate the effect of fetal placental growth factor on neonatal adverse growth pattern. Additionally, fetal placental growth factor with fetal doppler parameters could be a promising predictive biomarkers to intervene neonates at risk for adverse childhood outcomes.
Keywords
Fetal doppler parameters; Fetal placental growth factor; Full term newborns; Neonatal anthropometric measures; Third trimester
Download this article as:| Copy the following to cite this article: Abdelhamid E. R, Kamhawy A. H, Sherif L. S, Ahmed H. H, Saleh M. T, Salem S, Gad M. A. Association Between Cord Blood Placental Growth Factor Level, Fetal Doppler Parameters and Neonatal Growth Measures. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Abdelhamid E. R, Kamhawy A. H, Sherif L. S, Ahmed H. H, Saleh M. T, Salem S, Gad M. A. Association Between Cord Blood Placental Growth Factor Level, Fetal Doppler Parameters and Neonatal Growth Measures. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/4btAI6n |
Introduction
Among the most complicated fetal organs that achieves pleiotropic effects over the fetal growth is the placenta. It disconnects the maternal circulation from the fetal circulation, with which it is in touch via various surfaces, i.e. the syncytiotrophoblast presents the placenta to the maternal circulation and the endothelium is in touch with the fetal blood 1.
Early in the pregnancy, the development of placental vascularity starts and undergoes modifications throughout gestation. On the maternal aspect, the circulation of the uteroplacenta is stabilized by the termination of the first trimester 2. On the fetal part, the initial villi of the placenta start to evolve at thirteen’s day of conception, and the vascularization of the fetoplacental villi starts at twenty one’s day of conception 3. The adaptation of maternal vascularization comprises modulating of the spiral arteries of the uterus by invasive trophoblasts derived from fetus to permit a reduction in blood flow resistance into the placental intervillous space 4. So the placental vasculature growth and function are fundamental to reinforce the development of the uterus. Vasculogenesis, the de novo consistence of blood vessels and angiogenesis, the diverging and modulating of the presenting vasculature, intercede placental villi expansion and maturation, forming the materno-fetal interface 5.
Placental Growth Factor (PlGF) is mostly implicated in the angiopoiesis of the placental chorion and the preservation of healthy growth and development of the placenta. PlGF binds primarily to the Flt1 (soluble fms-like tyrosine kinase-1) receptor and remodels spiral arteries to allow for sufficient blood supply to the placenta 6. PlGF is primarily synthesized in the placenta and is also produced from umbilical vein endothelial cells 7. Interestingly, PlGF has been reported to be expressed in cord blood 8. Moreover, Makrydimas et al. 9 observed that the human amniotic fluid of 7-9 week of gestation has a concentration of PlGF about half that of matched maternal serum. Over midgestation, PlGF and Vascular Endothelial Growth Factor (VEGF) showed enhanced levels in amniotic fluid but VEGF revealed a 6-fold higher level than PlGF 10.
PlGFs participate to the fetoplacental circulation development which starts in early gestation and evolves all over pregnancy. Fetal size has been shown to be affected by the fetoplacental circulatory system 11. Unluckily, at the end of pregnancy no maternal PlGF levels were detectable and throughout pregnancy, the PlGF attains its high level about thirty one’s week of conception, then it turns down 12. Of note, around the ninteen weeks of conception the levels of maternal PlGF are approaching with its level to that of the time of delivery. Interestingly, a 10-fold difference between maternal and fetal circulation regarding P1GF levels at the time of delivery with no significant correlation between them 13.
Doppler ultrasound is utilized to determine the blood flow of the umbilical artery (UA) and fetal middle cerebral artery (MCA) 14. The cerebroplacental ratio (CPR) is measured by the pulsatility index (PI), that is applied to assess the oxygenation of the fetus 15. In the third trimester, aberration of Doppler findings is usually accompanied by adverse perinatal outcome. Almost all of the clinical research works, on the utilization of Doppler parameters, have been concerned with the estimation of small-for-gestational-age (SGA) fetuses, who are at high threat for unfavorable perinatal consequences16.
Up to our knowledge, the focus of most studies was on maternal PlGF rather than the fetal P1GF, thus the purpose of the current approach was to appraise the interrelation between fetal PlGF, fetal Doppler parameters, gestational age as well as neonatal growth parameters assessed by birth weight, birth length, head circumference and mid arm circumference.
Subjects and methods
This cross-sectional investigation was performed on 50 pregnant women in their third trimester and their newborns. Pregnant women were selected randomly from attendees at Al-Galaa Maternity Educational Hospital throughout the time frame from August 2022 to September 2022. The inclusion criteria comprised pregnant mothers with age range from 18 and 35 years, experiencing their first pregnancy, with a single full term child (> 37weeks) and the expected mode of delivery was normal vaginal or cesarean section. Exclusion criteria included pregnant mothers with age more than 35 years, multiparity, diabetic and those suffering from preeclampsia, antiphospholipid syndrome, connective tissue diseases, chronic infections, as well as those who engaged in alcoholism or smoking throughout pregnancy and those with any birth complications involving perinatal asphyxia.
The present investigation acted in accordance with the guidelines of the Committee of ethics for Medical Research of the National Research Centre(Code No. 3416072022). After explaining the background, objectives and advantages of the research, formal written agreement to participate in the study was taken from all candidates who included in this approach. Also, the informed consent was written by the mothers on the authority of their neonates who were incorporated in the research.
-All the pregnant women were submitted to the full history taking, general and obstetric examination, determining gestational age estimated by last menstrual period (LMP) and assured by assessment of crown-rump length at 11–13 weeks, establishing the lack of physical deformation of the fetus or any genetic situation by ultrasound examination performed during the previous period of antenatal follow up.
–Routine third trimester ultrasound examination was done; fetal biometrics and doppler studies for candidates > 37 weeks were done by a consultant via utilizing sonography device GE Voluson P8 with RAB 2-6 RS Real-time convex transducer 2–5 MHZ (Chicago, IL, USA). The following items of sonography were determined: estimated fetal weight (EFW) which was quantified in an automated manner according to the formula of Hadlock’s, mean umbilical arterial pulsatility index (UAPI) and mean middle cerebral artery pulsatility index (MCAPI). Based on the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) instructions in 2010, the subsequent sonographic items were employed to determine fetal weight and size; biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC), and femur diaphysis length (FDL), where Astraia software (copyright 2000‑2009, version 1.20.0 Build 139) was used for entering data and generating reports.
– Umbilical artery doppler: A free-floating cord was estimated, and the sample gate volume was placed over it, measuring 1 cm (two-third over the artery and one-third over the vein). The angle between the ultrasound beam and the direction of blood flow was maintained as close as possible to 0°. Measurement of 3‑6 waves in the image must avoid any fetal or breathing movements throughout the measurement.
The optimum method of MCA Doppler assessment was as follows.17
Brain axial section measurement (including thalami and sphenoid wings) and magnified.
Using of color flow mapping to recognize the circle of Willis and the proximal MCA
Placing of the pulse-wave Doppler gate at the proximal third of the MCA, close to the origin in the carotid artery.
The angle between the ultrasound beam and the direction of blood flow must be maintained as close as possible to 0°.
Recording of at least three to 10 successive waveforms.
Auto trace measurement should be used to calculate PI.
The detection of abnormal fetal blood flow was established by identifying diastolic block or reverse flow in the umbilical artery, as well as observing signs of centralization in fetal circulation, which included the increased resistance in the umbilical artery and/or the reduced resistance in the middle cerebral artery. Furthermore, the mode of delivery was evaluated to emphasize fetal distress as a crucial indicator for choosing the optimum delivery methods like vacuum extraction, forceps delivery, or cesarean section.
About 3ml blood of umbilical cord of the pregnant women was withdrawn, before placental separation. This collection was carried out from a portion of the umbilical cord that was promptly clamped and separated during delivery. The collected blood was then placed into plain tubes. Then the blood specimens were centrifuged under cooling at 1800 x g to isolate sera that were maintained at – 70°C until analysis. This included cord blood placental growth factor (PlGF) that was estimated by enzyme-linked immunosorbent assay (ELISA) commercial kit according to operating instructions.
The clinical assessments for all newborns, including chest, abdomen, heart, and central nervous system examination were performed by pediatrician. Also, the anthropometric measurements of the newborns were assessed prior breast feeding started. The weight of the newborns in kilograms was taken without diapers utilizing a digital electronic scale (Laka) designed for infants. The length of each newborn (in centimeters) was estimated in the supine situation, utilizing a stadiometer (Seca 416) consists of a fixed headboard and a movable foot board. Circumferences of the head and mid upper arm circumferences (in centimeters) were also estimated by inelastic tape 18.
Statistics
Data analysis was performed utilizing the SPSS statistical package software for windows version 26 (SPSS Inc, Pennsylvania, USA). Quantitative analyses were expressed as Mean ± Standard deviation (SD). Qualitative data were illustrated as frequency and percentage. Non-parametric analyses was presented by median and range. The analysis of data was done to examine the statistical significant difference between groups; differences between parametric variables were established using 2-tailed unpaired t-test. Pearson’s correlation coefficients were applied to assess correlations between the data displaying parametric distribution. Multiple linear regression analysis for PlGF as a dependent factor with the infant anthropometric measurements as predictor factors (Stepwise regression) was performed. P value < 0.05 was accounted significant difference and P < 0.005 was counted highly significant difference, at a confidence interval of 95%.
Results
Table (1) shows the correlation between fetal doppler parameters (UAPI, MCAPI, EFW) and PlGF. UA PI showed significant negative correlation with neonatal weight and PlGF. (P<0.001). Whereas UA PI showed insignificant negative correlation with MCA PI and EFW (P>0.01). MCA PI and EFW showed insignificant positive correlation with neonatal weight and PlGF(P>0.01). MCAPI showed insignificant negative correlation with EFW and UA PI (P>0.01). EFW showed insignificant negative correlation with MCA PI and UA PI (P>0.01).
Table (2) shows the anthropometric data and PlGF level in cord blood of the studied neonates. Also, the gestational age was included in Table (2). The level of cord blood PlGF ranged from 102.33±173.33 pg/ml with an average value of 142.67±17.93. The gestational age of the participants ranged from 32 to 39 weeks with the mean value of 36.8±2.00.
Table (3) shows correlation between all the studied neonatal anthropometric parameters, gestational age and PlGF level in cord blood. The findings detonated there is a significant positive correlation between gestational age (GA) and the neonatal anthropometric measurements including mid arm circumference (MAC), head circumference, weight, length, weight Z score, length Z score, head Z score, weight to length (W/L) and weight to length Z score (W/L Z Score). Also, significant positive correlation between PlGF and gestational age as well as the neonatal anthropometric measurements were found except head Z Score.
Table (4) represents multiple linear regression analysis for PlGF with UA PI and neonatal weight. Results indicated that UA PI and neonatal weight are significant predictors for fetal PlGF (P<0.001).
Table 1: Correlation between fetal doppler parameters (UAPI, MCAPI, EFW) and cord blood PlGF level .
|
|
PlGF |
Neonatal weight |
Fetal doppler parameters |
|||
|
MCA PI |
EFW |
UA PI |
||||
|
MCA PI |
Pearson Correlation |
0.414 |
0.408 |
1 |
-0.184 |
-0.406 |
|
Sig. (2-tailed) |
0.070 |
0.074 |
0.437 |
0.075 |
||
|
EFW |
Pearson Correlation |
0.261 |
0.247 |
-0.184 |
1 |
-0.166 |
|
Sig. (2-tailed) |
0.266 |
0.294 |
0.437 |
0.484 |
||
|
UA PI |
Pearson Correlation |
-0.933** |
-0.964** |
-0.406 |
-0.166 |
1 |
|
Sig. (2-tailed) |
<0.001 |
<0.001 |
0.075 |
0.484 |
||
|
** Correlation is significant at the 0.01 level (2-tailed). |
||||||
MCA PI: Middle cerebral artery pulsatility index
EFW: Estimated fetal weight
UA PI: Umbilical artery pulsatility index
Table 2: Gestational age, anthropometric data and placental growth factor level (PlGF) in the studied neonates
|
Range |
Mean |
SD |
|
|
Gestational Age (GA) (weeks) |
37 – 39 |
38.80 |
2.00 |
|
Neonatal Weight (Kg) |
2.40 – 3.50 |
2.8972 |
0.43900 |
|
Neonatal Length (cm) |
47.0 – 53.0 |
49.780 |
1.3559 |
|
W/L (Kg/cm) |
0.0 – 0.1 |
0.084 |
0.0370 |
|
Neonatal Mid Arm Circumference (MAC) (cm) |
6.5 – 11.0 |
8.988 |
1.1726 |
|
Neonatal Head Circumference (cm) |
30.0 – 36.0 |
32.600 |
1.5518 |
|
Weight Z Score (Kg) |
(- 8.04) – (- 4.88) |
-6.9864 |
0.96699 |
|
Length Z Score (cm) |
(-1.78) – 1.87 |
-0.0304 |
0.87401 |
|
W/L Z Score (Kg/cm) |
(-9.99) – (- 6.23) |
-8.7848 |
1.15131 |
|
Head circumference Z Score (cm) |
(-2.79) – 0.54 |
-1.0352 |
0.90455 |
|
PlGF (pg/ml) |
102.33 – 173.33 |
142.6660 |
17.92704 |
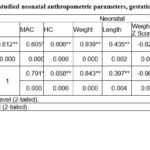 |
Table 3: Correlation between all the studied neonatal anthropometric parameters, gestational age and PlGF level in cord blood. |
Table 4: Linear regression analysis for cord blood PlGF level with UA PI and neonatal weight.
|
Model |
Unstandardized Coefficients |
Standardized Coefficients |
T |
Sig. |
||
|
B |
Std. Error |
Beta |
||||
|
1 |
(Constant) |
196.293 |
4.955 |
|
39.614 |
<0.001 |
|
UA PI |
-45.535 |
4.129 |
-0.933 |
-11.029 |
<0.001 |
|
|
2 |
(Constant) |
30.087 |
7.645 |
|
3.935 |
<0.001 |
|
Neonatal weight |
38.428 |
2.563 |
0.962 |
14.995 |
<0.001 |
|
UA PI: Umbilical artery pulsatility index
Dependent Variable: PlGF
Discussion
Fetal growth is commonly modulated by the function of placenta, with the placenta serving the fundamental respiratory, hepatic and renal functions of the fetus. The early problems of the placenta can occur du to incomplete trophoblast invasion leading to a remodeling failure of the myometrial arteries and uteroplacental blood flow reduction that is generally correlated with pre-eclampsia and fetal growth restriction19
Placental growth factor (PlGF) is a glycoprotein consisting of two subunits and belongs to the vascular endothelial growth factor (VEGF) family. It has been shown that PlGF has powerful proangiogenic impacts that lead to early placental vascular development 20. PlGF has a 53% homology to VEGF 21 and it is a pleiotropic growth factor (angiokine) capable of stimulating blood vessel formation and stabilization of many tissues 22.
The focus of our interest in the current research was to establish the interrelation between fetal PlGF, fetal Doppler parameters and neonatal growth parameters. The assessment of growth parameters at birth aids to predict the subsequent growth and development and risk of diseases. It has been demonstrated that the genetic, nutritional and the intrauterine conditions affect the fetal growth. Indeed growth parameters and gestational age assist in identifying the risk of neonatal pathology.
In our study, the abnormal fetal circulation was estimated by assessment of the latest Doppler ultrasound findings before delivery. Fetal blood flow was investigated in the umbilical and in the middle cerebral arteries and was correlated to PlGF level. Our findings indicated that regarding UAPI, there is a significant negative correlation between PlGF and neonatal weight. However, insignificant negative correlation was recorded between UAPI with MCAPI and estimated fetal weight (EFW). Gomez-Roig etal.23 stated that in the third trimester, UA Doppler PI and PlGF estimations aid for identifying the pregnancies at the great risk of negative perinatal outcomes owing to intrauterine growth restriction (IUGR). They concluded that, even though testing of joint doesn’t provide any no predictive advantage over UA Doppler PI alone, both diagnostic methods can be used interchangeably for this objective. Similarly, Molvarec et al. 24found slight or very slight level of PlGF in normal blood flow fetuses and in the neonates with IUGR and the PlGF is negatively correlated with PI values in the umbilical and uterine arteries as well. The correlation between serum levels of the angiogenic growth factor in the fetus and Doppler ultrasound measurements of the uterine and umbilical arteries in IUGR mirrors the fetal disorders severeness. The incorporation of both parameters can be helpful in the monitoring for early prognosis of pregnancy complications in the future25. Also, in agreement with our results, Taylor et al. 26reported that, in women with normal blood pressure and with IUGR, PlGF levels are reduced versus normal controls. Thus, PlGF may provide useful information to recognize fetuses demanding immediate delivery, and those at stake of subsequent unfavorable consequences not distinguished by fetal flow Doppler ultrasonography.
The data in the present research indicated significant positive correlation between umbilical cord blood PlGF and GA. Interestingly, the levels of fetal PlGF have been found to be correlated with fetal growth; reduced values of fetal PlGF were accompanied by delivery of SGA neonates 6. Fetuses with SGA represent a heterogeneous category comprising fetuses who are less developed than normal and those with fetal growth restriction (FGR) leading to a low birth weight 27. Differences of umbilical cord blood PlGF levels have been observed between neonates with SGA and AGA 28. This is in accordance to the findings of the present investigation.
The anthropometric parameters of the new-born population are considered as a significant scientific research tools to study the determinants and consequences of weakened or exaggerated fetal growth 29. Our results showed significant correlation between the gestational age and all anthropometric measures. In accordance with our findings, Thawani et al. 30 mentioned that gestational age has an excellent linear correlation with birth weight, crown heel-length, mid-upper arm-circumference, and head circumference.
The most fundamental factor for growth, and development of newborns is the gestational age (GA), because newborns morbidity and mortality are mainly related to GA as well as anthropometric parameters like length, birth weight (BW), head, arm, and chest circumferences 31. Recently, a positive correlation between GA and anthropometric variables has been demonstrated within newborns. From anthropometric measurements, BW was the most commonly used anthropometric indicator of birth size 32. Birth weight (BW) is a crude summary of fetal growth, and the similar birth weight may be the consequence of many different paths of growth 33. Mid arm circumference (MAC) was also correlated well with GA; it is considered as a good individual anthropometric parameter for GA assessment in neonates 34. Also, neonatal MAC within 72 h of birth is a good representative for BW in the developing nations and it is accounted as easy, convenient and significant anthropometrical parameter in detection of low birth weight newborn babies 35. Measuring the neonatal foot length, chest circumference and MAC have been found to be reasonable tools for estimating low BW (< 2500 g) and prematurity (gestational age < 37 weeks) 36. Paulsen et al.37observed that foot length, chest circumference and MAC all correlated well with BW and GA and had reasonable sensitivity and specificity for the detection of SGA. Moreover, a positive correlation has been detected between foot length as well as arm/head and chest circumferences and GA, BW in addition to body length 38. Gandhi et al.39 conducted a study in a Western Indian population about using head circumference as a primitive tool for estimation of GA in neonates and they found a strong correlation between GA and head circumference.
Placental growth factor(PlGF) is generated by trophoblasts which promote proliferation, migration and activation of endothelial cells 10. PlGF has been known to cross placenta, thus PlGF of fetal and maternal or placental origin is existed in the umbilical cord blood. However, minute correlations were observed between the level of PlGF in the umbilical cord blood on one side and maternal levels of PlGF and weight of placenta on the other. This indicates the predominance of fetal origin of PlGF in the umbilical cord blood 28.
The current findings showed significant positive correlation between cord blood PlGF and the all anthropometric parameters of the neonates implicated in the present study. The interrelation between PlGF of fetal origin and the clinical outcome fetal growth restriction (FGR) has been reported 40. Broere-Brown et al. 28 demonstrated that fetal PlGF is correlated with the growth of the fetus, with steady findings for BW, manner of growth, and the clinical outcome FGR. These investigators reported that there is an interrelation between fetal PlGF levels and fetal growth measures. Correlative studies have demonstrated that the elevated maternal soluble Flt1 (sFlt1), PlGF receptor, and the reduced PlGF, along with the decreased umbilical cord blood PlGF, are linked with low BW 6. These findings confirmed those of Broere-Brown et al. who established that the lower PlGF levels in the cord blood were linked with a lower BW 28Regarding the systemic impacts of sFlt-1 on fetal growth and development, it has been found that increased sFlt-1 umbilical levels are inversely correlated with fetal growth in normal pregnancies 6and sFlt-1 levels are also inversely associated with body weight percentile in preterm infants 41. It is well known that the bioavailability of PlGF is reduced by binding to its receptor sFLT-1 42. Garcia-Manau et al. concerned with the ratio of sFlt-1/PlGF in the detection of FGR, exploring that median values sFlt-1/PlGF enhanced alongside the FGR severity and confirmed an inverse association between the values of sFlt-1/PlGF ratio and GA at the moment of delivery43.
It has been stated that low PlGF MoM quintiles are also related to FGR. Thus, fetal PlGF might be an optimistic biochemical marker to estimate the growth of the fetus and FGR aberrations retrospectively, allowing follow-up of these neonates 28FGR is usually proved by a low BW and a high probability for boring neonate with SGA 44. Thus, the clinical outcome FGR is frequently recognized as SGA 45.
Neonates who have FGR may represent a susceptible category with a high chance of less efficient hearts in childhood 46. Thus, low levels of PlGF in the cord blood could be an important indication of this high risk. Anyhow, it must account the probability that fetuses with small weight deliver little PlGF. In that situation the behind machinery of the fetuses being small induce the probable counteractive consequences in the future life accompanied with FGR rather than PlGF itself. Fetuses with FGR are frequently delivered with a normal BW, but still represent a susceptible class with a high risk for cardiovascular diseases in childhood. The epidemiologic studies have proposed a correlation between low BW and/or FGR and elevated rate of cardiovascular mortality in future life. Moreover, experimental and clinical researches have evidenced that persistent nutrient and oxygen deficiency that are coupled with FGR stimulate adaptive cardiovascular alterations that might elucidate this association. FGR led to metabolic programming that may enhance metabolic syndrome risk and in turn cardiovascular morbidity in the adult life. Furthermore, FGR is highly connected with fetal cardiac and arterial remodeling and a subclinical
situation of cardiovascular dysfunction47. Recognition of this susceptible subjects by the follow-up of P1GF in the postnatal period, allows prohibiting the prospective deleterious outcomes in later life 28. PlGF could be considered a promising tool for identifying the neonates at risk for adverse consequences 48.
Conclusion
The data in the present approach shed light on the impact of fetal placental growth factor (PGF) level, which may not represent maternal level of placental growth factor, on the neonatal adverse growth pattern. In addition, fetal placental growth factor with fetal Doppler parameters may be considered a promising predictive biomarker for identifying those neonates at risk for detrimental childhood outcomes.
Acknowledgements
Not applicable.
Conflict of Interest
The authors declare that they have no competing interests.
Funding Source
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors’ contributions
E.R.A. suggested the idea of the manuscript, A.H.K. performed the anthropometric measurements of the neonates, L.S.S. performed the statistical analysis, H.H.A. conducted the biochemical analysis of PlGF and wrote the manuscript, M.T.S. carried out the clinical examination of the neonates, S.S. carried out the clinical examination of the pregnant women at delivery time and collected the blood samples from the umbilical cord, M.A.G. participated in writing the manuscript, All authors reviewed the manuscript.
References
- Desoye G, Hauguel-De Mouzon S. The human placenta in gestational diabetes mellitus the insulin and cytokine network. Diabetes Care. 2007; 30 (2): S120:S126.
CrossRef - Wang Y, Zhao S. Vascular biology of the placenta. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. PMID: 21452443.
- Demir R, Kayisli UA, Cayli S, Huppertz B. Sequential steps during vasculogenesis and angiogenesis in the very early human placenta. Placenta. 2006; 27:535–539. doi:10.1016/j.placenta.2005.05.011
CrossRef - Pollheimer J, Vondra S, Baltayeva J, Beristain AG, KnöflerM. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front Immunol. 2018; 9:2597. doi: 10.3389/fimmu.2018.02597
CrossRef - Weckman AM , Ngai M , Wright J, McDonald CR, Kain KC.The impact of infection in pregnancy on placental vascular development and adverse birth outcomes . Frontiers Microbiology 2019; 1924. doi: 10.3389/fmicb.2019.01924
CrossRef - Bergen NE, Bouwland-Both MI, Steegers-Theunissen RP, Hofman A, Russcher H, Lindemans J, et al. Early pregnancy maternal and fetal angiogenic factors and fetal and childhood growth. the Generation R Study. Human Reproduction.2015;30:1302-1313. doi:10.1093/humrep/dev070
CrossRef - Torry DS, Mukherjea D, Arroyo J, Torry RJ. Expression and function of placenta growth factor: implication for abnormal placentation. J. Soc. Gynecol. Invest.2003;10:178-188
CrossRef - Staff AC, Braekke K, Harsem NK, T. Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia, Eur. J. Obstet. Gynecol. Reprod. Biol.2005;122 (1): 33-39
CrossRef - Makrydimas G, Sotiriadis A, Savvidou MD, Spencer K, Nicolaides KH. Physiological distribution of placental growth factor and soluble Flt-1 in early pregnancy. Prenat Diagn. 2008; 3:175-179.
CrossRef - Kalampokas E, Vrachnis N, Samoli E, Rizos D, Iliodromiti Z, Sifakis S, et al. Association of adiponectin and placental growth factor in amniotic fluid with second trimester fetal growth. In Vivo. 2012; 26(2):327-333.
- Chau K, Hennessy A, Makris A. (2017). Placental growth factor and pre-eclampsia. J Hum Hypertens. 2017; 31(12):782–786. doi.org/ 10.1038/jhh.2017.61
CrossRef - Tsiakkas A, Duvdevani N, Wright A, Wright D, Nicolaides KH. Serum placental growth factor in the three trimesters of pregnancy: effects of maternal characteristics and medical history. Ultrasound Obstet Gynecol.2015; 45:591-598.
CrossRef - Algeri P, Ornaghi S, Bernasconi DP, Cappellini F, Signorini S, Brambilla P, et al. Feto-maternal correlation of PTX3, sFlt-1 and PlGF in physiological and preeclamptic pregnancies. Hypertens Pregnancy.2014;33:360-370
CrossRef - Ropacka-Lesiak M, Korbelak T, Świder-Musielak J, Breborowicz G. Cerebroplacental ratio in prediction of adverse perinatal outcome and fetal heart rate disturbances in uncomplicated pregnancy at 40 weeks and beyond. Arch Med Sci.2015; 11:142-148.
CrossRef - Akolekar R, Syngelaki A, Gallo DM, Poon LC, Nicolaides KH. Umbilical and fetal middle cerebral artery Doppler at 35–37 weeks’ gestation in the prediction of adverse perinatal outcome. Ultrasound Obstet Gynecol.2015; 46:82-92.
CrossRef - Khalil AA, Morales-Rosello J, Elsaddig M, Khan N, Papageorghiou A, Bhide A, et al. The association between fetal Doppler and admission to neonatal unit at term. Am J Obstet Gynecol.2015; 213(1): 57.e1-57.e7.
CrossRef - Bhide A, Acharya G, Bilardo CM, Brezinka C, Cafici D, Hernandez- Andrade E, et al. ISUOG practice guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet Gynecol.2013; 41:233-239.
CrossRef - Ramagopal Shastry CK, Poornima R. Bhat B. Anthropometric measurements of newborns. Int J Contemp Pediatr .2015;2(2):85-89.
CrossRef - Kaufmann P, Black S, Huppertz B. Endovascular trophoblastinvasion: implications for the pathogenesis of intrauterinegrowth retardation and preeclampsia. Biol Reprod 2003;69:1–7
CrossRef - Kasdaglis T, Aberdeen G, Turan O, Kopelman J, Atlas R, Jenkins C, et al. Placental growth factor in the first trimester: relationship with maternal factors and placental Doppler studies. Ultrasound Obstet Gynecol.2010; 35:280-285
CrossRef - Du H, Li P, Pan Y, Li W, Hou J, Chen H, et al. Vascular endothelial growth factor signaling implicated in neuroprotective effects of placental growth factor in an in vitro ischemic model. Brain Research.2010;1357:1-8
CrossRef - Dewerchin M, Carmeliet P. PlGF: a multitasking cytokine with disease restricted activity. Cold Spring Harb Perspect Med. 2012; 2: a011056.
CrossRef - Gomez-Roig MD, Mazarico E, Sabria J, Parra J, Oton L, Vela A. Use of placental growth factor and uterine artery doppler pulsatility index in pregnancies involving intrauterine fetal growth restriction or preeclampsia to predict perinatal outcomes. Gynecol Obstet Invest.2015;80(2):99-105.
CrossRef - Molvarec A, Gullai N, Stenczer B, Fügedi G, Nagy B, Rigó J Jr. Comparison of placental growth factor and fetal flow Doppler ultrasonography to identify fetal adverse outcomes in women with hypertensive disorders of pregnancy: an observational study. BMC Pregnancy Childbirth.2013;13:161.
CrossRef - Schlembach D, Wallner W, Sengenberger R, Stiegler E, Mörtl M, Beckmann MW et al. Angiogenic growth factor levels in maternal and fetal blood: correlation with Doppler ultrasound parameters in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol.2007;29(4):407-413.
CrossRef - Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol.2003; 188:177-182.
CrossRef - Figueras F, Gratacos E. Stage-based approach to the management of fetal growth restriction. Prenat Diagn.2014; 34:655-659.
CrossRef - Broere-Brown Z, Sarah Schalekamp-Timmermans S, Jaddoe V, Steegers E. Fetal growth and placental growth factor umbilical cord blood levels. Fetal Diagn Ther.2018; 43(1):26-33. doi: 10.1159/000475547.
CrossRef - Cheikh Ismail L, Knight H, Bhutta Z, Chumlea W, for the International fetal and newborn growth consortium for the 21st century (INTERGROWTH-21st). Anthropometric protocols for the construction of new international fetal and newborn growth standards: the INTERGROWTH-21st Project. BJOG. 2013;120 (Suppl. 2): 42–47.
CrossRef - Thawani R, Dewan P, Faridi M, Khanna Arora S, Kumar R. Estimation of gestational age, using neonatal anthropometry: a cross-sectional study in India. J Health Popul Nutr.2013; 31(4):523-530.
CrossRef - Oluwafemi O, Njokanma F, Disu E, Ogunlesi T. The current pattern of gestational age-related anthropometric parameters of term Nigerian neonates. S Afr J CH.2013; 7(3):100-104.
CrossRef - Ayed M, El-Bastwese R, Mahmoud T, Thabet A, Mohamed M. The relation between gestational age and anthropometric measurements among newborns. Egyptian Journal of Health Care.2021; 12(1): 404-417
CrossRef - Law C, Egger P, Dada O, Delgado H, Kyelberg E, Lavin P, et al. Body size at birth and blood pressure among children in developing countries. Int J Epidemiol.2001;30(1):52-57.
CrossRef - Tiruneh C. Estimation of gestational age using neonatal anatomical anthropometric parameters in Dessie Referral Hospital, Northeast Ethiopia. Risk Management and Healthcare Policy. 2020; 13:3021-3029
CrossRef - Rajesh N , Kiran P. Identification of an anthropometric surrogate to low birth weight in newborns: A hospital based cross sectional study. International Journal of Community Med Public Health.2018;5(5):2066-2071.
CrossRef - Kc A, Nelin V, Vitrakoti R, Aryal S, Malqvist M. Validation of the foot length measure as an alternative tool to identify low birth weight and preterm babies in a low-resource setting like Nepal: a cross-sectional study. BMC Pediatr.2015;15:43.
CrossRef - Paulsen C , Nielsen B , Msemo O , Møller S , Ekmann J , Theander T, et al. Anthropometric measurements can identify small for gestational age newborns: a cohort study in rural Tanzania. Paulsen et al. BMC Pediatrics.2019;19:120 doi.org/10.1186/s12887-019-1500-0
CrossRef - Deia K, Khalaf Y, Al- Rawi K, Maaesah S. The anthropometric measurements in full-term neonates in Baghdad. International Journal of Current Research.2018;10(7):71229-71232.
- Gandhi D, Masand R , Purohit A. A simple method for assessment of gestational age in neonates using head circumference. Global Journal for Research Analysis.2014; 3(5): 211- 213.
CrossRef - Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal 2009; 2 (59):re1. doi: 10.1126/scisignal.259re1.
CrossRef - Voller SB, Chock S, Ernst LM, Su E, Liu X, Farrow KN, et al. Cord blood biomarkers of vascular endothelial growth (VEGF and sFlt-1) and postnatal growth: a preterm birth cohort study. Early Hum. Dev.2014; 90:195-200. doi: 10.1016/j.earlhumdev.2014.01.003
CrossRef - Saffer C, Olson G, Boggess KA, Beyerlein R, Eubank C, Sibai BM. Determination of placental growth factor (PlGF) levels in healthy pregnant women without signs or symptoms of preeclampsia. Pregnancy Hypertens. 2013; 3(2): 124–132.
CrossRef - Garcia-Manau P, Mendoza M, Bonacina E, Garrido-Gimenez C, Fernandez-Oliva A, Zanini J, Catalan M, Tur H, Serrano B, Carreras E. Soluble fms-like tyrosine kinase to placental growth factor ratio in different stages of early-onset fetal growth re-striction and small for gestational age. Acta Obstet. Gynecol. Scand. 2021; 100: 119–128.
CrossRef - Sundrani D, Khot V, Pisal H, Mehendale S, Wagh G, Joshi A, et al. Gestation dependent changes in angiogenic factors and their associations with fetal growth measures in normotensive pregnancy. PLoS One.2013;8: e54153.
CrossRef - Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol.2016; 48:333-339
CrossRef - Crispi F, Bijnens B, Figueras F, Bartrons J, Eixarch E, Le Noble F, et al. Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation.2010;121:2427-2436.
CrossRef - Crispi F, Miranda J, Grataco´s E. Long-term cardiovascular consequences of fetal growth restriction: biology, clinical implications,and opportunities for prevention of adult disease. Expert Reviews 2018; 218: S869-S879.
CrossRef - Parchem JG, Brock CO, Chen H, Kalluri R, Barton JR, Sibai BM. Placental growth factor and the risk of adverse neonatal and maternal outcomes. Obstetrics Gynecology.2020;135(3):665-673.
CrossRef







