Manuscript accepted on :02-02-2023
Published online on: 18-10-2023
Plagiarism Check: Yes
Reviewed by: Dr. Gandham Rajeev
Second Review by: Dr. Abdulrahman Rasheed Mahmood
Final Approval by: Dr. Ayush Dogra
Hemanth T , Renuka P*
, Renuka P* and V. M. Vinodhini
and V. M. Vinodhini
Department of Biochemistry, SRM Medical College Hospital and Research Centre, Faculty of Medicine and Health Sciences, SRM Institute of Science and Technology, Kattankulathur – 603203, Kanchipuram, Chennai, TN, India.
Corresponding Author E-mail:renukap@srmist.edu.in
DOI : https://dx.doi.org/10.13005/bpj/2812
Abstract
Introduction and Aim: Diabetes mellitus is a burgeoning worldwide problem. For monitoring the glycemic state in diabetes, glycated hemoglobin (HbA1c) is one of the standardized tests. Measurement of uncertainty shows the magnitude of the doubt about a measurement result. The current study aims to estimate the measurement uncertainty (MU) of HbA1c in our laboratory using the method suggested by EURACHEM/CITAC and advocate its utilization as a quality standard in clinical laboratories as part of the quality control program to enhance the reliability of their results. Materials and Methods: The HbA1c levels were measured using a high-performance liquid chromatography method. The “Top Down” technique proposed by EURACHEM/ CITAC was used to calculate the uncertainty of HbA1c. This method allows the inclusion of the bias and imprecision of the HbA1c method. Bias value was calculated from data of external quality assessment results of the EQAS program and two levels of internal quality control results data was used to calculate the imprecision. Results: The measurement uncertainty (95% confidence interval) of HbA1c was estimated to be 4.2%. When measurement uncertainty was taken into account, the acceptable range for an HbA1c value of 6.5% typically used to diagnose DM was between 6.2 to 6.77. Conclusion: The EURACHEM/CITAC method is valuable for calculating MU of HbA1c and is a viable way that can be utilized as an extra analytical target in HbA1c testing. Furthermore, the data can assist clinicians in determining the degree of confidence that may be placed in test results.
Keywords
EURACHEM/CITAC; Glycated hemoglobin; Measurement uncertainty; Top-down approach
Download this article as:| Copy the following to cite this article: Hemanth T, Renuka P; Vinodhini V. M. The Use of Internal and External Quality Control Data for the Calculation of Measurement Uncertainty of Glycated Hemoglobin. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Hemanth T, Renuka P; Vinodhini V. M. The Use of Internal and External Quality Control Data for the Calculation of Measurement Uncertainty of Glycated Hemoglobin. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3tCwGHm |
Introduction
Diabetes mellitus (DM) is a multifactorial chronic metabolic disorder characterized by persistent hyperglycemia affecting large part of the population 1. According to the International Diabetes Federation (IDF), nearly 463 million people aged 20-79 years had diabetes in 2015, with the figures predicted to increase by another 200 million by 2040 if current trends persist.
The use of glycated haemoglobin for monitoring diabetes started in the 1980s. In 2009, the World Health Organization issued guidelines on the utility of HbA1c as a diagnostic test. The guidelines also stressed upon the need for maintaining stringent quality assurance measures of the measurand 2. Pre-diabetes is an asymptomatic type of diabetes mellitus in which blood glucose levels are elevated but not high enough to be classified as diabetes. Pre-diabetes can be clinically identified through HbA1c values between 5.7% and 6.4% 3. Type 2 Diabetes mellitus (T2DM) is diagnosed when the HbA1c level exceeds the pre- diabetes values (≥ 6.5%) 4.
In recent years, since the HbA1c values produced by clinical laboratories have such a significant impact on the diagnosis of diabetes and patient monitoring, it is critical for any clinical laboratory to continuously monitor the performance of their methods, ensuring that these methods achieve proper analytical performance and ensure that the results are accurate 5.
Measurement uncertainty is defined as the “nonnegative parameter characterizing the dispersion of the quantity values being attributed to a measurand, based on the information used” according to the Guidelines to the Expression of Uncertainty in Measurement” (GUM) which was first published in 1993 6,7. Measurement uncertainty indicates with a certain probability that the true value lies within the limits of uncertainty 8.
The calculation of uncertainty of measurement of glycated hemoglobin by using the method proposed by EURACHEM/CITAC is the core objective of our study and suggesting the adaptation of this parameter as an analytical tool used by clinical laboratories as part of their quality control to ensure the accuracy of their HbA1c results. The knowledge of the interval within which the true value of the glycemic status of the patient lies would enable the clinicians to determine the protocol for treatment with confidence.
Materials and Methods
The study was conducted at SRM General Hospital central lab and the data generated from the results of Internal Quality Control at SRM General Hospital Central lab (Biorad laboratory Inc., USA) in the time period from January to December 2020 and External Quality control results provided by Christian Medical College (CMC), Vellore, Tamil Nadu, India.
High performance liquid chromatography method
The high-performance liquid chromatography method (D-10 Biorad laboratories Inc., USA) was used to estimate the HbA1c values.
Estimation of measurement uncertainty
The statistical method used to obtain the uncertainty of measurement of HbA1c was the “top down” approach, described in the EURACHEM/CIATAC guide 9. This method will allow to calculate the uncertainty of HbA1c by using results of quality control data. Formula for that
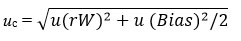
where the uncertainty component related to random error u(RW) and systemic errors u(Bias) are calculated separately by using the quality control data.
Step-1
The calculation of uncertainty of within-laboratory reproducibility u(RW) used Bio-Rad control level 1 CV% and level 2 CV %. It yields the uncertainty associated with random error.
Step-2
In order to calculate the u(Bias)(which is associated with systemic error), we have calculated RMS bias and RMS ucref which we can calculate by using the data from EQAS.
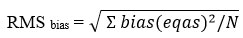
(N- number of external quality control, EQAS- external quality control results)
Bias is calculated by using following formula

Step – 3
RMS ucref, is standard uncertainty component for the certified or assigned value. Calculated by, formula
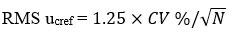
Step- 4
U bias calculated by using the RMS bias and RMS ucref values with the help of the following formula
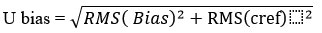
Step- 5

For calculating expanded uncertainty include a coverage factor of K= 2, which provides an expanded uncertainty at approximately the 95% confidence level. Formula is:
U = K ×uc
The U results were compared with TAE for the HbA1c test.
Results
The retrospective review study was conducted in the central lab of SRM Medical College Hospital & Research Centre (SRM MCH & RC). The data consisted of 142 level 1 and level 2 internal quality control results and 12 months external quality control results (Table 1 & 2).
Table 1: Imprecision results of control sera level 1and level 2
|
|
Control 1 |
Control 2 |
|
HbA1c expected provider concentration |
5.4 |
9.6 |
|
Number of IQC samples analyzed |
142 |
142 |
|
Mean of our IQC results |
5.34 |
10.01 |
|
CV % of our lab |
2.22 |
2.06 |
Table 2: External quality control assessment program data of 1 year
|
|
Results |
|
Peer group Monthly average participants |
483 |
|
Peer group coefficient of variance (CV%) |
6.45 |
|
Peer group HbA1c mean concentration |
6.32 |
|
HbA1c mean concentration of laboratory |
6.40 |
Table 3. The measurement of uncertainty for HbA1c
|
The measurement of uncertainty for HbA1c |
||
|
Internal quality control |
Level 1 (mean and CV%) |
5.32 – 2.22% |
|
Level 2 (mean and CV%) |
10.01 – 2.05% |
|
|
uRW |
2.14 |
|
|
External quality control |
EQAS (mean and CV%) |
6.32 – 6.45% |
|
n |
483 |
|
|
RMS bias |
2.0 |
|
|
Ucref |
0.29 |
|
|
Standard, combined and expanded uncertainty values |
Standard uncertainty |
2.0 |
|
Combined uncertainty |
2.1 |
|
|
Expanded uncertainty |
4.2 |
|
CV%- Coefficient variation, RMS bias- Root mean squares of biases, Ucref- Uncertainty component from the certified or nominal value, uRW- Uncertainty of within-laboratory reproducibility.
The results of the uncertainty estimation of HbA1c in our study are shown in table 3. HbA1c 6.5% is recommended as a cut-off value for diagnosing DM. The MU for HbA1c was estimated at ±4.2%, when the MU was taken into account, the acceptable range for a value of 6.5% was about 6.23 to 6.77.
Discussion
Measurement of HbA1c plays an important role in monitoring the glycemic status of diabetic patients. International organizations like the NGSP and IFCC have issued specific guidelines for standardization of the parameter 10 . Individual laboratories should take responsibility to ensure the adherence to the analytical performance goals to achieve HbA1c results that are fit for interpretation purposes.
Various fields of metrology find uncertainty of measurement to be an important parameter. The usage of MU in clinical laboratories is limited. ISO/IEC17025 suggest the inclusion of uncertainty of measurement in test reports whenever relevant 11 . The complexity of measuring this parameter as given by GUM deterred its usage in many laboratories. The EURACHEM/CITAC guide provides a relatively easy method for calculating uncertainty measurement using the internal & external quality control data.
Within laboratory reproducibility and bias due to the method and laboratory condition can be assessed by the internal & external quality controls. Day-to-day variation and sample repeatability together form the within-laboratory reproducibility. Bias represents the systemic error and bias variation can be evaluated using external quality assessment program 12. We have utilized imprecision, bias, uncertainty of bias in the calculation of MU in the current study to accord the actual dispersion of values pertaining to the measured parameter i.e., HbA1c.
Lenter-Westra et al demonstrated that HbA1c results generated by methods accredited by NGSP are not always useful for clinical usage. Standardization of the methods used for estimation of HbA1c is still insufficient 13. Assessment of reliability of a test parameter is essential to achieve a level of confidence in the analytical precision of the test result. MU provides the reliability and defines the predicted variability in a laboratory result in case of repetition. Knowledge of MU helps in improved evaluation of clinical decisions.
In 2009, International Expert Committee published a report recommending that HbA1c can be used to diagnose diabetes when the levels are ≥ 6.5 % 14,15. Hence, the knowledge of MU becomes important for decisions involving diabetes diagnosis. Unal et al demonstrated the effect of measurement uncertainty on the clinical decision levels of HbA1c in 1555 patients out of the 10212 subjects in a retrospective study 16. In our study, we found the expanded uncertainty to be 4.2%. The uncertainty of HbA1c value was below the total allowable error of ± 6% in our laboratory. When it is applied to the HbA1c of 6.5%, the accepted value would be between 6.23 % and 6.77 %. The interpretation of the result should be made with the knowledge of the measurement uncertainty. Assessment of the reliability of a test enhances its utility in the clinical field. There will be alteration in values that are close to the decision limits when appraised with the measurement uncertainty and requires careful interpretation. Evaluation of HbA1c reports with MU will indicate the actual limits and enable the clinicians to analyse and interpret the reports with a stated level of confidence. A limitation of our study is that the estimation of bias is ideally performed using reference materials which is not viable in most clinical laboratories. Also, matrix related bias was not eliminated since the commutability of control samples was not studied.
Conclusion
Our laboratory results demonstrated that the expanded uncertainty is 4.2% which is below the total allowable error of ± 6% suggested by NGSP. According to ISO17025, the laboratories should provide MU on request. Periodic calculation of the uncertainty of measurement of glycated hemoglobin is recommended to be a part of quality control program of the laboratory. Awareness of concept of MU would provide the clinician with a level of confidence that can be assigned to the test results and interpret them accordingly.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There are no funding sources
References
- IDF Diabetes Atlas 9th edition 2019 [Internet] [cited 2021 Apr 16]. Available from: https://www.diabetesatlas.org/en
CrossRef - International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334.
CrossRef - Shubrook J, Butts A, Chamberlain JJ, Johnson EL, Leal S, Rhinehart AS, et al. Standards of medical care in diabetes—2017 abridged for primary care providers. Clin Diabetes. 2017;35(1):5–26.
CrossRef - American Diabetes Association (ADA) Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:S62–9.
CrossRef - Diagnosis and Classification of Diabetes Mellitus. American Diabetes Association Diabetes Care 2014 Jan; 37(Supplement 1): S81-S90.
CrossRef - Vocabulario Internacional de Metrología, Conceptos fundamentales y generales, y términos asociados (VIM). Centro Español de Metrología, Available at http://www. cem.es/sites/default/files/vim-cem-2012web.pdf, (2012).
CrossRef - Bureau International des Poids et Mesures. Rapport BIPM-80/3, Report on the BIPM Inquiry on Error Statements. Sèvres:BIPM; 1980;4:2016. http://www.BIPM.org/utils/common/pdf/rapportBIPM/1980/03.pdf
CrossRef - Dallas Jones GR. Measurement uncertainty for clinical laboratories – a revision of the concept. Clinical Chemistry and Laboratory Medicine. 2016 Aug;54(8):1303-1307. DOI: 10.1515/cclm-2016-0311. PMID: 27176746.
CrossRef - EURACHEM/CITAC Guide, Quantifying Uncertainty in Analytical Measurement. 3rd ed. EURACHEM/CITAC 2012. Available at: https://www.eurachem.org/ images/stories/Guides/ pdf/QUAM2012_P1.pdf.
- Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, Lernmark A, Metzger BE, Nathan DM. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2011 Jun;57(6): e1-e47. doi: 10.1373/clinchem.2010.161596. Epub 2011 May 26. PMID: 21617152.
CrossRef - ISO/IEC 17025 (Ed.), General Requirements for the Competence of Testing and Calibration Laboratories, International Organization for Standardization, 2017.
CrossRef - Galindo-Méndez M, Sánchez-López A, Cruz-Fuentes L. The estimation of uncertainty of measurement of glycated hemoglobin as an analytical performance specification and in the interpretation of its results. Clin Biochem. 2019 Jan; 63:92-96. doi: 10.1016/j.clinbiochem.2018.10.012. Epub 2018 Oct 25. PMID: 30595159.
CrossRef - Lenters-Westra E, Slingerland RJ. Six of eight hemoglobin A1c point-of-care instruments do not meet the general accepted analytical performance criteria. Clin Chem. 2010 Jan;56(1):44-52. doi: 10.1373/clinchem.2009.130641. Epub 2009 Nov 19. PMID: 19926777.
CrossRef - Hattersley A, Bruining J, Shield J, Njolstad P, Donaghue K; International Society for Pediatric and Adolescent Diabetes. ISPAD Clinical Practice Consensus Guidelines 2006-2007. The diagnosis and management of monogenic diabetes in children. Pediatr Diabetes. 2006 Dec;7(6):352-60. doi: 10.1111/j.1399-5448.2006.00217.x. Erratum in: Pediatr Diabetes. 2007 Feb;8(1):49. PMID: 17212604.
CrossRef - International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009 Jul;32(7):1327-34. doi: 10.2337/dc09-9033. Epub 2009 Jun 5. PMID: 19502545; PMCID: PMC2699715.
CrossRef - Kubranur Unal, Gokce Filiz Atikeler. The evaluation of measurement uncertainty of HbA1c and its effect on clinical decision levels. Int J Med Biochem. 2018; 1(2): 53-56.
- Keutmann S, Zylla S, Dahl M, Friedrich N, Landgraf R, Heinemann L, Kallner A, Nauck M, Petersmann A. Measurement Uncertainty Impacts Diagnosis of Diabetes Mellitus: Reliable Minimal Difference of Plasma Glucose Results. Diabetes Ther. 2020 Jan;11(1):293-303. doi: 10.1007/s13300-019-00740-w. Epub 2019 Dec 16. PMID: 31845101; PMCID: PMC6965559.
CrossRef







