Manuscript accepted on :10-08-2023
Published online on: 15-11-2023
Plagiarism Check: Yes
Reviewed by: Dr. Saumya Bipin
Second Review by: Dr. Rifat-E-Nur Hossain
Final Approval by: Dr. Ayush Dogra
Praveen Thaggikuppe Krishnamurthy1 , Merina Benny2*
, Merina Benny2* , Benny Antony2
, Benny Antony2 , Binu T Kuruvilla2
, Binu T Kuruvilla2 and Nishant Kumar Gupta2
and Nishant Kumar Gupta2
1Department of Pharmacology, JSS College of Pharmacy, JSS Academy of Higher Education and Research, Ooty, Tamil Nadu, India.
2Development Laboratory, Arjuna Natural Private Ltd., Erumathala PO, Aluva, Kerala, India.
Corresponding Author E-mail: research@arjunanatural.com
DOI : https://dx.doi.org/10.13005/bpj/2774
Abstract
Amaranth is one of the popularly grown leafy vegetables in tropical regions globally and contains a large amount of nitrate. The present study's objective was evaluation of acute and repeated dose toxicity of amaranth extract as per the OECD guidelines. The acute oral toxicity was conducted in 6 female rats (150-170 g; 8-10 Weeks old) as per OECD 423 guidelines. The amaranth extract had no adverse/toxic effects and no mortality was noted at the dose of 2000mg/kg. The oral LD50, therefore, was considered greater than 2000mg/kg. The sub-chronic (28-day repeated dose) toxicity was studied in 40 rats (150-170 g; 8-10 Weeks old) as per OECD 407 guidelines whereas chronic (365-days repeated dose) toxicity study was conducted in 200 rats (150-170 g; 8-10 Weeks old) as per OECD 452 guidelines. Sub-chronic study confirmed the safety of amaranth extract at the highest dose of 1000 mg/kg/day. The 1000 mg/kg in rats was considered as NOEL (No Observed Adverse Effect Level). The chronic toxicity study established a NOEL of 180 mg/kg in rats. In the repeated dose toxicity studies, body weight, food consumption, blood profile, biochemistry parameters and histopathology of major organs were similar in test and control groups. The current study results indicated that amaranth extract was safe upon acute, sub-chronic and chronic administration in rats, under testing conditions and at dose levels employed.
Keywords
Chronic toxicity; OECD guidelines; Red spinach; Safety profile; Toxicity study
Download this article as:| Copy the following to cite this article: Krishnamurthy P. T, Benny M, Antony B, Kuruvilla B. T, Gupta N. K. Safety Evaluation of Amaranth Extract by Acute, Sub-Chronic and Chronic Exposure in Rats. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Krishnamurthy P. T, Benny M, Antony B, Kuruvilla B. T, Gupta N. K. Safety Evaluation of Amaranth Extract by Acute, Sub-Chronic and Chronic Exposure in Rats. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3G09OVe |
Introduction
Vegetables and fruits in diet are considered essential for long life and human health maintenance. These are considered rich in several vitamins, minerals and other potentially metabolically active compounds, e.g. polyphenols1. Apart from these nutritious compounds, nitrate (NO3ˉ) present in vegetables has unique role in vasodilation2,3. The facultative anaerobic bacteria present in the oral cavity, facilitates the conversion of NO3ˉ ions into nitrite (NO2ˉ) ions4. Once NO2ˉ is formed, it further converts to NO (nitric oxide) via various pathways5. During low oxygen saturation (also called hypoxia) in blood, the conversion of NO2ˉ to NO takes place at faster rate6. Endothelial nitric oxide synthase (eNOS or NOS3) is the primary source of NO in the vascular endothelium. Under a hypoxia state, the expression of eNOS decreases, resulting in low NO production. In such state, formation of NO3ˉ to NO2ˉ and then conversion to NO works as an alternate system for NO production inside the body7. NO is considered as one of the primes signaling molecule with multiple roles in humans. These include maintenance of muscles contraction, flow of blood in arteries and veins, homeostasis of certain molecules like calcium and glucose etc8,9.
It has been scientifically proved that NO2ˉ level can be improved in significant manner by ingesting NO3ˉ in diet. This helps in lowering the blood pressure by dilating the blood vessels10-12. Consumption of NO3ˉ in diet also increases endurance to exercise as a response to physiological benefits13. In a study by Stokes et al., C-reactive protein level was decreased after NO3ˉ and NO2ˉ intake in high cholesterol fed mice. It also decreased the vascular inflammation and significantly reversed the endothelial dysfunctions14.It has been documented that during old age, the supply of amino acid L-arginine (an important NOS-substrate) and tetrahydrobiopterin (one of the cofactors) reduces15. This along with poor level of NO2ˉ, makes the regular nitric oxide pathway less efficient during ageing16. Apart from this, overproduction of O2– (free radical superoxide) in old age, decreases the bioavailability of NO by formation of peroxy-nitrites17. This reduced availability of NO may increase the chances of endothelial dysfunctions during ageing process18 and may cause arterial hyper-tension19 of old age. Thus, increase of NO3ˉ consumption in the diet of old age peoples may be beneficial for overall vascular health and adequate supply of NO in bioavailable form.
The vegetables like Spinach, Cabbage and underground edible parts like beetroot are known to contain high percentage of NO3ˉ. Amaranth (also known as red spinach) is one of the popular vegetables rich in various nutrients along with significantly higher amount of NO3ˉ present in leaves20. It is cultivated as gluten free pseudo-cereal primarily in Asia, Mexico, South America and all tropical places of the world21. Amaranth is a fast-growing plant and easy to maintain and consume as leafy vegetables throughout the year. The leaves as well as seeds of amaranth are considered highly nutritious22,23. Both leaves and seeds are rich sources of various proteins. Quantitatively, leaves contain about 15-30% protein whereas seeds contain 15–45% of fresh matter. The leaves also contain Vitamin C (one of the widely known antioxidant), dietary fibers and traces of essential minerals24,25. The composition of amino acid present in amaranth proteins is considered well balanced, highly bioavailable and good functional characteristics26. Apart from these nutrients, amaranth leaves are also rich in secondary plant metabolites, which may provide potential health benefits27. Recent research has indicated that leaves and other aerial parts of amaranth are important sources of phenolic compounds28-30. Among these, hydroxycinnamic acids, benzoic acids, flavonols and their glycosides have been reported in amaranth leaves and flowers31. Other phytochemicals present in amaranth with antioxidant activity are betalains, especially betacyanins32. The contents of these pigments vary among amaranth species and genotypes33.
To get clinical benefits, heavy intake of vegetables rich in NO3ˉ is practically very difficult in routine day-to-day life. Along with nitrates, a large amount of oxalic acid (an anti-nutrient) also gets inside the body and may result in kidney damage on prolonged use. Moreover, it has been reported that food rich in NO3ˉ didn’t increase the nitrate levels in blood whereas consumption of NO3ˉ in the form of dietary supplement increased the same in old age peoples34. In another published study, the absorption of NO3ˉ from extract of amaranth leaves (2 g dose) was studied where single dose of extract, significantly increased (p<0.001) the plasma NO3ˉ levels in healthy adults as compared to the subject’s consumed placebo35. The authors concluded that one dose of extract from red spinach leaves can enhance the NO3ˉ levels in plasma for more than eight hours. The higher levels of NO3ˉ may be beneficial in various sports activities and routine exercises.
Apart from multiple benefits of dietary NO3ˉ, a few studies have reported some adverse effects/toxicity of synthetic potassium and sodium nitrate in animals36. These were mainly due to development of methemoglobinemia. At the same time, toxic effects of potassium nitrate on some biochemical parameters of rats were completely ameliorated by simultaneous feeding of ascorbic acid37. The rationale of current study is to establish safety of extract from amaranth leaves with high dietary nitrates and to show its high safety profile compared to toxicity associated with synthetic NO3ˉ and NO2ˉ.
Materials and Methods
Test sample and animals
Commercial batch of Oxystorm® from Arjuna Natural Private Ltd., Kochi, Kerala, India was used as test sample of Amaranth extract. It is standardized to contain approximately 9% dietary nitrate. Male and female Wistar albino rats weighing 150-170 g were kept at animal house conditions (Temperature 24±2°C; Relative humidity 55-70%; 12/12 h light/dark cycle). Filtered potable water and extruded rodent diet supplied by M/s. Amruth labs, Bangalore, India was used ad libitum. Acute and sub-chronic toxicity studies were approved by IAEC of JSS College of Pharmacy, Ooty, Tamilnadu, India (approval no. JSSCP/IAEC/CADRAT/2014-15). SD rats of 160-180 gram were used for the long-term chronic toxicity and study was approved by IAEC of Arjuna Natural Private Ltd., Kochi, Kerala, India (approval no. ANEL/ IAEC/ 2016-I/ 1607015). SD rats were chosen for long term study due to longer life span of SD rats as compared to Wistar rats. The animal house conditions for long term study were same as mentioned for acute and sub-chronic toxicity study.
Acute toxicity study
OECD 423 guidelines were followed to conduct this study in stepwise manner38. Three rats were used in the each step to determine adequate classification of the test material (amaranth extract) by acute toxicity test. Six Wistar albino rats (female only) weighing 150-170 g (Age, 8-10 Weeks) were used in this study and acclimatized for 7 days before each step’s commencement. The amaranth extract was dissolved in distilled water and administered to rats by oral route at 2000 mg/kg body weight. A metal canula fitted to a syringe was used for this purpose. Based on the results, the next set of animals were administered with a 2000 mg/kg dose of the test item. The rats were carefully observed by an experienced veterinarian for 14 days. Special attention was paid during initial 4 hours on day 1 of the study. Body weight of all the rats was noted at baseline, day 7 and day 14. On the last day of study, necropsy was conducted by a veterinarian to see any gross lesions or hemorrhage in major organs.
Sub-chronic (28 days repeated dose) toxicity study
OECD 407 guidelines were followed to conduct sub-chronic toxicity study in rats39. Forty rats (M/F: 1/1; 150-170 g; 8-9 weeks old) were included in this study and randomly distributed into four groups. Ten rats comprising of 5 males and 5 females were kept in each group. Acclimatization period was one week in standard animal house conditions. Amaranth extract was dissolved in water and administered at 100, 500 and 1000 mg/kg to the rats of group 1, 2 and 3 as low, medium and high dose, respectively. Required quantity of extract was daily weighed and freshly dissolved in water before administration to the rats. Extract feeding was continued for 28 days using metal cannula attached with syringe. The fourth group of ten rats (M/F: 1/1) was fed with distill water alone for the same duration (28 days) and was considered as the control group.
Daily cage side observations were done by a qualified veterinarian for any abnormal behaviour or symptom. At the end of the study, sensory reactivity towards different stimuli (e.g., various reflexes, visual, auditory and proprioceptive stimuli), measurement of grip strength and motor coordination assessment were performed as per the standards published procedures40-43. In brief, flexion reflex (tests spinal cord) was assessed by pinching the toes of rat with forceps, the response was to move the foot away. Grasping reflex (tests cerebral cortex) was assessed by picking up the rat and palm was touched with a wire; the response was to grip the wire. Righting reflex was tested by putting the rat on its back and it turns over immediately. Auditory startle was assessed by putting the rat on a level surface in quiet environment and then a loud hand clap was given. The rat flexed forelimbs, extended hind-limbs and arched the body. For assessment of grip strength, the animal was kept on the top of the wire-bottomed cage. The tail was clenched at the base and the animal was pulled along the surface to measure its capacity to hold on to the wired surface. The motor coordination was evaluated by Rotarod apparatus. At the end of the experiment, animals were fasted for 16 h and blood samples were collected from retro orbital plexus of all the animals. The blood samples were used as such (with K3EDTA as anti-coagulant) for hematology whereas serum was separated by centrifugation at 3000 rpm for 15 min and used for the biochemical estimations. Routine urine analysis was also conducted as per the standard procedures. Body weight of rats was recorded weekly and on the last day of study, all the rats were sacrificed and major tissues were preserved in 10% buffered formalin for histopathology using rotary microtome.
Chronic toxicity study
OECD 452 guidelines were followed to conduct chronic toxicity study44. Two-hundred rats (100 male/100 female) were used to conduct this study. The rats were divided into four main groups of 40 rats in each group. Ratio of male/female rats was kept 1:1 for all the groups. An acclimatization period of seven days was followed before start of dosing of extract. First three group of rats were fed at 45, 90 and 180 mg/kg of amaranth extract as low medium and high dose groups. Distill water was fed to the fourth group of rats and designated as control group. Dosing was continued once daily for one year duration.
Apart from four main study groups, two groups of 20 animals in each (10M/10F) were fed with amaranth extract 180 mg/kg and distill water, respectively and designated as ‘Recovery groups’. These groups were also fed for the duration of one year but after the end of one year period, these recovery group animals were observed for one more month (without feeding of extract) for any reversible effect or delayed toxicity.
Daily observations on any abnormal behaviour or toxic symptom were conducted by a qualified veterinarian. Blood samples were collected at the end of study period and serum was separated by centrifugation to conduct biochemistry. Hematology was performed with as such whole blood (EDTA was used as an anti-coagulant). Terminally, the rats were sacrificed and all the major organs were collected, preserved in formalin and studied for histopathological changes.
Statistical analysis
Bartlett’s test was conducted to analyze the homogeneous nature of data by GraphPad Prism Software. The data was further analyzed by ANOVA and if ‘F’ was found significant, an individual comparison of means of control and treated groups was done using Dunnett’s test. P value <0.05 was considered significant.
Results
Acute toxicity study
The body weight gain of all the rats was similar and in normal range over the study duration of 2 weeks (Table 1). All the animals were healthy and no animal died after feeding of amaranth extract at 2000 mg/kg dose. The behavior of all the rats was normal as observed by veterinarian. The animals didn’t show any symptom of toxicity or abnormality throughout the study period. The cavities and orifices were normal when observed on the day of sacrifice. There was no change in skin or eye color and mucous membrane was also normal. The gross necropsy revealed that animals were healthy and all the internal organs were normal. The LD50 of amaranth extract was calculated as >2000 mg/kg in rats. As per the OECD 423 guidelines, the extract falls in the category 5 of Globally Harmonized System.
Table 1: Body weight (g) and mortality data in acute toxicity study of amaranth extract
|
Dose in mg/kg |
Rat |
M/F |
Body weight (g) |
No. dead / No. tested |
||||
|
Initial |
Day 8 |
Weight change (day 8 – Initial) |
Day 15 |
Weight change (day 15 – Initial) |
||||
|
2000 |
1 |
Female |
158 |
165 |
7 |
169 |
11 |
0/6
|
|
2 |
Female |
161 |
170 |
9 |
176 |
15 |
||
|
3 |
Female |
157 |
163 |
6 |
174 |
17 |
||
|
4 |
Female |
149 |
152 |
3 |
163 |
14 |
||
|
5 |
Female |
150 |
155 |
5 |
172 |
22 |
||
|
6 |
Female |
149 |
154 |
5 |
171 |
22 |
||
Sub-chronic toxicity study
There was no mortality in extract treated or
control rats. All the rats were clinically fit and their behavior was normal
throughout the study period. Gain in body weight was almost similar in all the
groups. Similarly, there was no significant difference in the food intake
pattern of all the groups. In this study, no significant changes in the haematology
(Table 2-3) and bio-chemistry profile of control and treated rats were observed
after 28 days. These parameters for treated groups were comparable to respective
control group of rats (Table 4-5).
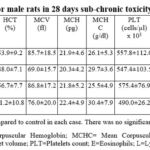 |
Table 2: Hematology profile for male rats in 28 days sub-chronic toxicity study of amaranth extract |
 |
Table 3: Hematology profile for female rats in 28 days sub-chronic toxicity study of amaranth extract |
Table 4: Clinical chemistry data for male rats in 28 days sub-chronic toxicity study of amaranth extract.
|
Group & |
FBS (mg/dl) |
ALKP (U/L) |
CHO (mg/dl) |
BUN (mg/dl) |
Cre (mg/dl) |
T.Pro (gm/dl) |
Alb (gm/dl) |
SGOT (U/L |
SGPT (U/L)) |
|
Control (0 mg/kg) |
85.2±5.9 |
144.3±8.1 |
118.7±8.5 |
44.8±14.1 |
0.9±0.0 |
11.9±1.9 |
7.3±0.6 |
119±12.9 |
74±11.2 |
|
Low dose (100 mg/kg) |
84.8±6.2 |
146.6±12.3 |
112.1±7.6 |
46.9±14.4 |
0.9±0.0 |
12.6±2.0 |
7.7±1.0 |
130.5±12.7 |
69.4±10.5 |
|
Medium dose (500 mg/kg) |
87.0±4.6 |
151.5±8.8 |
108.1±6.9 |
51.3±7.1 |
0.9±0.1 |
11.1±1.6 |
7.7±1.0 |
126.4±20.8 |
68.8±7.0 |
|
High dose (1000 mg/kg) |
85.5±4.2 |
149.3±10.6 |
113.0±8.1 |
42.8±9.0 |
0.8±0.0 |
12.6±2.4 |
7.5±0.7 |
125.9±5.4 |
74.5±3.6 |
Data presented as Mean±SD; n=5. ANOVA, p>0.05 as compared to control in each case. There was no significant difference between treated and control groups.
FBS=Fasting Blood Sugar; ALKP=Alkaline phosphatase; CHO=Cholesterol; Cre=Creatinine; BUN=Blood urea nitrogen; SGOT=Serum glutamic oxaloacetic transaminase; SGPT=Serum glutamic pyruvic transaminase; T.Pro=Total Protein; Alb=Albumin.
Table 5: Clinical chemistry data for female rats in 28 days sub-chronic toxicity study of amaranth extract.
|
Group & |
FBS (mg/dl) |
ALKP |
CHO (mg/dl) |
BUN |
Cre (mg/dl) |
T.Pro (gm/dl) |
Alb (gm/dl) |
SGOT |
SGPT |
|
Control (0 mg/kg) |
88.7±6.2 |
145.0±3.7 |
105.4±4.2 |
43.6±10.0 |
0.9±0.1 |
13.8±2.1 |
7.3±0.9 |
118.4±14.3 |
68.1±6.3 |
|
Low dose (100 mg/kg) |
85.7±5.4 |
137.5±4.2 |
112.0±12.0 |
46.4±13.0 |
0.9±0.1 |
11.6±2.0 |
7.5±0.8 |
124.0±16.0 |
71.9±6.5 |
|
Medium dose (500 mg/kg) |
83.2±6.2 |
150.3±13.4 |
111.8±9.6 |
42.7±6.3 |
0.9±0.0 |
13.2±2.5 |
7.4±1.0 |
123.7±21.7 |
69.0±8.1 |
|
High dose (1000 mg/kg) |
87.3±2.3 |
141.7±14.0 |
110.2±12.7 |
45.9±11.5 |
0.9±0.1 |
11.6±1.8 |
7.5±0.4 |
125.4±13.9 |
71.6±5.5 |
Data presented as Mean±SD; n=5. ANOVA, p>0.05 as compared to control in each case. There was no significant difference between treated and control groups.
FBS=Fasting Blood Sugar; ALKP=Alkaline phosphatase; CHO=Cholesterol; Cre=Creatinine; BUN=Blood urea nitrogen; SGOT=Serum glutamic oxaloacetic transaminase; SGPT=Serum glutamic pyruvic transaminase; T.Pro=Total Protein; Alb=Albumin.
The histological observations of all the vital organs in control and treated groups were same. There was no treatment related abnormality (Figure 1-2). Some haemorrhage and alveolar oedema was observed in the lungs of extract treated (high dose) as well as control rats. In few rats hydronephrosis and cysts in kidney was also noted but it was similar for control and high dose extract treated rats. Liver of a few rats in both the groups have shown vacuolation and cellular swelling at some places. These findings were considered normal/incidental by the histopathologist and concluded as non-toxic nature of the test extract.
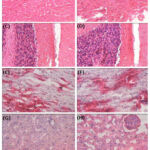 |
Figure 1: Microscopic sections of major organs in 28 days sub-chronic toxicity (X100). |
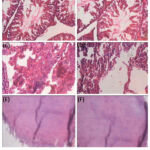 |
Figure 2: Microscopic sections of major organs in 28 days sub-chronic toxicity (X100). |
In the treated as well as control group of rats, body weight and organ weight on 28th day was in normal range. The organ weight to body weight ratios were comparable to respective control rats for males as well as females. In the medium dose (500 mg/kg) males, there was significant reduction in fasting body weight and a significant enhancement in weights of gonads and heart. In addition, medium dose females showed increase in absolute gonads weight. However, the same was not found with high dose rats (treated at 1000 mg/kg) and there was no dose correlation was observed, therefore, considered incidental.
In the 28-days repeated dose study with amaranth extract, the maximum dose of 1000 mg/kg in rats didn’t produce any toxic effect and all the findings were comparable to respective control counterparts. The NOEL (No Observed Adverse Effect Level) in this study was found as 1000 mg/kg in rats.
Chronic toxicity study
The chronic toxicity study was conducted at slightly low dosages to observe the accumulated effect over a period of one year. There was no death of rats at any dose level of extract treated or control group of animals. The body weight and food intake of all the rats was in normal range. When compared to the control rats, there was no abnormality noted with extract treated rats as far as visible clinical symptoms are concerned.
The
level of RBC, WBC, Hemoglobin etc was in normal range in extract treated as
well as control group of rats. Full hematological profile is presented as Table
6-7 for the rats of both the sexes. Furthermore, the liver functions, kidney
functions and lipid profile of all the rats was normal after one year of extract
treatment. The whole biochemistry data of control and amaranth extract fed rats
were similar when side by side comparison was conducted (Table 8-9). The gross
pathology and histopathology of all the collected organs was normal in all the
control and treated rats.
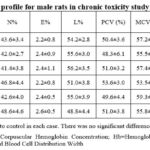 |
Table 6: Hematology profile for male rats in chronic toxicity study of amaranth extract |
 |
Table 7: Hematology profile for female rats in chronic toxicity study of amaranth extract |
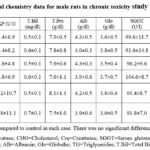 |
Table 8: Clinical chemistry data for male rats in chronic toxicity study of amaranth extract. |
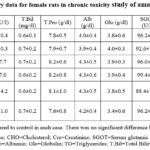 |
Table 9: Clinical chemistry data for female rats in chronic toxicity study of amaranth extract. |
Discussion
The traditional Japanese and Mediterranean food habits are considered most healthy all over the world. These are found to increase the life span and less occurrence of heart related disorders45. Both of these diets contain plenty of fresh vegetables and fruits. Japanese diet is also rich in fish whereas olive oil is one of the ingredients in Mediterranean diet. These diets do not contain red meat except a few occasional dishes. One of the common things is the presence of high dietary nitrate content in both of these diets. In Mediterranean diet, vegetables (leafy) are used as such. These may include red-spinach, lettuce and rocket salad etc. Whereas Japanese diet contains ta cai, spinach, garland chrisantemum, etc46. Food rich in vegetables is normally considered to bring down the blood pressure and less occurrence of fatal coronary heart disease, nonfatal myocardial infarction or acute stroke47.
The ADI (Acceptable Daily Intake) for nitrate is 3.7 mg per kg of body weight as set by the European Food Safety Authority. This equals to 0.06 mmol/kg body weight in human. For an average built human of roughly 70 kg body weight, it will be equal to 260 mg daily48. This ADI was set on the basis of toxicity and safety studies conducted with synthetic nitrate. Since the natural nitrate supplements like amaranth or red spinach extract contains a lot of anti-oxidants and other phyto-nutrients, these extracts are safer than synthetic nitrates. Moreover, the recommended dose of amaranth extract is 1000 to 2000 mg/day for an adult. This equates to around 90 to 180 mg of nitrate per day which is well within the set ADI limits.
There are a number of side effects are known due to ingestion of synthetic nitrates. These include toxicity to reproductive organs, methylation of hemoglobin (methemoglobinemia) and other endovrine or metabolic disorders. In one study, male Wistar rats were treated with sodium nitrate at 19 mg/kg, 66 mg/kg and 150 mg/kg once daily by oral route for a period of 10 days49. Methemoglobinemia was observed in all the rats and nitrate got accumulated in the liver of high dose rats. The functions of liver were also got impaired as evidenced by increased levels of transferases, lactates, triglycerides, and glucose in medium (66 mg/kg) as well as high dose (150 mg/kg) groups. In their investigations, histopathology further confirmed the inflammation of liver cells, necrosis, steatosis etc in high dose group. In contrast to the study by González et al., in present study 28 days repeated feeding of amaranth extract at highest dose of 1000 mg/kg daily (equivalent to 90 mg nitrate/kg per day) to rats did not induce any observable toxic effect or liver injury. The polyphenols and other anti-oxidants present in the amaranth extract might have a protective effect on liver and other organs by avoiding production of nitrosamines.
Acute toxicity in rats and mice is considered first step in toxicity evaluation. In the present study, non-toxic nature of amaranth extract at 2000 mg/kg in rats confirmed the safety of the test material as LD50 was computed as >2000 mg/kg in rats. The 28 days study by daily feeding to rats at maximum dose of 1000 mg/kg in rats is another evidence of safety of amaranth extract. Therefore, the NOEL of amaranth extract in rats was found as 1000 mg/kg. This corresponds to 11.2 g for a 70 Kg human50.
The sub-chronic toxicity had certain limitations. A few histopathological findings in lungs, kidney and liver of control as well as amaranth extract treated group were noted. These were considered to be safe as seen in such studies, and were considered as incidental. Similarly, the male rats of 500mg/kg dose had slight decrease in fasting weight and an enhancement in weights of gonads and heart. The females of 500 mg/kg group showed a remarkable enhancement in the weight of gonads. However, same was not found with 1000 mg/kg daily dose group and there was no correlation with dose was observed, therefore, considered incidental.
Since food supplements are supposed to be taken for long term, the chronic toxicity study was also conducted by feeding amaranth extract to rats for one year duration. There was no toxic sign or symptoms observed in chronic toxicity study at low, medium or high dose of amaranth extract. The biochemical, hematological and histopathological observations further confirmed the safety of amaranth extract in rats. In this study, the three dosages 45, 90 and 180 mg/kg in rats were chosen to represent the human equivalent dose of 500, 1000 and 2000 mg. In this study, the gross observation of behavior, appearance and toxicological findings like changes in pupil size, color of skin and unusual respiratory pattern was normal, thus detailed ophthalmic examination using ophthalmoscope was not done. In sub-chronic toxicity study, the neurological and functional examinations (proprioceptive stimuli) was normal in all rats examined, thus detail examination on these parameters was not conducted in the chronic toxicity study. These parameters are optional as stated in the OECD 452 guidelines.
Several human clinical studies have also been reported with amaranth extract (red spinach extract). In one such study, acute effect of amaranth extract extract (1000 mg dose) was determined on vascular reactivity in peripheral conduit and resistance arteries51. In another study on healthy human subjects, amaranth extract (1000 mg dose which equals to 90 mg nitrate) was orally given as a single dose to study the exercise performance and endurance. The authors found that amaranth extract delayed the ventilatory threshold and response was ergogenic52. Interestingly, there were no adverse effects reported in the above human studies which further confirm the safety of amaranth extract in human. In chronic toxicity study 180mg/kg was the maximum dose in rats. While converting to human dose, it will be approximately 2 g per day for a 70 Kg human. Overall, the results of these studies confirm the safety of amaranth extract in rats at the tested dosages.
Conclusion
The amaranth extract tested in the present study has shown non-toxicity of the product at 2000 mg/kg in acute toxicity test. There were no adverse effects at 1000 mg/kg daily dosing for 28-days in sub-chronic toxicity study and this dose was considered as NOEL. In the chronic toxicity study also, the extract was non-toxic at 180 mg/kg daily in rats. Thus, it can be concluded that amaranth extract is safe at the doses tested in this study as per the guidelines laid by OECD.
Acknowledgement
The authors from JSS College of Pharmacy, Ooty, Tamilnadu acknowledge Arjuna Natural Private Ltd., Aluva, Kerala for providing Amaranth extract (Oxystorm®) as gift sample.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding source
This research received no specific grant from any funding agency in the public, commercial, private, or not-for-profit sectors.
References
- Cermak NM, Gibala MJ, van Loon LJ. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int. J. Sport Nutr. Exerc. Metab., 2012; 22(1): 64-71.
CrossRef - Gilchrist M, Winyard PG, Benjamin N. Dietary nitrate: good or bad? Nitric Oxide, 2010; 22(2): 104-109.
CrossRef - Visioli F, Bogani P, Grande S, Galli C. Mediterranean food and health: building human evidence. J. Physiol. Pharmacol., 2005; 56(Suppl 1): 37-49.
- Duncan C, Dougall H, Johnston P, et al. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat. Med., 1995; 1(6): 546-551.
CrossRef - Zhang Z, Naughton D, Winyard P, Benjamin N, Blake D, Symons M. Generation of nitric oxide by a nitrite reductase activity of xanthine oxidase: A potential pathway for nitric oxide formation in the absence of nitric oxide synthase activity. Biochem. Biophys. Res. Commun., 1998; 249(3): 767–772.
CrossRef - Bryan NS. Nitrite in nitric oxide biology: cause or consequence? A systems-based review. Free Radic. Biol. Med., 2006; 41(5): 691-701.
CrossRef - Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov., 2008; 7(2): 156-167.
CrossRef - Cooper CE, Giulivi C. Nitric oxide regulation of mitochondrial oxygen consumption II: molecular mechanism and tissue physiology. Am. J. Physiol. Cell Physiol., 2007; 292: C1993-2003.
CrossRef - Dejam A, Hunter CJ, Schechter AN, Gladwin MT. Emerging role of nitrite in human biology. Blood Cells Mol. Dis., 2004; 32(3): 423-429.
CrossRef - Bailey SJ, Fulford J, Vanhatalo A, et al. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol., 2010; 109(1): 135–148.
CrossRef - Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med., 2006; 355(26): 2792-2793.
CrossRef - Vanhatalo A, Bailey SJ, Blackwell JR, et al. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol., 2010; 299(4): R1121- R1131.
CrossRef - Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. (Oxf), 2007; 191(1): 59-66.
CrossRef - Stokes KY, Dugas TR, Tang Y, Garg H, Guidry E, Bryan NS. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol., 2009; 296(5): H1281- H1288.
CrossRef - Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J. Physiol., 2008; 586(4): 1161-1168.
CrossRef - Kleinbongard P, Dejam A, Lauer T, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic. Biol. Med., 2003; 35(7): 790-796.
CrossRef - Kang LS, Reyes RA, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: role of NO and H2O2. Am. J. Physiol. Heart Circ. Physiol., 2009; 297(3): H1087- H1095.
CrossRef - Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a ‘set up’ for vascular disease. Circulation, 2003; 107(1):139-146.
CrossRef - Fagard RH. Epidemiology of hypertension in the elderly. Am. J. Geriatr. Cardiol., 2002; 11(1):23-28.
CrossRef - Fasuyi AO, Dairo FAS, Adeniji AO. Tropical vegetable (amaranthus cruentus) leaf meal as alternative protein supplement in broiler starter diets: bionutritional evaluation. J. Cent. Eur. Agr., 2008; 9(1): 23-34.
- Rastogi A, Shukla S. Amaranth: A new millennium crop of nutraceutical values. Crit. Rev. Food Sci. Nutr., 2013; 53(2): 109–125.
CrossRef - Repo-Carrasco-Valencia R, Peña J, Kallio H, Salminen S. Dietary fiber and other functional components in two varieties of crude and extruded kiwicha (Amaranthus caudatus). J. Cereal Sci., 2009; 49(2): 219–224.
CrossRef - Venskutonis PR, Kraujalis P. Nutritional components of amaranth seeds and vegetables: A review on composition, properties, and uses. Compr. Rev. Food Sci. Food Saf., 2013; 12(4): 381–412.
CrossRef - Sarker U, Islam MT, Rabbani MG, Oba S. Genotypic variability for nutrient, antioxidant, yield and yield contributing traits in vegetable amaranth. J. Food Agri. Environ., 2014; 12: 168–174.
CrossRef - Sarker U, Islam MT, Rabbani MG, Oba S. Genotypic diversity in vegetable amaranth for antioxidant, nutrient and agronomic traits. Indian J. Genet. Plant Breed., 2017; 77: 173–176.
CrossRef - López DN, Galante M, Raimundo G, Spelzini D, Boeris V. Functional properties of amaranth, quinoa and chia proteins and the biological activities of their hydrolyzates. Food Res. Int., 2019; 116: 419–429.
CrossRef - Tang Y, Tsao R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Mol. Nutr. Food Res., 2017; 61(7): 1600767.
CrossRef - Kraujalis P, Venskutonis PR, Kraujalienė V, Pukalskas A. Antioxidant properties and preliminary evaluation of phytochemical composition of different anatomical parts of amaranth. Plant Foods Hum. Nutr., 2013; 68(3): 322–328.
CrossRef - Jiménez-Aguilar DM, Grusak MA. Minerals, vitamin C, phenolics, flavonoids and antioxidant activity of Amaranthus leafy vegetables. J. Food Compos. Anal., 2017; 58: 33–39.
CrossRef - Neugart S, Baldermann S. Ngwene B, Wesong J, Schreiner M. Indigenous leafy vegetables of Eastern Africa—A source of extraordinary secondary plant metabolites. Food Res. Int., 2017; 100(3): 411–422.
CrossRef - Li H, Deng Z, Liu R, Zhu H, Draves J, Marcone M, Sun Y, Tsao R. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, flower and stalk extracts of three Amaranthus species. J. Food Compos. Anal., 2015; 37: 75–81.
CrossRef - Miguel MG. Betalains in some species of the Amaranthaceae family: A review. Antioxidants, 2018; 7(4), 53.
CrossRef - Cai Y, Sun M, Wu H, Huang R, Corke H. Characterization and quantification of betacyanin pigments from diverse Amaranthus species. J. Agric. Food Chem., 1998; 46(6): 2063–2070.
CrossRef - Miller GD, Marsh AP, Dove RW, Beavers D, Presley T, Helms C, Bechtold E, King SB, Kim-Shapiro D. Plasma nitrate and nitrite are increased by a high-nitrate supplement but not by high-nitrate foods in older adults. Nutr. Res., 2012; 32(3): 160-168.
CrossRef - Subramanian D, Gupta S. Pharmacokinetic study of amaranth extract in healthy humans: A randomized trial. Nutrition, 2016; 32(7-8): 748-753.
CrossRef - Aly HA, Mansour AM, Abo-Salem OM, Abd-Ellah HF, Abdel-Naim AB. Potential testicular toxicity of sodium nitrate in adult rats. Food Chem. Toxicol., 2010; 48(2): 572-578.
CrossRef - Azeez OH, Mahmood MB, Hassan JS. Effect of nitrate poisoning on some biochemical parameters in rats. Iraq J. Vet. Sci., 2011; 25(2): 47-50.
CrossRef - OECD (2002), Test No. 423: Acute Oral toxicity – Acute Toxic Class Method, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, https://doi.org/10.1787/9789264071001-en.
CrossRef - OECD (2008), Test No. 407: Repeated Dose 28-day Oral Toxicity Study in Rodents, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, https://doi.org/10.1787/9789264070684-en.
CrossRef - Crofton KM, Howard JL, Moser VC, Gill MW, Reiter LW, Tilson HA, MacPhail RC. Interlaboratory comparison of motor activity experiments: implications for neurotoxicological assessments. Neurotoxicol. Teratol., 1991; 13(6): 599-609.
CrossRef - Gad SC. A neuromuscular screen for use in industrial toxicology. J. Toxicol. Environ. Health., 1982; 9(5-6): 691-704.
CrossRef - Moser VC, McDaniel KL, Phillips PM. Rat strain and stock comparisons using a functional observational battery: baseline values and effects of amitraz. Toxicol. Appl. Pharmacol., 1991; 108(2): 267-283.
CrossRef - Tupper DE, Wallace RB. Utility of the neurological examination in rats. Acta. Neurobiol. Exp., 1980; 40(6): 999-1003.
- OECD (2018), Test No. 452: Chronic Toxicity Studies, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, https://doi.org/10.1787/9789264071209-en.
CrossRef - Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med., 2009; 169(7): 659–669.
CrossRef - Tsuji KMS, Morita Y, Shibata T, et al. Naturally occurring of nitrite and nitrate existing in various raw and processed foods. J. Food Hyg. Soc. Japan, 1993; 34(4): 294–302.
CrossRef - Kapil V, Webb AJ, Ahluwalia A. Inorganic nitrate and the cardiovascular system. Heart, 2010; 96(21): 1703–1709.
CrossRef - Katan MB. Nitrate in foods: harmful or healthy? Am. J. Clin. Nutr., 2009; 90: 11–12.
CrossRef - González Delgado MF, González Zamora A, Gonsebatt ME, Meza Mata E, García Vargas GG, Calleros Rincón EY, Pérez Morales R. Subacute intoxication with sodium nitrate induces hematological and biochemical alterations and liver injury in male Wistar rats. Ecotoxicol. Environ. Saf., 2018; 166: 48-55.
CrossRef - Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm., 2016; 7(2): 27-31.
CrossRef - Haun CT, Kephart WC, Holland AM, Mobley CB, McCloskey AE, Shake JJ, Pascoe DD, Roberts MD, Martin JS. Differential vascular reactivity responses acutely following ingestion of a nitrate rich red spinach extract. Eur. J. Appl. Physiol., 2016; 116(11-12): 2267-2279.
CrossRef - Moore AN, Haun CT, Kephart WC, Holland AM, Mobley CB, Pascoe DD, Roberts MD, Martin JS. Red Spinach Extract Increases Ventilatory Threshold during Graded Exercise Testing. Sports (Basel), 2017; 5(4): 80.
CrossRef







