Manuscript accepted on :14-11-2023
Published online on: 25-12-2023
Plagiarism Check: Yes
Reviewed by: Dr. Amit Panaskar
Second Review by: Dr. Nicolas Padilla
Final Approval by: Dr. Luis Jesús Villarreal-Gómez
Abdulloh Machin1,2,3* , Octaviana Galuh Pratiwi4
, Octaviana Galuh Pratiwi4 , Imam Susilo3,5
, Imam Susilo3,5 , M. Hamdan1,2
, M. Hamdan1,2 , Djoko Agus Purwanto3,6
, Djoko Agus Purwanto3,6 , Imam Subadi3,7
, Imam Subadi3,7 , Paulus Sugianto1,2
, Paulus Sugianto1,2 , Kenia Izzawa1,2, Dinda Divamillenia4
, Kenia Izzawa1,2, Dinda Divamillenia4 , Makhfudli3,8
, Makhfudli3,8 , Azizah Amimathul Firdha9
, Azizah Amimathul Firdha9 and Chrismawan Adianto10
and Chrismawan Adianto10
1Department of Neurology, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
2Dr. Soetomo General Academic Hospital, Surabaya, Indonesia
3Universitas Airlangga General Academic Hospital, Surabaya, Indonesia
4Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
5Department of Anatomical Pathology, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
6Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Universitas Airlangga, Surabaya, Indonesia
7Department of Physical Medicine and Rehabilitation, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
8Faculty of Nursing, Universitas Airlangga, Surabaya, Indonesia
9Indonesian Medical Association (IDI) Surabaya chapter, Surabaya, Indonesia
10Department of Pharmacy Practice, Faculty of Pharmacy, Universitas Airlangga, Surabaya, Indonesia
Corresponding Author E-mail: abdulloh.m@fk.unair.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2791
Abstract
Background: Stroke ranks among the leading global causes of mortality and disability. During an ischemic stroke, there is an increase in oxidative stress. When oxidative stress occurs, mTOR is activated, causing an increase in NLRP3 expression. Nucleotide-binding oligomerization domain-containing 3 (NOD3) (as known as NLRC3) exerts an inhibitory effect on NLRP3, specifically inhibiting the pyroptosis process in microglial cells. Additionally, NOD3 serves as an inhibitor of the mTOR pathway. Inhibition of mTOR can result in the downregulation of the NOD3 protein. Green tea (Camellia sinensis) has been correlated to neuroprotection, especially the active compound Epigallocatechin-3-gallate (EGCG), which has an antioxidant effect. In the microglia cell of the Rattus norvegicus middle cerebral artery occlusion (MCAO) model, this study aims to ascertain the changes in nucleotide-binding oligomerization domain containing 3 (NOD3) expression with the intervention of green tea, containing the active compound EGCG. Methods: Eleven male rats were randomly assigned to each of the six groups. The groups are sham = healthy-rats group, P0 = control group (MCAO), P1 = MCAO with EGCG 10mg/kg Body Weight treatment, P2 = MCAO with EGCG 20mg/kg BW Body Weight treatment, P3 = MCAO with EGCG 30mg/kg Body Weight treatment, and P4 = MCAO with green tea extract 'Meditea' 30mg/kg Body Weight treatment. The stroke condition was created using the MCAO model by locking the internal carotid artery with a bulldog clamp for 180 minutes. For seven days, the intervention was administered once daily. The coronal portion of the infarcted hemisphere brain tissue was then removed for immunohistochemical analysis. The Allerd score represented the number of NOD3 expressions. Results: NOD3 expression is downregulated in response to EGCG and green tea extract intervention. The 10mg/kg Body Weight treatment resulted in a significant difference in NOD3 expression (p = 0.001). Green tea's active components, EGCG and NOD3, have a correlation (r = -0.330; p = 0.007). Conclusions: Green tea may treat ischemic stroke because its active compound, EGCG, decreases NOD3 expression.
Keywords
EGCG; Green Tea; MCAO; NLRC3; Stroke
Download this article as:| Copy the following to cite this article: Machin A, Pratiwi O. G, Susilo I, Hamdan M, Purwanto D. A, Subadi I, Sugianto P, Izzawa K, Divamillenia D, Makhfudli M, Firdha A. A, Adianto C. Green Tea with EGCG Active Compound Decreases NLRC3 Expression in Middle Cerebral Artery Occlusion (MCAO) Rats Model. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Machin A, Pratiwi O. G, Susilo I, Hamdan M, Purwanto D. A, Subadi I, Sugianto P, Izzawa K, Divamillenia D, Makhfudli M, Firdha A. A, Adianto C. Green Tea with EGCG Active Compound Decreases NLRC3 Expression in Middle Cerebral Artery Occlusion (MCAO) Rats Model. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3GUJJHp |
Introduction
Stroke is characterized by a sudden onset of neurological dysfunction and stands as one of the prevailing global culprits for fatalities and incapacitation. Stroke can be categorized into two types: ischemic stroke (~ 80%) and hemorrhagic stroke (~ 20%). As a result of hemiparesis or hemiplegia, both ischemic and hemorrhagic stroke patients require the usage of supportive devices. Ischemic stroke affects all aspects of quality of life, with the exception of independence and the environment. The cerebral media arteries are the most frequent places of occlusion when ischemia occurs.1,2 The preferred treatment is intravenous thrombolysis with r-TPA, but only 2-8.5% of patients receive it since many are contraindicated or have passed the thrombolysis phase.3,4 Neuronal cell death plays an important role in ischemia-induced brain injury. High Mobility Group Box Protein 1 (HMGB1) and Phospholipase A2 (PLA2) are released from the injured and dead components of the neurovascular unit in ischemic-induced brain injury. High Mobility Group Box Protein 1 (HMGB1)binds to several Toll-Like Receptors (TLRs) on microglia cells to further upregulate NOD-like Receptor Protein-3 (NLRP3).5 A recent study shows that the mammalian target of rapamycin (mTOR) plays a role in brain ischemia.6 Another study also explains that mTOR regulates NLRP3 activation via reactive oxygen species (ROS) in murine lupus.7
Nucleotide-binding oligomerization domain containing 3 (NOD3) (as known as NLRC3) has an inhibitory effect on NLRP3, which inhibits the pyroptosis process in microglia cells. 8,9 NOD3 also plays a role as an inhibitor of the mTOR pathway. The inhibition of mTOR can induce the downregulation of the NOD3 protein.10
Green tea (Camellia sinensis) has a potential neuroprotection source due to the catechins. 11 Green tea contains high levels of flavonoids: Epigallocatechin-3-gallate (EGCG), accounting for about 59% of the total content of catechins, epigallocatechin, and others.12 Several studies have demonstrated the antioxidant effects associated with EGCG.13 However, there are important variations in the processing of herbs, so the differences in EGCG concentrations in different types of tea may be responsible for the different neuroprotective effects of some teas.14 EGCG can inhibit mTOR.15
Based on the information above, it is known that green tea, containing the active compound EGCG, has the potential to prevent neuron cell death in ischemia conditions. This study analyzes the neuroprotective effect of green tea on NOD3 expression. This study might offer an alternate approach to solving ischemic stroke problems.
Material and Methods
The study procedure was reviewed and approved by the Animal Care and Use Committee (ACUC) of the Faculty of Veterinary Medicine at Universitas Airlangga No. 2.KE.161.09.2018 on 25 September 2018.
Animal
Male Rattus norvegicus mice, aged four months, weighing 200-275 grams, were used for the study. They were acclimated to the animal housing environment for one week under specific conditions, which included a 12-hour light-dark cycle and a temperature of °C. During this time, they had unrestricted access to water and standard rodent food. The criteria for animal care and use, established by the Institutional Animal Ethics Committee at Universitas Airlangga in Indonesia, were followed for all experimental methods.
Within several steps, the rats were conditioned to be middle cerebral artery occlusion (MCAO). Ketamine 80 mg/kg Body Weight and xylazine 10 mg/kg Body Weight were used intraperitoneally to anesthetize Rattus norvegicus. Excision was performed in the right neck of Rattus norvegicus until carotid communis could be seen, and then the carotid artery was separated from the inner carotid and locked for 180 minutes using a small bulldog clamp. At the 180-minute mark, the bulldog clamp was removed, and the neck incision was sealed. The rat consciousness was observed while observing whether or not the stroke model had emerged.16 Although the bulldog clamp technique was simpler than the other technique, it made the MCAO model in Rattus norvegicus well.
Eleven male rats were randomly assigned to each of the six groups. The groups are sham = healthy rats’ group, P0 = control group, P1 = EGCG 10mg/kg Body Weight, P2 = EGCG 20mg/kg Body Weight, P3 = EGCG 30mg/kg Body Weight, and P4 = green tea extract ‘Meditea’ 30mg/kg Body Weight. The extract of EGCG or green tea was diluted with aquades, a concentration of 1 mg/ml, then delivered to the samples before they had a meal using rat sonde every morning. The intervention was given once a day for seven days.
After the seventh day, the samples were anesthetized using propofol 0.1 mg/100 g intravenous rodent. A coronal section of the brain tissue from the infarcted hemisphere was removed. The rat brains were then preserved in 4% formalin and subsequently encased in a paraffin block for immunohistochemical (IHC) analysis.17
Chemicals
Anti NOD3 pAb Lsbio ABIN625967, EGCG (from Xi’an Rongsheng Biotechnology CO., LTD, Keji 3rd Road, Xi’an, China) or green tea extract (obtained from PT. Dharma Putra Airlangga, Tegalsari, Surabaya, Indonesia) extract dose 10, 20, and 30 mg/kg Body Weight; standardized green tea extract label ‘Meditea’; aquadest; ketamine 80 mg/kg Body Weight; xylazine 10 mg/kg Body Weight; propofol 0,1 mg/100 gr IV; anti-NOD3 antibodies; and BSA 1 %.
Immunohistochemical Examination
Samples of brain tissue were preserved in a solution containing 4% buffered neutral formalin; then, they were encased in paraffin, sectioned, and subjected to immunohistochemical staining to assess the NOD3 expression under a light microscope.
Anti-NOD3 pAb Lsbio ABIN625967 was used for immunohistochemical detection of NOD3 expression. Immunohistochemical (IHC) staining was carried out with anti-NOD3 antibodies. The slides were added with enzyme conjugate, diluted with BSA 1%, and incubated for an hour at ambient temperature; a chromogen was applied for a duration of 10 minutes, and the slides were rinsed with flowing water. If appropriate, counterstain was also given.
The result of the IHC staining was examined using a microscope with a magnification of 400x microscope. Scoring guidelines according to DC Allred, MD’s proportion score is as follows: 0 indicates no marker expression in any specimens; 1 indicates > 0–1% marker expression; 2 indicates > 1–10% marker expression; 3 indicates > 10–33.3 % marker expression; 4 indicates > 33.3-66.6 % marker expression; 5 indicates > 66.6–100% marker expression; and strength score is as follows: 0 indicates negative marker expression; 1 denotes low expression, 2 represents moderate expression, and 3 signifies high expression. The ratio plus the strength score adds up to the total scores.18
Statistical Analysis
All variables were analyzed with the descriptive test. The data underwent analysis to check for normal distribution using the Kolmogorov-Smirnoff and Levene tests for variance homogeneity. The independent sample T-test was employed to examine the variance in EGCG effects among groups, while the Pearson test was utilized to assess the correlation between variables. Data was analyzed using the Statistic Package for Social Science Software, Version 21.Results
The result of the IHC staining for NOD3 expression examination under the microscope with a magnification of 400 x (figure 1).
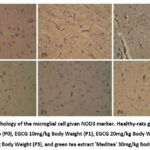 |
Figure 1: Morphology of the microglial cell given NOD3 marker. Healthy-rats group (Sham), control group (P0), EGCG 10mg/kg Body Weight (P1), EGCG 20mg/kg Body Weight (P2), |
The mean score of NOD3 expression in sham = 2,636, P0 = 5,636, P1 = 4,636, P2 = 4,000, P3 = 2,636, P4 = 2,909 (figure 2).
 |
Figure 2: The mean score of NOD3 expression in all treatment groups and the comparison result of each group with P0a and shamb, aSignificantly less than 0.05 compared to the P0 (control group). |
The research data was subjected to analysis using the Independent Sample T-test. To use this test, a few requirements must be met: each group’s data must have a normal distribution, The sample should be derived from separate data sources, and there should be uniform variance among the groups. This study’s homogeneity test, which used the Levene test, produced a sig value greater than 0.05 (p = 0.01), signifying that the data exhibited homogeneity; the normality test, which used the Kolmogorov-Smirnov test, also produced a sig value. > 0.05 (p = 0.01) indicates normally distributed data for this study. We compared every group with P0 and Sham.
The Independent sample T-Test result compared to sham in the NOD3 expression in the P0 = 0.00; P1 = 0.001; P2 = 0.001; P3 = 1.000 and P4 = 0.235. While if compared to P0, the results are Sham = 0.001; P1 = 0.001; P2 = 0.001; P3 = 0.001 and P4 = 0.001.
The relationship between these two variables was assessed through the Pearson correlation test. The significance is 0.007, with a value of r = – 0.330.
Discussion
This study divided Rattus norvegicus into six groups, each with 11 male rats. All groups except Sham were conditioned as stroke rats. We carried out middle cerebral artery (MCA) occlusion. The stroke condition was created using the MCAO model by locking the internal carotid artery with a bulldog clamp for 180 minutes. Due to its supply of a significant portion of the basal ganglia, internal capsule, and lateral brain surface, the MCA is the most often affected in stroke cases.19 Various studies have shown that other cells besides neurons have a role in the pathophysiology of ischemia. A functional “neurovascular unit” is also formed by combining the vascular, glial, and neuronal components.
We observed NOD3 expression in microglia cells, which play an essential role in pyroptosis, which leads to ischemic stroke.20,21 As a result, P0 demonstrated the highest mean expression of NOD3 in comparison to the other groups, while P3 had the smallest mean, followed by P4.
We also analyze the data using a descriptive test. Figure 2 shows the data on the mean total score of NOD3 marker expression in six groups of mice. The graph presents that the intervention of EGCG and green tea extract can reduce the NOD3 expression in microglia cells. Decreased expression of NOD3 indicates the inhibition of mTOR by EGCG. Xie et al. (2014) reported that inhibition of mTOR signalling modulates macrophages/microglia after focal ischemia. Inhibition of mTOR through the administration of rapamycin significantly reduced IL-1β expression in microglia, inhibiting the pyroptosis process, which plays an important role in ischemic stroke.22
In this study, the independent sample T-Test result in the expression of NOD3 in the P0-P2 groups showed a significant difference with Sham with a significance value of 0.001. However, P3 and P4 did not show a significant difference to Sham. These results indicate a significant repair of microglia cells in each treatment group until it is even close to normal in the P3 and P4 groups, as evidenced by the significance value of P3 and P4 with a sham> 0.005. P3 had the biggest significance value compared to Sham, meaning that P3 is the best treatment, followed by P4 because the IHC score is closest to the normal group.
Independent sample T-Test results on the expression of NOD3 in the P1, P2, P3, and P4 group showed a significant difference with P0 with a significance value of 0.001. This implies that every dosage of EGCG and tea extract exhibited a noteworthy impact compared to the control group. These findings align with Zhou’s (2014) previous studies, which revealed the downregulation of NOD3 protein after being induced by mTOR inhibition.10
The Pearson correlation test showed that the correlation was significant with a negative correlation. The negative correlation indicates that taking more green tea active ingredient EGCG or green tea extract will decrease the NOD3 expression markers in the brain of the Rattus norvegicus MCAO model.
Ischemic stroke is generally caused by thromboembolism, where the injured and dead neurovascular unit releases HMGB1 and Phospholipase A2 (PLA2), which are variations of DAMPs, HMGB1 binds to TLR receptors (TLR2, TLR4, and RAGE) in microglial cells inducing further upregulation of NLRP3.5 NLRP3 is recognized as a key mediator of the innate immune response to danger signals and increases inflammation. It induced IL-1B and IL18 activation, leading to pyroptosis. Pyroptosis plays a crucial role in ischemic stroke pathogenesis.23,24
A recent study by Perez-Alvarez et al. shows that mTOR plays a role in brain ischemia6, Li et al. explain that mTOR regulates NLRP3 activation via ROS in murine lupus7. Recently, it was discovered that NOD3 is an inhibitor of NLRP3. NOD3 competes with the ASC adapter to bind to the CARD domain (Caspase Recruitment Domain), which has previously bound to pro-caspase 1 so that pro-caspase 1 cannot be activated to caspase 1 and the pyroptosis process is inhibited.8 Moreover, research conducted by Hawley et al. revealed that NOD3 acts as a nutrient sensor for the mTOR signaling pathway.25
Green tea contains the active ingredient EGCG, which acts as an antioxidant. EGCG has been shown to inhibit mTOR in the NF-kB pathway. Research on the effect of EGCG on PI3K/mTOR signaling and protein translation inhibition shows that inhibition of PI3K/mTOR can be accomplished at EGCG doses ranging from 320-380 nM EGCG.23,26
Using the NOD3 marker is one of the strong points in this research since it is one of the NLR families that has not been studied much before compared to other NLR families. Our research can also be applied to humans because it uses markers found in the brain where MCAO occurs. Another strong point is the design of this research. It is true experimental research, so the researcher can control the confusing factors affecting the research result.
Apart from strong points, this research also has some limitations. We performed IHC as our method, which is a semi-quantitative method, so it is less valid when compared to quantitative methods. The marker used is only a NOD3 marker, so the neuroprotective effect of green tea (EGCG) on other markers and pathways is unknown. And also, the cells studied were only on microglia cells, so green tea’s neuroprotective effect on other cells in the brain was unknown.
Conclusion
The intervention of EGCG and green tea extract results in a downregulation of NOD3 expression. There was a noticeable change in NOD3 expression after the 10 mg/kg Body Weight treatment. EGCG (the active component of green tea) and NOD3, are correlated. We can conclude that Green tea, which contains the active ingredient EGCG, could be used to treat ischemic stroke.
Acknowledgement
This work was supported by all the staff of the Faculty of Medicine, Airlangga University – Dr. Soetomo General Academic Hospital.
Conflict of Interest
All authors in this article declared no potential conflict of interest.
Funding Source
No funding
References
- Banerjee S (ed.). Practical Approach to Peripheral Arterial Chronic Total Occlusions. 1st ed. 2017. Springer Singapore : Imprint: Springer: Singapore, 2017.
CrossRef - Ali DKA. Quality of Life of Patients with Ischemic Stroke versus Hemorrhagic Stroke: Comparative Study. Rese Jour of Pharm and Technol. 2018;11(11):4911.
CrossRef - Jeon S-B, Koh Y, Choi HA, Lee K. Critical Care for Patients with Massive Ischemic Stroke. J Stroke. 2014;16(3):146.
CrossRef - Willey JZ. Acute Ischemic Stroke. In: Lee K (ed). The NeuroICU Book, 2e. McGraw-Hill Education: New York, NY, 2017.
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. Journal of Leukocyte Biology. 2007;81(1):1–5.
CrossRef - Perez-Alvarez MJ, Villa Gonzalez M, Benito-Cuesta I, Wandosell FG. Role of mTORC1 Controlling Proteostasis after Brain Ischemia. Front Neurosci. 2018;12:60.
CrossRef - Li X, Zhang X, Pan Y et al. mTOR regulates NLRP3 inflammasome activation via reactive oxygen species in murine lupus. ABBS. 2018;50(9):888–896.
CrossRef - Eren E, Berber M, Özören N. NLRC3 protein inhibits inflammation by disrupting NALP3 inflammasome assembly via competition with the adaptor protein ASC for pro-caspase-1 binding. Journal of Biological Chemistry. 2017;292(30):12691–12701.
CrossRef - Karki R, Man SM, Malireddi RKS et al. NLRC3 is an inhibitory sensor of PI3K–mTOR pathways in cancer. Nature. 2016;540(7634):583–587.
CrossRef - Zhou JY. Characterization of NLRC3 and its Mechanism of Action in Regulating T cell Function and Activation. TSpace: University of Toronto, Toronto. 2014.
- Navarro-Yepes J, Zavala-Flores L, Anandhan A et al. Antioxidant gene therapy against neuronal cell death. Pharmacology & Therapeutics. 2014;142(2):206–230.
CrossRef - Assunção M, Santos-Marques MJ, Carvalho F, Andrade JP. Green tea averts age-dependent decline of hippocampal signaling systems related to antioxidant defenses and survival. Free Radical Biology and Medicine. 2010;48(6):831–838.
CrossRef - Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutrition Research. 2012;32(6):421–427.
CrossRef - Sosa PM, de Souza MA, Mello-Carpes PB. Green Tea and Red Tea from Camellia sinensis Partially Prevented the Motor Deficits and Striatal Oxidative Damage Induced by Hemorrhagic Stroke in Rats. Neural Plasticity. 2018;2018:1–8.
CrossRef - Harakeh S, Diab-Assaf M, Azar R et al. Epigallocatechin-3-gallate Inhibits Tax-dependent Activation of Nuclear Factor Kappa B and of Matrix Metalloproteinase 9 in Human T-cell Lymphotropic Virus-1 Positive Leukemia Cells. Asian Pacific Journal of Cancer Prevention. 2014;15(3):1219–1225.
CrossRef - Machin A, Purwanto DA, Sugianto P et al. Camellia sinensis with its active compound EGCG can decrease necroptosis via inhibition of HO-1 expression. EurAsian Journal of BioSciences. 2020; 14(1):1813-1820.
- Jafarzadeh M, Mousavizadeh K, Joghataei MT, Bahremani MH, Safa M, Asghari SM. A Fibroblast Growth Factor Antagonist Peptide Inhibits Breast Cancer in BALB/c Mice. Open Life Sciences. 2018;13(1):348–354.
CrossRef - Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155–168.
- Hui W, Wu C, Zhao W et al. Efficacy and Safety of Recanalization Therapy for Acute Ischemic Stroke With Large Vessel Occlusion: A Systematic Review. Stroke. 2020;51(7):2026–2035.
CrossRef - McKenzie BA, Mamik MK, Saito LB et al. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc Natl Acad Sci USA. 2018;115(26).
CrossRef - Barrington J, Lemarchand E, Allan SM. A brain in flame; do inflammasomes and pyroptosis influence stroke pathology?: Recent advances in inflammasome research. Brain Pathology. 2017;27(2):205–212.
CrossRef - Xie L, Sun F, Wang J et al. mTOR Signaling Inhibition Modulates Macrophage/Microglia-Mediated Neuroinflammation and Secondary Injury via Regulatory T Cells after Focal Ischemia. The Journal of Immunology. 2014;192(12):6009–6019.
CrossRef - Syed DN, Afaq F, Kweon M-H et al. Green tea polyphenol EGCG suppresses cigarette smoke condensate-induced NF-κB activation in normal human bronchial epithelial cells. Oncogene. 2007;26(5):673–682.
CrossRef - Kesavardhana S, Kanneganti T-D. Mechanisms governing inflammasome activation, assembly and pyroptosis induction. International Immunology. 2017;29(5):201–210.
CrossRef - Hawley SA, Boudeau J, Reid JL et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade,. J Biol. 2003;2(4):28.
CrossRef - Van Aller GS, Carson JD, Tang W et al. Epigallocatechin gallate (EGCG), a major component of green tea, is a dual phosphoinositide-3-kinase/mTOR inhibitor. Biochemical and Biophysical Research Communications. 2011;406(2):194–199.
CrossRef







