Manuscript accepted on :28-08-2023
Published online on: 18-12-2023
Plagiarism Check: Yes
Reviewed by: Dr. Tushar Sonawane and Dr. Amit Gupta
Second Review by: Dr. Doaa Khaled Ibrahim Mohamed
Final Approval by: Dr. Anton R Kiselev
Thukaa Z. Abdul-Jalil* , Ruaa Mohammed Ibrahim
, Ruaa Mohammed Ibrahim and Zahraa Suhail Nassir
and Zahraa Suhail Nassir
Department of Pharmacognosy, College of Pharmacy, University of Baghdad, Baghdad, Iraq.
Corresponding Author E-mail: thukaazuhaira@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2807
Abstract
Celery and coriander are vastly applied in modern medicine and traditionally because various medicinal and nutritional benefits depend on their medicinal characteristics. The study aimed to detect, isolate and compare extracts contents of phenolic acids (caffeic and p-coumaric acids) in ethyl acetate fraction of fresh and dry aerial parts of coriander (Coriandrum sativum L.) and celery (Apium graveolens L.) of the Apiaceae family. The extraction of these constituents was carried out by maceration method using 70% ethanol and fractionation was done by using petroleum ether, and ethyl acetate. The existence of caffeic and p-coumaric acids in aerial part extracts of two plants was identified by thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC). After qualitative determination of these active constituents, high-performance liquid chromatography analysis was also applied for quantification of caffeic and p-coumaric acid and the results showed that ethyl acetate extract of dry coriander showed higher contents of caffeic and p-coumaric acid than ethyl acetate extracts of fresh coriander and celery (dry and fresh). Depending on the results of TLC and HPLC, two bioactive compounds (caffeic acid and p-coumaric acid) were isolated and identified by Fourier transform infrared spectroscopy, proton nuclear magnetic resonance spectroscopy, and full scan product ion mode liquid chromatography mass spectrometry. This result opens the minds to the dry plant era and leads to counter fiction of what was known that fresh plant gives better results than the dry one.
Keywords
Apiaceae; Caffeic acid; Celery; Coriander; P-coumaric acid
Download this article as:| Copy the following to cite this article: Jalil T. Z. A, Ibrahim R. M, Nassir Z. S. Extraction, Isolation and Structure Elucidation of Two Phenolic Acids from Aerial Parts of Celery and Coriander. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Jalil T. Z. A, Ibrahim R. M, Nassir Z. S. Extraction, Isolation and Structure Elucidation of Two Phenolic Acids from Aerial Parts of Celery and Coriander. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3NF4c6R |
Introduction
Several therapeutic compounds have been detected in medicinal herbs today. These herbs are recorded in pharmacopeia throughout the world and play an important role in conventional medicine1. Coriandrum sativum L. (known as coriander) is a seasoning plant of the Apiaceae family, with origins in Mediterranean countries. This plant is grown commercially in Asia, Africa, and Europe2. All parts of the plant are edible while the common parts utilized in cooking are the dried seeds and the fresh leaf 3. Coriander (see Figure 1) has nutritional components such as protein, water, fiber, fat, ash, sugar, essential oils, and minerals4. The main active ingredients of coriander are fatty oils and essential oils5. It also contains coumarins, phthalides, phenolic acids (chlorogenic and caffeic acids), and flavonoids (quercetin and rutin)6,7. Oleic, linoleic, palmitic, ascorbic, and stearic acids are also present in the coriander plant and are very effective in lowering the blood cholesterol level8. Coriander is used in Indian folk medicine for the treatment of urinary, digestive, and respiratory disease; it also has diuretic, diaphoretic, stimulant, and carminative activity3. This plant exhibits a strong antioxidant effect because it is an excellent source of phytochemicals and polyphenols2. The coriander leaf contains higher antioxidant concentrations than its seeds5. It also possesses other pharmacological properties such as anti-mutagenic 9, antidiabetic 10, anti-spasmodic 11, antilipidemic effects 12, and others.
Apium graveolens L. (known as celery) is an annual plant of the Apiaceae family, native to outlying hills in Afghanistan, Punjab, and the North Western Himalayas13. The leaves, roots, and stems are vastly utilized in cooking such as salads, side dishes, and soups. Celery contains furanocoumarin, polyphenols, tannins, steroids, terpenoids, saponins, acids (glutamine, ascorbic, oxalic), vitamins B2, B1, A, PP, B9, B6, K, E, and mineral salts (14). It also contains essential oils as the main active constituents14. It is applied in traditional medicine to the bladder, kidney diseases, gout, gastric ulcer, gastritis, duodenal gout, obesity, diabetes, dermatitis, nephritis, rheumatism, and prostate inflammation14. The celery plant has different activities such as antidepressant, antimicrobial, antidiabetic13, hepatoprotective, estrogenic, antioxidant, antiestrogenic, cytotoxic15-17, anti-inflammatory and analgesic effects18,19.
 |
Figure 1: Photo of Iraqi Coriandrum sativum(A) and Iraqi Apium graveolens(B). |
Materials and Methods
Apparatus and Instruments
The following were used, Rotatory evaporator type Buchi attached to a vacuum pump Buchi-Germany), oven (Memmert-854, Buchi-Germany), and an electrically sensitive balance (Sartorius, Germany) with an Ultraviolet light type DESAGA HEIDELBERG of 254 nm and 366 nm waves lengths type DESAGA-Germany. Fourier transforms infrared spectra (FT-IR) spectra were scanned on Jusco/ Japan FT-IR-4200 at the University of Baghdad, College of Pharmacy, 1H NMR was carried out in al-Albayt University, Al-Mafraq, Jordan (Euro-vector EA 3000A/ Italy), LC/MS was carried out in Iraqi National Center for Drug Control and Research (LCMS-8040 series system, Shimadzu/ Japan) and HPLC analysis was carried out using SYKAM/ Germany at the directorate of the ministry of science and technology/environment and water center.
Chemicals and Reagents
Standard of p-coumaric acid and Caffeic acid were purchased from Biopurfy-China, Silica gel of GF 254nm with a thickness of 0.25 mm MERCK-USA while the 0.75mm from Sanpont-China, absolute both ethanol and methanol, HPLC grade acetonitrile, the ethyl acetate and petroleum ether were purchased from Schar lab S.L.-Spain and Toluene was purchased from the CDH-India while the formic acid was from GCC, U.K.
Plant material
The aerial parts of the plants (Apium graveolens L. and Coriandrum sativum) were collected from Baghdad city, which is located in the center of Iraq, in December 2021. Some aerial parts of each plant were dried and the other aerial parts were used fresh.
Plant collection licenses
We have received ethical approval and permission to collect celery and coriander from the Pharmacognosy Department at Baghdad University, and Iraqi Medical Research Center 1120,2020.
Extraction of phenolic acids
The extraction was performed according to the process with some modifications described by Iswantini et al. 20,21. The extraction was carried out for dry and fresh aerial parts (leaves and stem) of Apium graveolens L. and Coriandrum sativum L., taking (100 gm) of each dry plant and fresh parts and then macerated with 70 % ethanol (1000 ml) at room temperature separately. The extracted crudes were concentrated under reduced pressure at 50 ° C. Then successively fractionated with petroleum ether (250 ml*2) and ethyl acetate (250 ml*2). The ethyl acetate fractions were concentrated separately to obtain four fractions of ethyl acetate for each part of the plant.
Identification of phenolic acids by thin-layer chromatography (TLC)
The preliminary analysis of caffeic acid (C9H8O4) and p-coumaric acid(C9H8O3) in ethyl acetate fractions from fresh and dry aerial parts of Apium graveolens and Coriandrum sativum was achieved by TLC.
The GF254 plate of silica gel (readymade) was used as the stationary phase and toluene: ethyl acetate: formic acid (36:12:5) as developing solvent 21. The detection of developed plates is done first by visualizing under UV light at 254nm, then spraying with a chemical reagent for phenolic compounds such as ferric chloride (0.5%). The value of the retardation factor (Rf value)of the separated spots of caffeic and p-coumaric acids was calculated and matched to that of their standard.
Qualitative and quantitative estimations of phenolic acids by high-performance liquid chromatography (HPLC)
By HPLC (table 1), qualitative analysis of caffeic and p-coumaric acids in ethyl acetate fractions of celery and coriander plants is performed by matching the retention time of caffeic and p-coumaric acids in all samples with those of their standard at identical chromatographic conditions 22:
Table 1: HPLC conditions
|
Mobile phase(isocratic) |
Acetonitrile: Water (1:1) |
|
Column |
Shimadzu LC C18 (250 mm x 4.6 mm, 5 μm particle size) |
|
Column temperature |
ambient |
|
Flow rate |
1ml / min |
|
Injection volume |
20μL |
|
Injection concentration |
1 mg /ml |
|
Detection |
UV detector at λ 265 nm |
|
Sample |
Ethyl acetate fraction |
|
Standards |
Caffeic acid and p-coumaric acid |
In HPLC, quantitative estimations of p-coumaric and caffeic acids in ethyl acetate fractions of celery and coriander plants are made by drawing a calibration chart for which serial dilutions of the standard caffeic and p-coumaric acids were made by methanol (10, 20,30, and 40 ppm).
Isolation and purification of phenolic acids by preparative TLC plates
The isolation of caffeic and p-coumaric acids from the ethyl acetate fraction of the dry coriander aerial part(DC) is achieved by using preparative TLC. TLC plates (silica gel GF254 with thick 0.75mm and 20*20) were used and activated at 120° C before the application of the sample. The ethyl acetate fraction of DC is seen by a capillary tube on the plate in the form of a row and, according to standard processes, allowed the plate to develop in toluene: ethyl acetate: formic acid (36:12:5) as a solvent system. Under UV at 254nm, the separated bands were identified and with a needle marked. The two bands were rubbed off with standards similar to those of the p-coumaric and caffeic acids standards put in a beaker and then adding a sufficient quantity of absolute methanol, the flasks were later shaken on a warm water bath, kept cool in a refrigerator for crystallization about 4-5 hrs. The crystalline constituent was then filtered through double filter paper and dried for a pure constituent 23.
Identification and characterization of the isolated constituents
Fourier transforms infrared (FT-IR) spectroscopy
The FT-IR spectroscopy was performed in Baghdad University/College of Pharmacy/ Department of Pharmacognosy and Medicinal plants for each isolated constituent by using a KBr disc and then the structural assignments correlated for characteristic bands as illustrated in the results.
1H nuclear magnetic resonance spectroscopy (NMR) analysis
The spectra of the NMR were taken by dissolving the sample in dimethyl sulfoxide (DMSO) – d6 and later on running the NMR Spectrometer. All the chemical shifts were reported with tetramethyl silane (TMS) reference at 0 parts per million (ppm). NMR measurement was carried out at Al-Albayt University/Al-Mafraq/Jordan and the Euro-vector EA 3000A NMR spectrometer apparatus (300MHz for 1H-NMR) was used. Chemical shifts are given on a δ (ppm) scale with TMS as the internal standard.
Liquid chromatography/ mass spectroscopy LC/MS
Analytical LC/MS was done by using Shimadzu-LC / MS-8040 series system and coupled to a mass Spectrometer-Shimadzu with an electrospray interphase (ESI). The caffiec and p-coumaric acids isolated from Coriandrum sativum dried aerial parts were performed by C18, pore size 3.5 μm, length 15 cm, id 4.6μm column, the column was maintained at 42C0 temperature, the mobile phase was composed of 0.1% acetic acid and acetonitrile (V/V), the flow rate was 0.8 ml/min and the isocratic mode of elution was used. The volume of the injected standard and sample was 5 μL and Negative ionization mode was applied for structural elucidation 24,25.
Preparation of the isolated compounds
Two milligrams of caffeic acid and 2 mg of p-coumaric acid were dissolved in 2 mL of ethanol separately and after that filtrated using disposable filters of 0.45 μm pore size before utilization of LC/MS for analysis.
MS conditions
The mass analysis was performed by a Shimadzu mass spectrometer with an electrospray interphase (ESI) and then Chem Station was used for the chromatographic data processing. MS data were acquired in both modes the negative and positive ionization at a defined condition that is: nitrogen gas flow rate 3 l/min, heat block temperature 400 C°, desolvation line (DL) temperature 250 C°, drying gas flow rate 15 l/min and mass spectra were recorded using the fill scan mode in the range of 100-800 Daltons 26.
Results and Discussion
Caffeic and p-coumaric acids are curative phenolic acids that are used in nutrition and medicine. However, recent studies have reported their many pharmacological effects for the treatment of various disorders. Apium graveolens (Celery) and Coriandrum sativum(Coriander) are readily available in the supermarket, and they have many phytochemicals that are widely known for their various applications in the medical field and other uses.
The percentage yields (%w/w) of each ethyl acetate fraction for dry and fresh aerial parts of Apium graveolens and Coriandrum sativum are shown in Table 2.
Table 2: The percentages of ethyl acetate fractions of dry and fresh aerial parts of Coriandrum sativum and Apium graveolens.
|
Analyzed fraction |
Percentage yield (%w/w) |
|
Fresh Coriandrum sativum (FC) |
2.6 % |
|
Dry Coriandrum sativum (DC) |
4.7 % |
|
Fresh Apium graveolens (FA) |
2.4 % |
|
Dry Apium graveolens (DA) |
3.5 % |
Identification of phenolic acids by TLC
The identification of caffeic and p-coumaric acids was achieved by TLC in aerial parts of the ethyl acetate extracts of Apium graveolens and Coriandrum sativum as shown in Figure (2).
After observation of the spots under UV at 254 nm and by spraying with ferric chloride (0.5%), caffeic and p-coumaric acids in plant samples are detected by calculating their Rf values as shown in Table (3)
Table 3: The Rf values of separated phenolic acids (caffeic and p-coumaric acids) and their standards in one solvent system in TLC.
|
Rf value |
Standards |
Fresh Coriandrum sativum (FC) |
Dry Coriandrum sativum (DC) |
Fresh Apium graveolens (FA) |
Dry Apium graveolens (DA) |
|
caffeic acid |
0.371 |
0.371 |
0.371 |
0.371 |
0.371 |
|
p-coumaric acid |
0.513 |
0.513 |
0.513 |
0.513 |
0.513 |
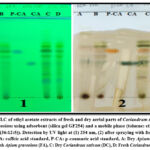 |
Figure 2: TLC of ethyl acetate extracts of fresh and dry aerial parts of Coriandrum sativum and Apium graveolens using adsorbent (silica gel GF254) and a mobile phase (toluene: ethyl acetate: formic acid (36:12:5)). |
Qualitative and quantitative estimations of phenolic acids by HPLC
HPLC was performed to determine the existence and quantity of caffeic and p-coumaric acids in the extracts of Apium graveolens and Coriandrum sativum (fresh and dry aerial parts).
For qualitative estimations, the result revealed that the retention time of p-coumaric and caffeic acids in different ethyl acetate extracts and the retention time of their standards were similar. This proved the presence of p-coumaric and caffeic acids in the samples as shown in Figures (3-8).
 |
Figure 3: HPLC analysis of caffeic acid standard
|
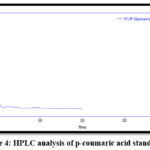 |
Figure 4: HPLC analysis of p-coumaric acid standard
|
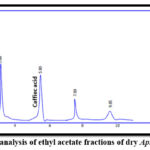 |
Figure 5: HPLC analysis of ethyl acetate fractions of dry Apium graveolens
|
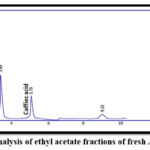 |
Figure 6: HPLC analysis of ethyl acetate fractions of fresh Apium graveolens
|
 |
Figure 7: HPLC analysis of ethyl acetate fractions of dry Coriandrum sativum
|
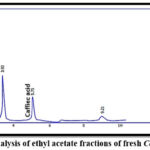 |
Figure 8: HPLC analysis of ethyl acetate fractions of fresh Coriandrum sativum
|
In quantitative estimations of p-coumaric and caffeic acids in fractions analyzed from coriander and celery plants by HPLC, a plot of the area vs. standard concentration for both caffeic and p-coumaric acids show a linear fit. The concentration of these phenolic acids in each fraction was determined by using a straight-line equation as in Figures (9,10).
The coriander and celery plants had shown different concentrations of p-coumaric and caffeic acids, as shown in Table 4.
The results indicated that the concentration of p-coumaric and caffeic acids is higher in the ethyl acetate fraction of the dry coriander aerial part (DC) than their concentrations in other ethyl acetate fractions. Therefore, p-coumaric and caffeic acids are isolated by preparative TLC from the ethyl acetate fraction of DC.
The result revealed that the HPLC method was efficient for the quantitative and qualitative determination of caffeic and p-coumaric acids.
Table 4: Concentration of caffeic and p-coumaric acids in fresh and dry aerial parts of coriander and celery.
|
Ethyl acetate fraction |
The concentration of caffeic acid (ppm) |
Concentration of p-coumaric acid (ppm) |
|
Dry Coriandrum sativum (DC) |
166.911 |
247.615 |
|
Fresh Coriandrum sativum (FC) |
166.883 |
211.357 |
|
Dry Apium graveolens (DA) |
120.878 |
181.256 |
|
Fresh Apium graveolens (FA) |
112.365 |
165.021 |
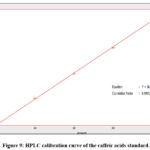 |
Figure 9: HPLC calibration curve of the caffeic acids standard.
|
 |
Figure 10: HPLC calibration curve of the p-coumaric acids standard.
|
Isolation of phenolic acids by preparative TLC plates
The development of the ethyl acetate fraction of the aerial part of dry coriander in toluene: ethyl acetate: formic acid (36:12:5) in preparative TLC plates results in the number of bands that represent several compounds as in Figure 11.
 |
Figure 11: Preparative TLC plates of the ethyl acetate fraction of the dry aerial part of coriander (sample = DC) developed in toluene: |
Identification and characterization of the isolated constituents
The isolated constituents that result from preparative TLC were subjected to different analysis identification techniques to determine their purity of them in addition to other several techniques that were applied to obtain the structural elucidation. These techniques include the following:
FTIR
The IR spectra of isolated caffeic and p-coumaric acids that gave similar results were matched with their standard; which indicated that isolated compounds are p-coumaric and caffeic acids as shown in Figures (12,13).
 |
Figure 12: FTIR spectra of A= isolated caffeic acid and B = standard caffeic acid.
|
 |
Figure 13: FTIR spectra of A= isolated p-coumaric acid and B= p-coumaric acid standard.
|
The isolated compounds’ characteristic FTIR absorption bands with their standards (p-coumaric and caffeic acids) are listed in Tables 5 and 6, respectively27,28.
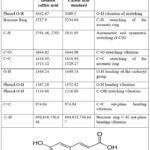 |
Table 5: FTIR absorption bands (cm-1) of the isolated caffeic acid.
|
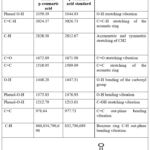 |
Table 6: FTIR (cm-1) absorption bands of isolated p-coumaric acid.
|
Nuclear magnetic resonance (NMR) spectroscopy
Is a sophisticated and powerful analytical technique for the elucidation of organic compound structures and identification of molecular interaction 28.
For that reason, the identification of isolated, p-coumaric, and caffeic acids was further confirmed by NMR spectroscopy application. The isolated constituents’ NMR spectra are in good agreement with works of literature on p-coumaric and caffeic acids as shown in Figures 14,15 and Tables 7 and 8 26,29,30.
 |
Table 7: Assignment of 1H-NMR spectral data of isolated caffeic acid.
|
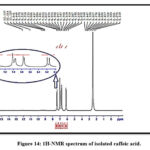 |
Figure 14: 1H-NMR spectrum of isolated caffeic acid.
|
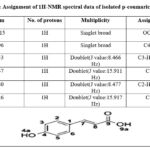 |
Table 8: Assignment of 1H-NMR spectral data of isolated p-coumaric acid.
|
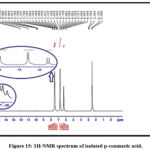 |
Figure 15: 1H-NMR spectrum of isolated p-coumaric acid.
|
LC/MS
The identification of isolated caffeic acid and p-coumaric acids were further confirmed by LC/MS. For structural scanning, full scan product ion was used instead of multiple reaction monitoring (MRM) since it was used for qualitative applications to obtain structural information.
According to the chromatographic conditions, Rt (retention times) of the analyzed constituents are 5.6 min for caffeic acid and 7.8 min for p-coumaric acid; in addition, the ions monitored by the MS method are illustrated in Table 9.
In the ionization conditions, most of the phenolic acids lost a molecule of COO (44 units) so ions detected by spectrometer are always in the form of [M-H-COO] ̅. All these data are in good agreement with the works of literature on p-coumaric and caffeic acids31-33.
Table 9: The specific ion of isolated caffeic acid and p-coumaric acid was monitored in the screening method.
|
Isolated compounds |
Rt (min) |
theoretical molecular weight (M) |
Calculated molecular weight (M) |
Fragmented ions (m/z) |
|
caffeic acid |
5.6 |
180.16 |
180 |
179(M-H), 135(M-H-CO2), 161(M-H-H2O), 134(M-H-HCO3) |
|
p-coumaric acid |
8.7 |
164.158 |
164 |
163(M-H), 119(M-H-CO2),113, and 101 (dissociation of the phenolic ring) |
These assignments were proved by full product ion scan liquid chromatography connected with a negative (-ve) ES ionization spectrum of caffeic acid (Figure 16) and p-coumaric acid (Figure 17).
 |
Figure 16: Full product ion scan LC chromatogram and mass spectrum of isolated caffeic acid. |
 |
Figure 17: Full product ion scan LC chromatogram and mass spectrum of isolated p-coumaric acid. |
Conclusion
Coriander (Coriandrum sativum L.) and celery (Apium graveolens L.) are excellent sources of caffeic and p-coumaric acids (phenolic acids). These compounds are considered a substantial class of natural components that are widely used in several countries as an ingredient in many herbal remedies and preparation. This work shows that the aerial parts extracts of the coriander and celery plant contain caffeic and p-coumaric acids, and quantitative estimation by HPLC shows that the concentrations of caffeic and p-coumaric acids in the dry coriander are higher than in the fresh coriander and celery (dry and fresh). According to the HPLC result, the caffeic and p-coumaric acids were isolated from dry coriander by using the preparative TLC method. This study confirms that coriander showed significantly higher p-coumaric and caffeic acids contents than celery.
Acknowledgement
None
Conflict on Interest
There is no conflict of interest
Funding Source
There are no funding sources.-
References
- Nhut PT, Quyen NT, Truc TT, Minh LV, An TN, Anh NH. Preliminary study on phytochemical, phenolic content, flavonoids, and antioxidant activity of Coriandrum sativum L. originating in Vietnam. InIOP Conference Series: Materials Science and Engineering 2020;991(1):012-022.
- Bhat S, Kaushal P, Kaur M, Sharma K. Coriander (Coriandrum sativum L.): Processing, nutritional and functional aspects. Afr J Plant Sci 2014; 8:5-33.
- Pathak NL, Kasture SB, Bhatt NM. Phytochemical screening of Coriander sativum Linn. Int J Pharm Sci Rev Res 2011; 9:159-163.
- Ahmed EH, Abadi RS, Mohammed AM. Phytochemical screening, chemical composition and antioxidant activity of seeds essential oil of Coriandrum sativum L. from the Sudan. International Journal of Herbal Medicine 2018; 6(1): 01-04.
- Karmakar UK, Rahman MA, Roy DN, Sadhu SK, Ali ME. Chemical and biological investigations of Coriandrum sativum Linn. IJPSR 2011; 2(4): 999-1006.
- Ghani A. Medicinal plants of Bangladesh, 2nd Ed. The Asiatic Society of Bangladesh, Dhaka, 2003; pp 382.
- Rathore SS, Saxena SN, Balraj S. Potential health benefits of major seed spices. Int J Seed Spices 2013; 3:1-12.
- Cortes-Eslava J, Gomez-Arroyo S, Villalobos-Pietrini R. Antimutagenicity of Coriander (Coriandrum sativum) juice on the mutagenesis produced by plant metabolites of aromatic amines. J Toxicol Lett 2004; 153:283-292.
- Eidi M, Eidi A, Saeidi A, Molanaei S, Sadeghipour A, Bahar M et al. Effect of Coriander seed (Coriandrum sativum L) ethanol extract on insulin release from pancreatic beta cells in streptozotocin-induced diabetic rats. J Phytother Res 2012; 23:404-406.
- Alison MG, Peter RF. Insulin-releasing and insulin-like activity of the traditional antidiabetic plant Coriander sativum (coriander). British J Nutr1999; 81:203-209.
- Sunil C, Agastian P, Kumarappan C, Ignacimuthu S. In vitro antioxidant, antidiabetic and antilipidemic activities of Symplocos cochinchinensis (Lour.) S. Moore bark. J Food Chem Toxicol 2012; 50:1547-1553.
- Nair SN, Varghese A, Meenu B, Rejimon G, Neeraja E D. Comparative evaluation of Coriandrum sativum Linn. and Apium graveolens for Antimicrobial activity. Research J. Pharm and Tech 2017; 10(2):541-544.
- Sarmanovna TZ. Phytochemical Study of Odorous Celery Root (Apium graveolens L.) Grown in the North Caucasus. Pharmacogn J 2019; 11(3): 527-530.
- Uddin Z, Shad AA, Bakht J, Ullah I, Jan S. In vitro antimicrobial, antioxidant activity, and phytochemical screening of Apium graveolens. Pak J Pharm Sci 2015; 28 (5):1699-1704.
- Popovic M, Kaurinovic B, Triviv S, Mimica-Dukic N, Bursac M. Effect of celery (Apium graeolens) extracts on some biochemical parameters of oxidative stress in mice treated with carbon tetrachloride. Phytother 2006; 20: 531-536.
- Edziri H, Ammar S, Souad L, Mahjoub MA, Aouni M, Mighri Z, Verschaeve L. In vitro evaluation of antimicrobial and antioxidant activities of some Tunisian vegetables. South Afri J Bot 2012; 78: 252-256.
- Shad AA, Shah HU, Bakht J, Choudhary MI, Ullah J. Nutraceutical potential and bioassay of Apium graveolens L. grown in Khyber Pakhtunkhwa-Pakistan. J Med Plant Res 2011; 5: 5160-5166.
- Asgarpanah J and Kazemivash N. Phytochemistry, pharmacology and medicinal properties of Coriandrum sativum L. African Journal of Pharmacy and Pharmacology 2012; 6(31): 2340-2345.
- R.A. Mans D, Grant A, Pinas N. Plant-Based Ethnopharmacological Remedies for Hypertension in Suriname. Herbal Medicine 2017.
- Iswantini D, Ramdhani TH, Darusman LK. In vitro, inhibition of celery (Apium graveolens l.) extract on the activity of xanthine oxidase and determination of its active compound. Indo J Chem 2012, 12 (3): 247-254.
- Medic-Saric M, Jasprica I, Smoicic-Bubalo A, Mornar A. Optimization of chromatographic conditions in thin layer. Croatica Chemica Acta 2004; 77(1-2):361-366.
- Shah Sh M, Abdul-Jalil Th Z. Qualitative and Quantitative Estimation or Chemical Constituents from Leaves and Roots of Iraqi Agave attenuata by GC-MS and RP-HPLC. Iraqi J Pharm Sci 2022; 31(Sppl):75-85.
- Dubber MJ, Kanfer I. High-performance liquid chromatographic determination of selected flavonols in Ginkgo biloba solid oral dosage forms. J Pharm Pharm Sci 2004;7(3):303-309.
- Adams M, Weidenmann M, Tittel G, Bauer R. HPLC-MS trace analysis of atropine in Lycium barbarum berries. Phyto Chem Anal 2006; 17: 279-283.
- MoCan A, Vlase L, Letitia AA, Vodnar D, Bischin C, Dumitrescu R.S., Crisan G. HPLC/MS analysis of Caffeic and chlorogenic acids from three Romanian Veronica species and their antioxidant and antimicrobial properties. Farmacia 2015; 63(6): 890-896.
- Kajdzanoska M, Gjamovski V, Stefova M. HPLC-DAD-ESI-MS Identification of phenolic compounds in cultivated Strawberry from Macedonia. Maced J Chem Chem Eng 2010; 29(2): 181-194.
- Al-Hussaniy HA, Al-Kuraishy HM, Abdulameer AG. The Use of Panax Ginseng to Reduce the Cardiotoxicity of Doxorubicin and Study its Effect on Modulating Oxidative Stress, Inflammatory, and Apoptosis Pathways. Macedonian Journal of Medical Sciences 2022; 10:715-719.
- Viswannth A, Rao L and Babu P. Simple isolation and characterization of p-coumaric acid from Cynodon dectylon Linn (pers). IJPAR 2017; 6(2): 314-318.
- Wong KC. Review of NMR spectroscopy: basic principle, concepts and application in chemistry. J Chem Educ 2014; 91: 1103-1104.
- Lopez-Martinez LM, Ortega HS, Navarro RE, Mundo RR, Aguilar GC. A 1HNMR investigation of the interaction between phenolic acids found in Mango (Manguifera indica VC Ataulfo) and Papaya (Carica papaya CV Maradol) and 1,1-diphenyl-2,2 picrylhydrazal (DPPH) free radicals. PLOS One 2015; 11: 1-11.
- Swislocka R, Sadowy MK, Kalinowska M, Lewandowski W. Spectroscopic (FTIR, FT-Raman, 1H and 13C NMR and theoretical studies) of p-coumaric acid and alkali metal p-coumarates. Spectroscopy 2012; 27: 35-48.
- Hossain MB, Rai DK, Brunton NP, Martin-Diana AB, Barry-Ryan C. Characterization of phenolic composition in Lamiaceae species by LC-ESI-MS/MS. J Agric Food Chem 2010; 58(19): 10576-10581.
- Wv Z, Ma X, Fang D, Qi H, Ren W, Zhang G. Analysis of caffeic acid derivative from Osmanthus xunnanensis using electrospray ionization quadrupole time-of-flight mass spectrometry. EJMS 2009; 15:415-429.







