Manuscript accepted on :07-04-2023
Published online on: 03-11-2023
Plagiarism Check: Yes
Reviewed by: Dr. Daya Shankar Gautam
Second Review by: Dr. Jagadeesh Kalavakunta
Final Approval by: Dr. Jihan Seid Hussein
Sumit Kumar1 , Adithi K2
, Adithi K2 , Supriya PS3
, Supriya PS3  and Shailaja S Moodithaya4*
and Shailaja S Moodithaya4*
1Department of Physiology, KVG Medical College and Hospital (Affiliated to RGUHS), Sullia, Karnataka, India.
2Department of General Medicine, K.S Hegde Medical Academy, Nitte (Deemed to be University), Deralakatte, Mangalore, Karnataka, India.
3Department of General Medicine, Father Muller's Medical College. Mangalore, Karnataka, India.
4Department of Physiology, K.S Hegde Medical Academy, Nitte (Deemed to be University), Deralakatte, Mangalore, Karnataka, India.
Corresponding Author E-mail: Shailaja.moodithaya@nitte.edu.in
DOI : https://dx.doi.org/10.13005/bpj/2816
Abstract
A plethora of research showed that vitamin D deficiency is one of the most prevalent dietary inadequacies in India. The condition is addressed as hypovitaminosis-D and often intervenes with the occurrence of diseases like cardio-metabolic disease. Though several epidemiological studies have supported this fact, there is an insufficiency of rational data to support the fact. Hence the current study investigates the relationship between vitamin D levels and markers of cardio-metabolic diseases among middle-aged adults. This study recruited 100 healthy middle-aged adults, and the participants underwent evaluation of their anthropometric measurements, Heart Rate Variability (HRV), and serum vitamin D levels. Analysis was done by taking consideration of total power (TP), absolute, and normalized power of high-frequency (HF), and low-frequency (LF) power spectrum including their ratio (LF/HF) as HRV indices. Data analysis was performed using the Pearson correlation test. Data analysis showed a negative correlation between vitamin D and anthropometric measurements and a positive correlation with cardiac sympathovagal balance as determined by waist circumference and LF/HF ratio. However, there was no discernible correlation between vitamin D levels and the BMI, fat percentage, Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), absolute power spectrum of HRV. The results of this study conclude that serum vitamin D levels affect central obesity and cardiac sympathovagal balance, indicating low levels of serum vitamin D might contribute higher risk for cardiovascular diseases.
Keywords
Fat percentage; Heart rate variability; Vitamin D; Waist-hip ratio
Download this article as:| Copy the following to cite this article: Kumar S, Adithi K, Supriya P. S. Moodithaya S. S. Cardio-Metabolic Indices in Relation to Serum Vitamin D Levels among Middle-Aged Adults. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Kumar S, Adithi K, Supriya P. S. Moodithaya S. S. Cardio-Metabolic Indices in Relation to Serum Vitamin D Levels among Middle-Aged Adults. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3FJzBkn |
Introduction
Vitamin deficiencies are an important health concern because they contribute to the pathogenesis, development, mortality, and morbidity loads of many chronic diseases, including cardiovascular diseases (CVD); it is a significant public health issue. Globally, CVDs account for more than 40% of all deaths, making them one of the main causes of illness and death1. Vitamin D (25(OH)D), acts as a steroid hormone2. 25(OH)D has a role in several human organ developments, including calcium homeostasis, bone formation, cardiovascular control, and muscular and brain activity among others3. Its deficiency is an autonomous risk feature for cardiovascular disease development4,5. One of the multifactorial properties of vitamin D is to regulate blood pressure by suppressing renin synthesis and thereby renin-angiotensin system (RAS), and it outperforms in anti-inflammatory, anti-hypertrophic, anti-thrombotic, and anti-diabetic properties. It also regulates the traditional cardiovascular risk elements. These elements regulate the progress of diseases including hypertension, metabolic disease, and malignancy, infectious and autoimmune illnesses, which are the main reasons for disease and death in advanced countries6,7.
The dilemma about 25(OH)D deficiency among different communities persists due to unavailable resources from different races. According to Holick et al., across all civilizations and age categories, more than 80% of the world’s population has a lower serum concentration of 25(OH)D8. However, a number of research9-11 reported a greater incidence rate of serum 25(OH)D insufficiency in the global populace. Epidemiological studies have stated the connection between a low concentration of 25(OH)D and various disease states. According to the prospective studies12–15, low 25(OH)D can accentuate the severity of cardio-metabolic risk factors16,17.
By keeping global population risk in mind, the risk of CVD can be deaccelerated by implementing a treatment to overcome vitamin D deficiency18. Though middle-aged adults are more prone to CVD as well as hypovitaminosis D, the accuracy of data that could establish the relation between 25(OH)D deficiency and cardio-metabolic risk elements is limited. Therefore, the current study attempted to investigate the relationship between serum vitamin D concentration and cardio-metabolic risk elements in the middle-aged populace.
Materials and Methods
It was observational research. A total of 100 subjects both male and female, between the age of 45 and 65, were enrolled in the study. Study subjects were selected from the patients undergoing routine checkups in the Medicine Department at the tertiary care Hospital, Deralakatte, Mangalore, Karnataka, India.
Volunteers were chosen based on the inclusion criterion of age group 45 to 65 years and volunteers with vitamin D levels (30-100 ng/ml). Patients with hypertension, Diabetic Mellitus, Chronic Kidney disease, Liver Cirrhosis, acute inflammatory-infectious illness, and patients who were on vitamin D supplements were not considered for the study.
The Institutional Ethical Committee approved the study after scrutinizing it in detail. A comprehensive medical history was taken from all subjects. BMI, fat percentage, and waist-hip ratio were calculated by using standard techniques19. The following indices, including HRV, mean blood pressure, pulse pressure, and Resting Heart Rate (RHR), were used to assess cardiovascular risk factors.
To assess HRV components, like TP, HF, LF, and Very Low Frequency (VLF) characteristics, Lead II ECG data with a sampling frequency of 1000Hz was collected for 5 minutes while the participant remained supine. The amount of HF power spectrum (0.15 to 0.4 Hz) was used to determine whether there had been any cardio-vagal changes. The LF power spectrum, which mainly reflects the cardio-sympathetic nervous system activity, was in the range of 0.04 to 0.15 Hz. Additionally, the LF/HF ratio depicts the sympathovagal balance20.
2 ml of Venous blood was drawn with all precautions from the median cubital vein for Vitamin D assessment. The sample was centrifuged to obtain serum and then vitamin D concentrations were analysed using an ELISA kit (Krishgen Biosystems, India) as per instructions given by the manufacturer.
Statistical Evaluation
The statistical data analysis was done using IBM SPSS software version 22.0. The link between vitamin D levels and markers of cardiometabolic risk was evaluated using Pearson’s correlation test. Statistics considered P values under 0.05 to be significant.
Results and Discussion
Table 1 displays descriptive statistics (Mean and SD) of the study population. The mean age of the study population is 52.36±5.83 years. The mean Vitamin D level of the study population is 28.58±11.47 ng/dl. The cardiovascular parameters included are blood pressure, resting heart rate and indices of heart rate variability. Body mass index, waist circumferences and percent fat considered as metabolic parameters in this study.
Table 2 indicates the inference statistics of the study results. Analysis shows a significant inverse relationship between vitamin D and cardiometabolic indices such as LF/HF ratio the measure of sympathovagal balance (p=0.039), RHR (p=0.06), and WC (p=0.000). However, the HRV indices namely TP, HF and LF in absolute units were not statistically significant associated with the level of vitamin D.
Vitamin D has roles in pathogenesis, progression, and consequences on mortality and morbidity of many chronic diseases, including cardiovascular disorders, which are significant public health issues. The current investigation’s objective was to ascertain if a middle-aged person’s low serum vitamin D levels were related to indicators of cardio-metabolic risk variables.
The findings of the study showed that waist circumference, an indicator of central obesity was significantly negatively correlated with serum Vitamin D levels. Also, a negative association was found between LF/HF indices of HRV, a surrogate of sympathovagal balance, and serum Vitamin D levels. However, no relationship was found between serum 25(OH)D insufficiency and SBP, DBP, and BMI among middle-aged adults. Further, the study’s findings also revealed that there was no conclusive association of absolute power of HF and LF indices of HRV with concentrations of serum 25(OH)D.
Analysis of the data revealed that statistically significant increase in waist circumference in decreased vitamin D concentrations indicating a greater proportion of abdominal fat (fig.1). According to previous research, greater abdominal fat storage is a risk element for CVD21. Abdominal obesity is linked to a slew of negative health consequences22. Increased waist circumference, a marker of abdominal fat, was found to be significantly predicted by low 25(OH)D status. This may imply that 25(OH)D insufficiency and the risk of cardio-metabolic disease are associated with obesity 23. Additionally, abdominal fat has been linked to higher cardiovascular disease risks, including high insulin levels, high blood pressure and heart disease24, which could impact circulating concentrations of 25(OH)D.
Receptors of Vitamin D present in adipose tissue are responsible for the synthesis of 1,25-dihydroxy vitamin D to an active form of vitamin D3. It suggests that vitamin D may be entangled in the control of adipose tissue25. Also, according to previous research 25(OH)D may have anti-obesity benefits by regulating the gene expression of adipocyte differentiation, lipolysis, and lipogenesis26. Other elements, including enhanced parathormone concentrations, have been connected to both decreased vitamin D concentration and a higher risk of obesity. . Though there are studies indicating obesity and vitamin D association27,28 there is lack of studies on association of vitamin D and cadiometabolic disease especially in middle aged population therefore, findings of this study would be additional contribution to the field of metabolic and cardiac diseases.
A significant inverse relationship between HRV parameters and vitamin D levels shows that low concentrations of 25(OH)D resulted in a decrease in variability of heart rate indicating altered autonomic cardiac autonomic modulation. (Table 2). A significant negative association of LF/HF ratio with vitamin D showing sympathetic dominance is significantly linked with decreased concentrations of serum 25(OH)D. In current research, the association of LF/HF ratio with low 25(OH)D levels indicates that serum insufficiency can alter cardiovascular risk. Studies have consistently shown that LF/HF ratio is a surrogate of cardiac sympathovagal balance. Further, relation of sympathetic dominance with cardiovascular morbidity is well documented. Therefore in this study we have included LF/HF ratio as one of the indices for diseases of cardiovascular risks. Studies have consistently shown that LF/HF ratio is a surrogate of cardiac sympathovagal balance29,30. Further, relation of sympathetic dominance with cardiovascular morbidity is well documented. Therefore, in this study we have included LF/HF ratio as one of the indices of cardiovascular risks.
LF/HF ratio, which is negatively correlated with vitamin D levels, cardiac autonomic dysfunction, and low blood 25(OH)D levels are coupled, which could start the pathophysiological process that raises cardiovascular disease risk in people with 25(OH)D insufficiency. Hence, this study concludes that the greater cardiac sympathovagal balance seen in subjects with low serum 25(OH)D concentrations could be attributed to cardio metabolic disorders.
Table 1 presents the population’s descriptive data. (N=100).
|
Parameters |
Mean ± SD |
|
Age (years) |
52.36 ± 5.83 |
|
BMI (kg/m2) |
24.96 ± 3.58 |
|
Waist Circumference (Cm) |
85.07 ± 11.305 |
|
Fat (%) |
28.25 ± 8.42 |
|
RHR (bpm) |
80.84 ± 3.96 |
|
SBP (mmHg) |
140.88 ± 10.2 |
|
DBP (mmHg) |
81.90 ± 6.46 |
|
TP (ms2) |
9218 ± 1966 |
|
LF (ab) (ms2) |
2187 ± 6405 |
|
HF (ab) (ms2) |
1551.6 ± 3382.4 |
|
LF/HF |
1.98 ± 1.95 |
|
Vitamin D (ng/ml) |
28.58 ± 11.47 |
Data is represented as Mean ± SD.
Abbreviations: Total Power (TP), Low Frequency (LF), High Frequency (HF), and LF/HF: Low Frequency High Frequency Ratio, Resting heart rate (RHR). Systolic blood pressure -SBP, and diastolic blood pressure – DBP.
Table 2: displays the relationship between concentrations of 25(OH)D and cardiometabolic parameters. (N= 100).
|
Cardio-metabolic indices |
Vitamin D Levels |
|
|
R-value |
P value |
|
|
TP (ms2) |
-.091 |
.371 |
|
LF (ab) (ms2) |
-.43 |
.677 |
|
HF (ab) (ms2) |
-.004 |
.972 |
|
LF/HF |
-.209 |
.039* |
|
SBP (mmHg) |
-.002 |
.984 |
|
DBP (mmHg) |
-.111 |
.275 |
|
RHR (bpm) |
-.275 |
0.06** |
|
BMI (kg/m2) |
0.16 |
.878 |
|
Waist Circumference (Cm) |
-.416 |
.000** |
|
Fat (%) |
2.73 |
0.06 |
The correlation test was used to analyse the data.
“r” stands for correlation strength.
** Correlation is significant at the 0.01 level (2-tailed).
* Correlation is significant at the 0.05 level (2-tailed).
Abbreviations: Total Power (TP), Low Frequency (LF), High Frequency (HF), and LF/HF: Low Frequency High Frequency Ratio, Resting heart rate (RHR). Systolic blood pressure – SBP, and diastolic blood pressure – DBP.
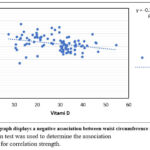 |
Figure 1: The graph displays a negative association between waist circumference and vitamin D. |
Limitations
A better understanding of the association of Vitamin D with cardio-metabolic indices would have been established by estimating the lipid profile in this study population as well as assessing central obesity using sensitive methods.
Conclusion
The findings of this study conclude that lower serum concentrations of 25(OH)D are associated with central obesity as well as cardiac sympathetic dominance, therefore a low level of vitamin D might increase the risk for cardiovascular diseases.
Acknowledgments
We thank the study participants for participation in this study and Nitte (Deemed to be University), for funding this study.
Conflict of Interest
The authors declare no conflict of interest.
Funding Sources
Financial support was received from Nitte (Deemed to be University). Grant number is N/RG/NUFR2/KSHEMA/2020/11
References
- Voutilainen S, Nurmi T, Mursu J, Rissanen TH. Carotenoids and cardiovascular health. Am J Clin Nutr 2006; 83 (6): 1265-71.
CrossRef - Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281.
CrossRef - Ibero-Baraibar, I.; Navas-Carretero, S.; Abete, I.; Martinez, J.A.; Zulet, M.A. Increases in plasma 25 (OH) D levels are related to improvements in body composition and blood pressure in middle-aged subjects after a weight loss intervention: Longitudinal study. Clin. Nutr. 2015, 34, 1010–1017.
CrossRef - Mousa, A.; Naderpoor, N.; de Courten, M.P.; Scragg, R.; de Courten, B. 25-hydroxyvitamin D is associated with adiposity and cardiometabolic risk factors in a predominantly vitamin D-deficient and overweight/obese but otherwise healthy cohort. J. Steroid Biochem. Mol. Biol. 2017, 173, 258–264.
CrossRef - Mozos, I.; Marginean, O. Links between Vitamin D Deficiency and Cardiovascular Diseases. Biomed. Res. Int. 2015, 2015, 109275.
CrossRef - Al Mheid, I.; Patel, R.S.; Tangpricha, V.; Quyyumi, A.A. Vitamin D and cardiovascular disease: Is the evidence solid? Eur. Heart J. 2013, 34, 3691–3698.
CrossRef - Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144, 138–145.
CrossRef - Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S.
CrossRef - Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj, F.G.; Josse, R.G.; Lips, P.; Morales-Torres, J. IOF Committee of Scientific Advisors (CSA) Nutrition Working Group. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820.
CrossRef - Van Schoor, N.M.; Lips, P. Worldwide vitamin D status. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 671–680.
CrossRef - Wahl, D.A.; Cooper, C.; Ebeling, P.R.; Eggersdorfer, M.; Hilger, J.; Hoffmann, K.; Josse, R.; Kanis, J.A.; Mithal, A.; Pierroz, D.D. A global representation of vitamin D status in healthy populations. Arch. Osteoporos. 2012, 7, 155–172.
CrossRef - Chonchol, M.; Scragg, R. 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney Int.2007,71, 134–139.
CrossRef - Barengolts, E. Vitamin D role and use in prediabetes. Endocr. Pract. 2010,16, 476–485.
CrossRef - Pham, T.M.; Ekwaru, J.; Setayeshgar, S.; Veugelers, P. The effect of changing serum 25-hydroxyvitamin D concentrations on the metabolic syndrome: A longitudinal analysis of participants of a preventive health program. Nutrients 2015,7, 7271–7284.
CrossRef - Pham, T.M.; Ekwaru, J.P.; Loehr, S.A.; Veugelers, P.J. The relationship of serum 25-hydroxyvitamin D and insulin resistance among nondiabetic Canadians: A longitudinal analysis of participants of a preventive health program.PLoS ONE 2015,10, e0141081.
CrossRef - Deleskog, A.; Hilding, A.; Brismar, K.; Hamsten, A.; Efendic, S.; Östenson, C.G. Low serum 25-hydroxyvitamin D level predicts progression to type 2 diabetes in individuals with prediabetes but not with normal glucose tolerance. Diabetologia 2012,55, 1668–1678.
CrossRef - Song, Y.; Wang, L.; Pittas, A.G.; Del Gobbo, L.C.; Zhang, C.; Manson, J.E.; Hu, F.B. Blood 25-hydroxyvitamin D levels and incident type 2 diabetes: A meta-analysis of prospective studies.Diabetes Care2013,36,1422–1428.
CrossRef - Qorbani M, Zarei M, Moradi Y, Appannah G, Djalainia S, Pourrostami K, Ejtahed HS, Mahdavi-Gorabi A, Naderali EK, Khazdouz M. Effect of vitamin D supplementation on cardiac-metabolic risk factors in elderly: a systematic review and meta-analysis of clinical trials. Diabetology & Metabolic Syndrome. 2022 Dec;14(1):1-5.
CrossRef - Anthro–Norton, K.; Olds, T.Anthropometrica: A Textbook of Body Measurement for Sports and Health Courses; UNSWpress: Randwick, Australia, 1996.
- Task Force of the European Society of Cardiology and the North American Society of Pacing and electrophysiology, 1996).
- Ko DH, Kim YH, Han JK. Relationship between cardiovascular disease risk factors, health behaviour and physical fitness according to visceral fat in older men. Journal of Men’s Health. 2022 May 27;18(6):128.
CrossRef - Arunabh, S.; Pollack, S.; Yeh, J.; Aloa, J.F. Body fat content and 25-hydroxyvitamin D levels in healthy women. J. Clin. Endocrinol. Metab. 2003, 88, 157–161.
CrossRef - Earthman CP, Beckman LM, Masodkar K, Sibley SD. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. International journal of obesity. 2012 Mar;36(3):387-96.
CrossRef - Sowers, J.R. Obesity as a cardiovascular risk factor. Am. J. Med.2003,115, 37–41.
CrossRef - Szymczak-Pajor I, Miazek K, Selmi A, Balcerczyk A, Śliwińska A. The Action of Vitamin D in Adipose Tissue: Is There the Link between Vitamin D Deficiency and Adipose Tissue-Related Metabolic Disorders? International Journal of Molecular Sciences. 2022 Jan 16;23(2):956.
CrossRef - Borojević A, Jauković A, Kukolj T, Mojsilović S, Obradović H, Trivanović D, Živanović M, Zečević Ž, Simić M, Gobeljić B, Vujić D. Vitamin D3 Stimulates Proliferation Capacity, Expression of Pluripotency Markers, and Osteogenesis of Human Bone Marrow Mesenchymal Stromal/Stem Cells, partly through SIRT1 Signaling. Biomolecules. 2022 Feb 18;12(2):323.
CrossRef - Paschou SA, Kosmopoulos M, Nikas IP, Spartalis M, Kassi E, Goulis DG, Lambrinoudaki I, Siasos G. The impact of obesity on the association between vitamin D deficiency and cardiovascular disease. Nutrients. 2019 Oct 14;11(10):2458.
CrossRef - Vranić, L., Mikolašević, I., & Milić, S. (2019). Vitamin D Deficiency: Consequence or Cause of Obesity? Medicina, 55(9). https://doi.org/ 10.3390/medicina55090541
CrossRef - Tuttolomondo A, Del Cuore A, La Malfa A, Casuccio A, Daidone M, Maida CD, Di Raimondo D, Di Chiara T, Puleo MG, Norrito R, Guercio G. Assessment of heart rate variability (HRV) in subjects with type 2 diabetes mellitus with and without diabetic foot: correlations with endothelial dysfunction indices and markers of adipo-inflammatory dysfunction. Cardiovascular Diabetology. 2021 Dec;20(1):1-2.
CrossRef - Malik M, Camm AJ, Bigger JT, Breithardt G, Cerutti S, Cohen RJ, et al. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996; 17(3):354–81.
CrossRef







