Manuscript accepted on :14-11-2023
Published online on: 25-12-2023
Plagiarism Check: Yes
Reviewed by: Dr. Kartik Salwe and Dr. Malar Selvi
Second Review by: Dr. Raja Azman Raja Awang
Final Approval by: Dr. Patorn Piromchai
Ngakan Ketut Wira Suastika1 * and Ketut Suega2
* and Ketut Suega2
1Department of Internal Medicine, Faculty of Medicine, Udayana University/Udayana University Hospital, Bali, Indonesia,
2Department of Internal Medicine, Faculty of Medicine, Udayana University/Professor Ngoerah Hospital, Bali, Indonesia,
corresponding Author E-mail: wira.suastika@unud.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2809
Abstract
Early identification of patients who may develop into clinical deterioration is necessary to prevent complications and death from COVID-19. This research aims to determine the association between lymphocyte-to-monocyte ratio (LMR) and survival in Coronavirus disease-2019 (COVID-19) patients. This study used a retrospective cohort design. We collected survival data retrospectively by tracing medical records to gather data on demographic, clinical, and laboratory characteristics. Mann-Whitney U test was used to determine the difference in LMR values in the survivor and non-survivor groups. A total of 502 subjects were involved in this study. The LMR value was significantly lower in the non-survivors group compared to the survivors group (p=0.001). We found an adjusted odds ratio (OR) of LMR of 3.62; 95% confidence interval (CI) 1.92-14.25; p=0.046). LMR can reflect the disease severity and can be used to predict prognosis.
Keywords
COVID-19; Lymphocyte; Monocyte; Survival
Download this article as:| Copy the following to cite this article: Suastika N. K. W, Suega K. Association between Lymphocyte-to-Monocyte Ratio and Survival in COVID-19 Infected Patients. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Suastika N. K. W, Suega K. Association between Lymphocyte-to-Monocyte Ratio and Survival in COVID-19 Infected Patients. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/48qslGi |
Introduction
The coronavirus disease 2019 (COVID-19) has caused a pandemic and extraordinary health impacts. Most patients show mild clinical symptoms, but some can experience severe clinical symptoms with severe acute respiratory distress syndrome.1,2. Death often occurs in patients with co-morbidities or the elderly3.
Because the symptoms of COVID-19 can develop into serious complications, it is crucial to identify patients who may experience clinical deterioration at an early stage. Routine examinations such as a complete blood count (CBC) can be a quick and easy examination to predict prognosis4,5. Studies show that the worsening of clinical symptoms in COVID-19 is closely related to immune system dysregulation6,7. Inflammatory biomarkers originating from the peripheral blood, such as neutrophils, lymphocytes, and platelets, have been studied to predict prognosis in ARDS patients8.
The severity of COVID-19 is related to the proportion of circulating immune cells9. Clinical symptoms in COVID-19 are influenced more by the exaggerated inflammatory response than by the direct effects of viral replication. Lymphopenia is the most common hematological change in patients with COVID-1910. In patients with severe clinical symptoms, a decrease in lymphocyte count may be accompanied by an increase in monocyte count11. Lymphocyte-to-monocyte ratio (LMR) can be used as a clinical biomarker to monitor disease progression in COVID-19 infection.
Few studies have reported the role of the LMR as a prognostic marker in COVID-19. This study aims to determine the association between LMR and survival so that LMR can be used to predict prognosis in COVID-19 patients.
Methods
Study design and sample
This study was conducted at Udayana University Hospital from January to April 2023 using a retrospective cohort design. Data collection is through a medical record review of all the variables studied. The sample was patients aged 18 years or older treated at Udayana University Hospital from June 2021 to June 2022. Patients who died unrelated to COVID-19 infection, had hematological diseases (such as aplastic anemia, myelodysplastic syndrome, and leukemia), and pregnancy was excluded from this study.
Data collection
SARS-CoV-2 infection was confirmed through naso-oropharyngeal swab examination and analyzed by polymerase chain reaction (PCR). The lymphocyte-to-monocyte ratio is calculated by comparing the absolute lymphocyte count and absolute monocyte count based on the results of a complete blood count examination. Survival data were collected from patient medical records and followed up retrospectively to determine demographic, clinical, and laboratory results.
Statistical analysis
We used median (interquartile range/IQR) to present numerical variables and frequency (percentage) to present categorical variables. We used the Mann-Whitney U test to determine the difference in LMR values between non-survivors and survivors. We used the Receiver Operating Characteristic (ROC) to determine the cut-off and area under the curve (AUC) of the LMR value in predicting death in COVID-19 patients. We performed multiple logistic regression analyses to determine factors that influence survival, such as age and underlying disease. Statistically significant if p<0.05. SPSS software version 25.0 was used for all statistical analyses.
Results
A total of 502 subjects were involved in this study. 71.5% of patients in the non-survivors group had an underlying disease. There are significant differences in LMR values in the survivors group compared to non-survivors (Table 1).
Table 1: Characteristics of the subject
|
|
Non-survivors (n=14) Median (IQR) |
Survivors (n=488) Median (IQR) |
p-value |
|
Age, years |
61 (44 – 74) |
42 (18 – 84) |
<0.001 |
|
Sex, n (%) |
|
|
|
|
Male |
11 (78.6) |
298 (60.4) |
0.266 |
|
Female |
3 (21.4) |
195 (39.6) |
|
|
Underlying disease |
|
|
|
|
Without underlying disease |
4 (28.6) |
395 (80.1) |
<0.001 |
|
With underlying disease |
10 (71.4) |
98 (19.9) |
|
|
Hemoglobin, gr/dl |
13.1 (11.6 – 15.9) |
13.90 (7.9 – 17.4) |
0.195 |
|
WBC, ×109 cells/L |
9.75 (5.78 -17.25) |
6.70 (2.36 – 15.98) |
0.001 |
|
Neutrophil count |
|
|
|
|
Absolute, ×109 cells/L |
8.11 (4.53 – 15.50) |
3.99 (1.99 – 13.78) |
<0.001 |
|
Relative, % |
84.05 (73.60 – 95.20) |
60.60 (40.20 – 93.50) |
<0.001 |
|
Lymphocyte count |
|
|
|
|
Absolute, ×109 cells/L |
0.70 (0.31 – 1.53) |
1.64 (0.31 – 5.92) |
<0.001 |
|
Relative, % |
10.20 (3.0 – 14.20) |
26.40 (1.29 – 53.60) |
<0.001 |
|
Monocyte count |
|
|
|
|
Absolute, ×109 cells/L |
0.55 (0.05 – 1.15) |
0.57 (0.09 – 1.66) |
0.031 |
|
Relative, % |
6.10 (0.70 – 14.2) |
8.90 (0.32 – 25.00) |
0.011 |
|
Platelet count, ×109 cells/L |
267 (122 – 446) |
235 (54 – 672) |
0.448 |
|
LMR |
1.49 (0.37 – 6.20) |
2.98 (0.44 – 9.66) |
0.001 |
Using ROC analysis, we obtained a cut-off value of LMR less than 2.0 to predict the occurrence of death in hospitalized COVID-19 patients (Graph 1 and Table 2).
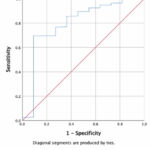 |
Graph 1: ROC analysis of the LMR variable. |
Table 2: Cut-off values, sensitivity, specificity, and AUC of LMR
|
|
Cut-off value |
Sensitivity |
Specificity |
Area under curve |
95% confidence interval |
p-value |
|
LMR |
<2.0 |
76.5% |
72.7% |
0.797 |
0.639 – 0.956 |
0.001 |
By including the variables age and presence of underlying disease in multiple logistic regression analysis, we obtained an adjusted odds ratio (OR) of LMR of 3.62 (95% CI 1.92-14.25; p=0.046) (Table 3).
Table 3: Crude odds ratio and adjusted odds ratio of LMR, age, and underlying disease
|
|
Odds ratio |
95% confidence interval |
P value |
Adjusted odds ratio |
95% confidence interval |
P value |
|
LMR |
8.62 |
2.241 -33.142 |
0.002 |
3.62 |
1.92 – 14.25 |
0.046 |
|
Age |
5.15 |
1.59 – 16.69 |
0.006 |
1.77 |
1.29 – 11.03 |
0.038 |
|
Underlying disease |
7.11 |
2.031 |
0.002 |
5.17 |
1.19 – 22.43 |
0.028 |
LMR:
Discussion
We found a significant association between LMR and survival in COVID-19. This association remained significant after the multivariate analysis included age and underlying disease variables. COVID-19 is a disease with systemic multiorgan disorders caused by SARS-CoV-2. The inflammatory mediators release can increase the activation of the immune system, which causes an inflammatory storm and tissue damage7. Inflammatory biomarkers can predict disease severity and assess therapy’s effectiveness.
SARS-CoV-2 causes a decrease in the number of lymphocytes in infected patients. Lymphocyte apoptosis due to immune-mediated mechanisms or direct viral effects on lymphocytes is the pathogenesis leading to lymphopenia12. Lymphocytes and monocytes are essential in the inflammatory cascade of COVID-19 infection. Studies show that in COVID-19 infection, the lymphocyte ratio is a better predictor for assessing the severity of infection than the total leukocyte count13.
Monocytes can be an excellent marker to assess the severity of infection in COVID-19 patients. Studies show that monocyte counts are higher in COVID-19 patients compared to flu patients14. Monocytes are essential in phagocytosis, antigen presentation, and inflammatory response15. The study by Zhou et al. in severe COVID-19 shows a significant increase in monocytes that produce interleukin-6 in the peripheral blood, indicating that monocytes have a significant role in the occurrence of cytokine storms16. Lower LMR values are associated with severe clinical symptoms and the need for mechanical ventilation in COVID-19 patients11,16.
LMR can be easily calculated from a complete blood count, provides fast results, is cheap, and is available in all health facilities, including areas with limited resources. Identification of patients at high risk at early onset of the disease is critical so that appropriate and aggressive therapy can be given to prevent complications and death.
This study is a retrospective cohort with a relatively large sample size. The limitation of this study is that it did not record the pattern of changes in LMR values and was a single-center study.
Conclusions
Lymphocyte-to-monocyte ratio is significantly lower in non-survivors. LMR can reflect the disease severity and will assist clinicians in identifying patients at risk for complications and death. More studies are needed to confirm these findings.
Acknowledgments
The author would like to thank the Chairperson of the Research and Community Service Institute of Udayana University and all those who have assisted in carrying out this study.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Funding Source
This research was funded by Udayana University PNBP Grants, Fiscal Year 2023 (Grant number: B/1.175/UN14.4.A/PT.01.03/2023)
References
- Guan W.J, Ni Z.Y, Hu Y, Liang W.H, Ou C.Q, He J.X, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med., 2020; 382(18): 1708-1720.
CrossRef - Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet., 2020; 395(10223): 497-506.
CrossRef - Rothan H.A and Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun., 2020; 109: 102433.
CrossRef - Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci., 2020; 63: 364-374.
CrossRef - Peng J, Qi D, Yuan G, Deng X, Mei Y, Feng L, et al. Diagnostic value of peripheral hematologic markers for coronavirus disease 2019 (COVID‐19): a multicenter, cross‐sectional study. J. Clin. Lab. Anal., 2020; 34(10): e23475.
CrossRef - Udugama B, Kadhiresan P, Kozlowski H.N, Malekjahani A, Osborne M, Li V.Y, et al. Diagnosing COVID-19: the disease and tools for detection. ACS nano., 2020; 14(4): 3822-3835.
CrossRef - Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis., 2020; 71(15): 762-768.
CrossRef - Wang Y, Ju M, Chen C, Yang D, Hou D, Tang X, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in acute respiratory distress syndrome patients: a retrospective study. J. Thorac. Dis., 2018; 10(1): 273.
CrossRef - Ding X, Yu Y, Lu B, Huo J, Chen M, Kang Y, et al. Dynamic profile and clinical implications of hematological parameters in hospitalized patients with coronavirus disease 2019. Clin. Chem. Lab. Med., 2020; 58(8): 1365-1371.
CrossRef - Erdogan A, Can F.E and Gönüllü H. Evaluation of the prognostic role of NLR, LMR, PLR, and LCR ratio in COVID‐19 patients. J. Med. Virol., 2021; 93(9): 5555-5559.
CrossRef - Lissoni P, Rovelli F, Monzon A, Privitera C, Messina G, Porro G.J, et al. Evidence of abnormally low lymphocyte-to-monocyte ratio in COVID-19-induced severe acute respiratory syndrome. J. Immuno. Allerg., 2020; 1(2): 1-6.
CrossRef - Liu Y, Du X, Chen J, Jin Y, Peng L, Wang H.H, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect., 2020; 81(1): e6-12.
CrossRef - Kalabin A, Mani V.R and Valdivieso S.C, Donaldson B. Role of neutrophil-to-lymphocyte, lymphocyte-to-monocyte and platelet-to-lymphocyte ratios as predictors of disease severity in COVID-19 patients. Infez. Med., 2021; 29(1): 46-53.
- Curtolo A, Oliva A, Volpicelli L, Ceccarelli G, D’Ettorre G, Borrazzo C, et al. Monocyte absolute count as a preliminary tool to distinguish between SARS-CoV-2 and influenza A/B infections in patients requiring hospitalization. Infez. Med. 2020; 28(4): 534-538
- Jakubzick C.V, Randolph G.J, Henson P.M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol., 2017; 17(6): 349-362.
CrossRef - Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev., 2020; 7(6): 998-1002.
CrossRef







