Manuscript accepted on :20-11-2023
Published online on: 29-12-2023
Plagiarism Check: Yes
Reviewed by: Dr. Hany Akeel and Dr. Neelufar Shama
Second Review by: Dr. Moumita Hazra
Final Approval by: Dr. Jihan Seid Hussein
Farizah Izazi1* , Hardiyono Hardiyono2
, Hardiyono Hardiyono2  and Angelica Kresnamurti2
and Angelica Kresnamurti2
1Department of Biology Pharmacy, Hang Tuah University, Surabaya, Indonesia
2Department of Clinical Pharmacy, Hang Tuah University, Surabaya, Indonesia
Corresponding Author E-mail: farizah.izazi@hangtuah.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2825
Abstract
Background: Sea urchin shells contain pigment compounds, such as PHNQ, which vary based on habitat conditions. These pigments, especially in darker shells, display diverse chemical compounds and increased antioxidant power. Diadema paucispinum is a type of sea urchin found in Sumenep-Madura, Indonesia, which has yet to be extensively studied for its antioxidant potential. Aim: To identify the class of compounds present in the 96% ethanol extract of Diadema paucispinum (EEDP) from Sumenep-Madura, Indonesia, and to evaluate the antioxidant activity of this extract. Methods: The research utilized phytochemical screening for extracts, FTIR analysis of simplicia and extracts, and antioxidant tests with DPPH and ABTS. Results: The study identified the presence of alkaloid, flavonoid, saponin, and tannin compounds in the extract. Antioxidant activity, determined by the IC50 value, was found to be 6084 µg/ml using the DPPH method and 756.3 µg/ml with the ABTS method, while IC50 of Vitamin C was 3,25 ppm with DPPH method and 2,09 ppm for ABTS method. Conclusion: According to the study's findings, Diadema paucispinum extract found in Sumenep-Madura contains alkaloids, flavonoids, saponins, and tannins. The IC50 value of EEDP was more significant than 200 ppm, indicating that 96% EEDP sea urchin did not have antioxidant activity when compared to vitamin C as a standard compound.
Keywords
Antioxidant; ABTS; Diadema paucispinum; DPPH; FTIR analysis; Sea urchin
Download this article as:| Copy the following to cite this article: Izazi F, Hardiyono H, Kresnamurti A. Antioxidant Activity and Phytochemical Profile of Diadema paucispinum from Sumenep-Madura, Indonesia. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Izazi F, Hardiyono H, Kresnamurti A. Antioxidant Activity and Phytochemical Profile of Diadema paucispinum from Sumenep-Madura, Indonesia. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3vjYwZR |
Introduction
Sea urchin, locally known as sea urchin/tehe-tehe, is a type of Echinodermata phylum, Echinoidea class, with a round shape covered with long moving spines 1. Sea urchins act as bioindicators in controlling vegetation growth in the sea because their leading food is seaweed and microalgae 2. Sea urchins are nocturnal foragers, while during the day, they hide in coral crevices 3. Diadema paucispinum is found in the Sumenep-Madura Sea, and is not utilized by fishermen, so that many sea urchin habitats die in the waters around the coast and become marine litter.
Japan is one of the countries that utilize sea urchin as a potential food due to the high protein content in its gonads. The utilization of the shell has yet to be widely studied and is only thrown away, even though the sea urchin shell contains pigment compounds, including PHNQ, which has the potential as a high antioxidant and potential as a cosmetic ingredient 4. These pigment compounds vary in sea urchins. It depends on the habitat conditions in which they live 5. The darker or more colorful the shell pigments, the more diverse the absorption of chemical compounds and the higher the antioxidant power 6,7, so exploring the metabolite content of sea urchin shells based on their habitat is essential. Using sea urchin shells and gonads will increase their antioxidant activity 7.
Sea urchins have a hard shell and contain gonads containing essential amino acids, ß-carotene, and DHA [8;9]. In the analysis of Diadema Sp gonads, 80% polyunsaturated fatty acids (PUFA) were found, such as eicosapentaenoic acid (EPA), and other components like arachidonic acid, carotenoids, and containing antioxidant compounds, such as echinenone, ß-carotene and fucoxanthin 10,11. Nutrients in sea urchin gonads include vitamins A, B9, PUFAs, and flavonoids, predicted suppress chronic inflammation 12. Sea urchin shells contain bioactive compounds known as polyhydroxy naphthoquinone (PHNQ) derivatives such as Spinochrome A, B, C, D, E, Echinocrome, and Echinamine A, B, E. PHNQ in the shells is a pigmen specific compound in sea urchin, proves to have pharmacological potential actions as antioxidants and anti-inflammatory agent 13.
Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) are the most widely recognized free radicals. ROS mediates intracellular damage to lipids, proteins, carbohydrates, and nucleic acids. ROS are highly reactive because they are unstable (have unpaired electrons). Oxidative stress occurs when an imbalance between oxidant and antioxidant molecules increases ROS production, resulting in tissue damage and inflammation. Antioxidant compounds can slow down/prevent the oxidation process that produces free radicals and break down chains that can damage cells and tissues, so antioxidants can be used as a therapeutic option for inflammation, which is generally caused by ROS 14.
Inflammatory processes can arise acutely or chronically, cause local or systemic impacts, and cause pathological abnormalities. The primary treatment for inflammation is to relieve pain and stop tissue damage. The drugs given are steroids and non-steroidal anti-inflammatory drugs (NSAIDs), which have serious side effects, such as an increased risk of gastric ulcers and upper gastrointestinal bleeding 15.
Several studies derived from marine biota on anti-inflammatory agents also have strong antioxidant potential. Sea urchin is a marine biota with anti-inflammatory, antioxidant, and antidiabetic activities 3. The anti-inflammatory activity of methanol extract of the sea urchin Echinometra mathaei showed high anti-inflammatory activity 16. In the 70% ethanol extract of Echinometra mathaei, one type of sea urchin from Weh Island, Sabang, proved to be a more potent anti-inflammatory than diclofenac sodium at a dose of 100 mg/KgBB 17.
Research on Diadema fauscispinum itself is mostly just its morphological, anatomical, and phylogenetic properties. Activity testing and active metabolite content of this species have not been found, primarily based on the closeness of its genus, Diadema setosum. Based on the closeness of the genus, Diadema sp. known has PHNQ content in both the shell and gonads 18. The more researched type of long-spine black sea urchin is Diadema setosum, which has been studied for its gonad extract as a burn agent in rabbits and has the potential for 100% burn healing in 1 to 2-degree burns 19. The potential of Diadema sp as a burn medicine is related to its anti-inflammatory properties. Diadema sp research in Indonesia states that this sea urchin is proven to have antibacterial activity 20,21, reduce IFN, increase IL-10 22, have antioxidant activity 23,24, have flavonoids, steroids, saponins 24, and do not cause toxicity in the BSLT test 25. Diadema research in Egypt and Vietnam found phenolic content, PHNQ, antioxidant activity, and antibacterial properties 4,26:27. In addition, Diadema sp research shows low toxicity26,28. Diadema fauscispinum was choosen in this study because Diadema species is the most found species in Indonesian coral seas. It will be utilized as much as possible for health and beauty. The antioxidant potential of sea urchin is excellent and has potential for development in various treatments; besides, its antioxidant power is related to its potential as an essential ingredient for cosmetic preparations.
Methods and Material
Research Materials
Preparation of research test materials includes drying shells (simplicia) and extraction with 96% ethanol. The ethanol extract of Diadema paucispinum was prepared by maceration using 96% ethanol; the filtrate was collected and evaporated with a rotary vacuum evaporator to obtain a thick extract. The percentage yield of the extract was calculated against the weight of the initial simplicia used. The extract’s phytochemical screening and FTIR analysis identified the secondary metabolite profile.
Chemical and Materials
Aquadest, 96% ethanol, FeCl3, Mg powder, HClconct, Dragendroff reagent, Mayer reagent, acetic acid anhydrous, H2SO4, and Libermann-Burchard reagent.
Phytochemical Screening
Phytochemical Screening test: 3 g sample extract, reagent, and opinions. The sample extract is then subjected to multiple operations and observed for color changes, precipitation, or other phenomena as directed by the process, the test based on Shaikh and Patil research 29.
Infrared Spectroscopy Profile Analysis
Infrared spectroscopy profile analysis takes samples of 0.5-1.5 mg of chemicals inserted into the sample holder, then scans using Infrared spectroscopy.
Antioxidant activity
Preparation of DPPH reagent.
Weigh 4 mg of DPPH dissolved with methanol to 100 ml (40 ppm).
Preparation of ABTS reagent
Reagent 1. A 7 mM ABTS solution was made by weighing 19.2045 mg of ABTS dissolved in 5 ml of distilled water.
Reagent 2. Make a 2.45 mM potassium persulphate solution by weighing 3.31 mg of potassium persulphate in 5 ml of distilled water. Mix 5 ml of reagent 1 and 5 ml of reagent 2, and incubate in a dark room for 12-16 hours. Dissolve the ABTS reagent mixture with distilled water until the absorbance value is about 0.7 at a wavelength of 734 nm.
Preparation of vitamin C standard
Prepare a concentration series by diluting a solution of 10 ppm, 20 ppm, 30 ppm, and 40 ppm. Measurement with a spectrophotometer is done by mixing each sample concentration series as much as 0.1 ml plus 0.9 ml ABTS reagent / DPPH reagent, incubating for 6 minutes, then measuring the absorbance at a wavelength of 734 nm.
Sea urchin (Diadema paucispinum) sample preparation
Prepare a master solution with a concentration of 50,000 ppm by weighing 253.5 mg of sea urchin sample dissolved with 96% ethanol to a volume of 5 ml. A concentration series was made by diluting the previous solution into concentrations of 5.070 ppm, 10.140 ppm, 15.210 ppm, 20.280 ppm, 25.350 ppm, 30.420 ppm, and 40.560 ppm.
Measurement of DPPH/ABTS Binding Activity
Measurement with a spectrophotometer was carried out by mixing each sample concentration series of 0.2 ml plus 0.8 ml DPPH/ABTS reagent, incubated for 30 minutes, then measuring the absorbance at a wavelength of 515 nm for DPPH and 734 nm for ABTS.
The antioxidant activity is measured based on the formula:

Data analysis of IC50 DPPH/ABTS
inhibition against extract concentration was calculated based on the regression
equation of % inhibition.
Results and Discussion
Determination results of sea urchin (Diadema paucispinum)
Sea urchins were determined at the Biology Service Unit, Faculty of Science and Technology, Universitas Airlangga, East Java. Determination results showed that the samples used were sea urchins with the species Diadema paucispinum. The following is the classification of Diadema paucispinum sea urchin we found.
Kingdom : Animalia
Phylum : Echinodermata
Subphylum : Echinozoa
Class : Echinoidea
Subclass : Euechinoidea
Infraclass : Aulodonta
Superorder : Diadematacea
Order : Diadematoida
Family : Diadematidae
Genus : Diadema
Species : Diadema paucispinum
Synonyms : Centrechinus paucispinus
Macroscopic Testing Results
In the macroscopic test, observations are made by looking at the physical appearance to determine the characteristics of sea urchins (Diadema paucispinum). The results of the macroscopic test determination of sea urchin (Diadema paucispinum) are shown in the figure and table below.
 |
Figure 1: Sea urchin (Diadema paucispinum). |
Table 1: Macroscopic test results of sea urchin (Diadema paucispinum)
|
Parameters |
Observation results |
Reff Standard * |
|
Shape Color Size |
Oval round Brownish black Width : 7,3 cm Length : 7,8 cm Height : 4,5 cm |
– – – |
|
Requirement (*) = Standard reference not found |
||
Microscopic Testing Results
The following results are microscopic observations of sea urchin (Diadema paucispinum) shells simplicial. Microscopic observations in chloralhydrate under 100 times magnification showed many prism-shaped calcium oxalate crystals, needle-shaped calcium oxalate crystals, and more-shaped calcium oxalate crystals. It was also found in oil cells and trichomes
Extraction Results of Sea urchin (Diadema paucispinum)
Sea urchin (Diadema paucispinum) was extracted using maceration method with 96% ethanol solvent. The extraction results obtained a yield of 4.87% which is the amount of compounds that are attracted during the extraction process using 96% ethanol solvent. The resulting yield is relatively smaller at 4.87% of the dry weight of 600g. The higher the percent yield value obtained, the more extracts obtained.
Phytochemical Screening Test Results
The following is a phytochemical screening test of 96% ethanol extract of sea urchin (Diadema paucispinum) shown in the table below.
Table 2: Phytochemical Screening Results of 96% Ethanol Extract Diadema paucispinum
|
Compounds |
Reagents |
Test *) |
Color results |
Results |
|
Alkaloid |
Mayer reagens |
Mayers test |
Forms a yellowish-white precipitate |
+ |
|
Flavonoid |
Extract + methanol + Mg + HCl conc. |
Shibata’s test |
Form dark red colour (flavonols) |
+ |
|
Saponin |
Hot water |
Foaming test |
foaming form 1,5 cm height |
+ |
|
Tannins |
FeCl3 5% |
Braymer’s test |
Blue-Greenish ring |
+ |
|
Terpenoid
|
0.5 mL Chloroform + 0.5 mL anhydrous acetic acid + 1-2 mL concentrated sulfuric acid (H2SO4) |
– |
A red rose colour : Not formed |
– |
|
Steroid |
Anhydrous acetic acid + concentrated sulphate (H2SO4) |
Libermann-Burchard test |
An array of colour change: Not formed |
– |
* literature based on Shaikh and Patil (2020)
The table above shows the results of the phytochemical screening test of 96% ethanol extract of sea urchin (Diadema paucispinum). Phytochemical screening tests include steroids, tannins, flavonoids, saponins, alkaloids, and terpenoids. The results of the chemical content test showed that 96% ethanol extract of sea urchin (Diadema paucispinum) positively contained alkaloid compounds, flavonoids, saponins, and tannins and negatively contained steroid compounds, and terpenoids.
Results of FTIR Analysis of Sea Urchin (Diadema paucispinum)
Samples of sea urchin were obtained, and simplicia and extracts with FTIR were analyzed to determine the differences in functional groups that appear in FTIR data between simplicia and 96% ethanol extract. The following are spectra images and interpretation of FTIR data.
 |
Figure 2: FTIR spectra and functional group analysis of shell simplicia of sea urchin. |
Notes : A is the OH group of alcohol, phenol; B is the C-H bond of the alkene which strengthens the OH group of the phenol alcohol; C is the weak triple C bond of alkyne (usually found in the aromatic group); D is an ester with a C-O-H bond that strengthens the OH group of phenol alcohol with a CH3 bond; E is a C-O bond, this bond exists because in A there is an OH group of alcohol, phenol. E strengthens the carboxylic acid; F is the =C-H double bond in aromatics.
 |
Figure 3: FTIR spectra and functional group analysis of 96% ethanol shell extracts of sea urchin. |
Notes : A is the OH group of alcohol, phenol; B is the alkene C-H bond that strengthens the OH groups of phenol alcohols and amides
C is the aromatic ring; D is an ester with a C-O-H bond that strengthens the OH group of phenol alcohol; E is an ester with a C-O-H bond that strengthens the OH group of phenol alcohol
The FTIR results above between simplicia and extracts show a slight difference. It appears in the FTIR spectra of the extract. In the simplicia sample, there is no splitting process known as extraction so the results seen in the spectra generally start with the OH, CH, CO, and CH3 groups. In simplicia, the aromatic and ester ring structures are visible. The extracted sample looks more detailed, and this is due to the extraction process in which the sample is broken down so that the spectra results are more detailed, one of which is the presence of N-H, C = C, and C-O groups. The details of the types of bonds in the extract could be seen which show the presence of aromatic alcohols, esters and phenols, in addition to the presence of amide groups. The extraction process can break down the chemical content of plants or animals. It can be concluded that the extraction process affects the groups produced by FTIR. The two FTIR spectra results above strengthen the results of phytochemical screening, which are positive for flavonoids, tannins, saponins, and alkaloids. Specific groups of these compounds are found in the spectra above. They start from alcohol, aromatic, ketone, ether, and double bond.
Results of Antioxidant Test of EEDP with DPPH method
DPPH is one of the methods that can be used in antioxidant testing. The presence of antioxidants in 96% ethanol extract of Diadema paucispinum sea urchin shells will neutralize the DPPH radical by giving electrons to DPPH, resulting in a color change from purple to yellow or the intensity of the purple solution reduced.
The higher the concentration of antioxidant chemicals in a sample, the more compounds will donate electrons or hydrogen atoms to DPPH free radicals, causing DPPH color fading. When DPPH is exposed to substantial concentrations of antioxidant chemicals, it turns from dark purple to yellow. This shift in DPPH color is also connected to the energy that DPPH free radicals carry. When in a radical state, DPPH is unstable (reactive) and has a high energy because it constantly reacts to find its electron pair, but once found, DPPH becomes more stable (low energy).
The IC50 value is a number that represents a 50% reduction in DPPH oxidation (capable of lowering DPPH oxidation by 50%). Several 0% indicates that there is no antioxidant activity. In comparison, a value of 100% means total attenuation and the test must be repeated by diluting the test solution to determine the activity concentration limit. The results of the calculations are placed into the regression equation (Y=AX+B), with the extract concentration (ppm) as the abscissa (X-axis) and the % reduction value (antioxidant) as the coordinate (Y-axis). This equation calculates the IC50 of each sample, which is represented by a y value of 50 and the x value obtained as IC50. A chemical is stated to be a powerful antioxidant if the IC50 value is less than 50 ppm, a potent antioxidant if the IC50 value is 50-100 ppm, a medium antioxidant if the IC50 value is 100-150 ppm, and a weak antioxidant if the IC50 value is 150-200 ppm. that the antioxidants in the sample reduced because they were easily damaged by the external environment, limiting their activity in reducing DPPH free radicals.
Table 3: Antioxidant Activity Category.
|
No |
Category |
IC50 |
|
1 |
Very strong |
< 50 ppm |
|
2 |
Strong |
50 – 100 ppm |
|
3 |
Moderate |
100 – 150 ppm |
|
4 |
Weak |
150 – 200 ppm |
Table 4: Percentage of Vitamin C Immersion as Antioxidant by DPPH Method.
|
ppm (preparation) |
ppm (test) |
DPPH Abs |
Abs |
% immersion |
|
5070 |
1014 |
0.857 |
0.785 |
8.401400233 |
|
10140 |
2028 |
0.857 |
0.711 |
17.03617270 |
|
15210 |
3042 |
0.857 |
0.617 |
28.00466744 |
|
20280 |
4056 |
0.857 |
0.571 |
33.37222870 |
|
25350 |
5070 |
0.857 |
0.485 |
43.40723454 |
|
30420 |
6084 |
0.857 |
0.428 |
50.05834306 |
|
40560 |
8112 |
0.857 |
0.396 |
53.79229872 |
|
50700 |
10140 |
0.857 |
0.339 |
60.44340723 |
 |
Figure 4: Graph of % Vit C Immersion versus Grade. |
Table 5: Percentage of Immersion of Sea Urchin Extract Samples in DPPH
|
ppm (preparation) |
ppm |
% immersion |
|
5070 |
1014 |
8.401400233 |
|
10140 |
2028 |
17.03617270 |
|
15210 |
3042 |
28.00466744 |
|
20280 |
4056 |
33.37222870 |
|
25350 |
5070 |
43.40723454 |
|
30420 |
6084 |
50.05834306 |
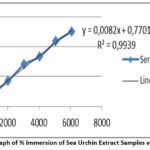 |
Figure 5: Graph of % Immersion of Sea Urchin Extract Samples versus Levels. |
The results and curves in the two tables above explain that the sea urchin extract sample has a better regression when compared to vitamin C. The value of r close to 1 states that the more linear the data. This regression equation will be able to determine the size of the IC50.
Results of ABTS Method Antioxidant Testing of Sea urchin (Diadema paucispinum) samples
ABTS method antioxidant activity testing of sea urchin (Diadema paucispinum) samples is based on the ability of antioxidant compounds to stabilize free radical compounds by donating proton radicals. The ability of sea urchin extract (Diadema paucispinum) to stabilize free radical compounds can be seen from the change in color of the blue-green test solution to colorless or reduced color intensity. The results of antioxidant activity of sea urchin extract (Diadema paucispinum) and vitamin C as a comparison. The data shown in the table below.
Compared to other procedures, the ABTS method reacts rapidly, and the test is straightforward to repeat. A high IC50 value suggests that the antioxidant activity is strong. The IC50 value in the sample solution is the concentration required to lower 50% of the ABTS free radical activity. The lower the content in the sample, the lower the absorbance value and the higher the antioxidant activity.
Table 6: Percentage of Vitamin C Immersion as Antioxidant by ABTS Method
|
ppm (prepartion) |
ppm (test) |
ABTS |
Abs (734 nm) |
% immersion |
|
5070 |
253.5 |
0.597 |
0.519 |
13.06532663 |
|
10140 |
507.0 |
0.597 |
0.366 |
38.69346734 |
|
15210 |
760.5 |
0.597 |
0.283 |
52.59631491 |
|
20280 |
1014.0 |
0.597 |
0.211 |
64.65661642 |
|
25350 |
1267.5 |
0.597 |
0.186 |
68.84422111 |
|
30420 |
1521.0 |
0.597 |
0.161 |
73.03182580 |
|
40560 |
2028.0 |
0.597 |
0.194 |
67.50418760 |
|
50700 |
2535.0 |
0.597 |
0.242 |
59.46398660 |
 |
Figure 6: Graph of % Immersion of Vit C in ABTS Reagent versus Grade. |
Table 7: Percentage of Immersion of samples of Sea urchin Extract as Antioxidant by ABTS Method.
|
ppm (preparation) |
ppm |
% immersion |
|
|
5070 |
253.5 |
13.0653266 |
|
|
10140 |
507 |
38.6934673 |
|
|
15210 |
760.5 |
52.5963149 |
|
|
20280 |
1014 |
64.6566164 |
|
 |
Figure 7: Graph of % Immersion of Sea urchin Samples in ABTS Reagent versus Levels |
Table 8: Antioxidant Activity of Vitamin C and Diadema paucispinum Extracts
|
Samples |
IC50 DPPH method |
IC50 ABTS method |
|
Vitamin C |
3,25 ppm |
2,09 ppm |
|
Diadema paucispinum extract |
6084 ppm |
756,3 ppm |
In determining the antioxidant activity, the IC50 parameter is used, which is the sample concentration required to capture DPPH radicals by 50%, where the smaller the IC50 value, the stronger the antioxidant activity. Meanwhile, Molyneux (2004) categorizes an IC50 value below 50 ppm as a powerful antioxidant, an IC50 value of 50 – 100 ppm as a potent antioxidant, an IC50 value of 100 – 150 ppm as a medium antioxidant, and an IC50 value of 150 – 200 ppm as a weak antioxidant. Based on the results of antioxidant activity testing of sea urchin extract (Diadema paucispinum) with DPPH and ABTS immersion methods, the extract did not have antioxidant properties because the IC50 value was > 200 ppm. In contrast, Vitamin C as a standard drug had a solid value as an antioxidant..
Conclusion
Based on the results of the study, it was found that 96% ethanol extract of Diadema pauscispinum sea urchin found in Sumenep-Madura contains alkaloids, flavonoids, saponins, and tannins. Antioxidant activity of 96% ethanol extract of Diadema pauscispinum sea urchin showed with IC50 value greater than vitamin C, this indicates that 96% ethanol extract of Diadema pauscispinum sea urchin did not have antioxidant activity compared with vitamin C as a standard compound.
Acknowledgement
None
Conflict of Interest
There are no conflicts of interest.
Funding Sources
This project was funded by Ministry of Research and Technology for Higher Education (Kemendikbudristek DIKTI) contract number 032/SP2H/PT/LL7/2023; B/13/HIB-EX.PB/UHT.C7/VI/2023.
References
- Rahim SAKA, Nurhasan R., Status of Sea Urchin Resources in the East Coast of Borneo, Journal of Marine Biology. 2016; (2016), https://doi.org/10.1155/2016/6393902
CrossRef - Pinna S., Pais A., Campus P., Sechi N., Ceccherelli., Habitat Preferences of the Sea Urchin Paracentrotus lividus. Marine Ecology Progress Series. 2012; 445:173-180.
CrossRef - Soleimani S., Moein S., Yousefzadi M., Bioki NA., Determination of in vitro antioxidant properties, anti-inflammatory effects and A-amylase inhibition of purple sea urchin extract of Echinometra mathaei from the Persian Gulf. Jundishapur Journal of Natural Pharmaceutical Products. 2017:12(3). https://doi.org/10.5812/jjnpp.36547
CrossRef - Nhu Hieu, VM., Thanh Van TT., Hang CTT., Mischenko NP., Sergey AF., Truong HB., Polyhydroxynaphthoquinone Pigment From Vietnam Sea Urchins as a Potential Bioactive Ingredient in Cosmeceuticals. Natural Product Communications, 2020;15(11):1-8. https://doi.org/10.1177/ 1934578X20972525
CrossRef - Nyawira A. Muthiga, Timothy R. McClanahan, Chapter 18 – Diadema, Editor(s): John M. Lawrence, Developments in Aquaculture and Fisheries Science. Elsevier. 2013; 38:257-274. ISSN 0167-9309, ISBN 9780123964915, https://doi.org/10.1016/B978-0-12-396491-5.00018-6.
CrossRef - Soleimani S, Yousefzadi M, Rezadoost H. Quantitative and Qualitative Identification of Polyhydroxylated Naphthoquinone Pigments from Shell and Spine of Echinometra Mathaei of the Persian Gulf. JMBS 2018; 9 (4) :501-506 URL: http://biot.modares.ac.ir/article-22-24437-en.html
- Yusuf M, Atthamid NFU, Indriati S, Saleh R, Latief M, Rifai A. Optimization Ultrasonic Assisted Extraction (UAE) of Bioactive Compound and Antibacterial Potential from Sea Urchin (Diadema setosum). Curr Res Nutr Food Sci. 2020; 8(2). doi : http://dx.doi.org/10.12944/CRNFSJ.8.2.22
CrossRef - Abubakar L, Wangi C, Uku J, Ndirangu S. Antimicrobial activity of various extracts of the sea urchin Tripneustes gratilla (Echinoidea). African Journal of Pharmacology and Therapeutics. 2012;1(1):19- 23.
- Dincer T and Cakli S. Chemical composition and biometrical measurements of the Turkish Sea urchin (Paracentrotus lividus, Lamarck, 1816). Critical Reviews in Food Science and Nutrition. 2007;47(1): 21–26.
CrossRef - Jinadasa BKKK, De Zoysa HKS, Jayasinghe GDTM, & Edirisinghe EMRKB. Determination of the biometrical parameters, biochemical composition and essential trace metals of edible sea urchin (Stomopneustes variolaris) in Sri Lanka. Cogent Food & Agriculture. 2016;2(1):1-12. DOI: 10.1080/23311932.2016.1143343
CrossRef - Chen G., Weng ZX., Chi CL., Juang P., Jian WQ., Feng C., Yue J. A comparative analysis of lipid and carotenoid composition of the gonads of Anthocidaris crassispina, Diadema setosum and Salmacis sphaeroides. Food chemistry. 2010;120 (4): p. 973-977.
CrossRef - Kim W and Lee H. Advances in nutritional research on regulatory T-cells. Nutrients. 2013;5(11):4305–15.
CrossRef - Brasseur L, Demeyer M, Decroo C, Caulier G, Flammang P, Gerbaux P, Eeckhaut I. Identification and quantification of spinochromes in body compartments of Echinometra mathaei’s coloured types. R. Soc. Open Sci. 2018. 5: 171213. http://dx.doi.org/10.1098/rsos.171213
CrossRef - Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., & Bitto A., Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev. 2017;2017:8416763. doi:10.1155/2017/8416763
CrossRef - Lanza FL, Chan FK, Quigley EM., Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728–738.
CrossRef - Soleimani, S., Moein, S., Yousefzadi, M., Bioki, N.A., Rezadoost, H. Identification and antioxidant of polyhydroxylated naphthoquinone pigments from sea urchin pigments of Echinometra mathaei. Med Chem Res. 2016b ;25:1476–1483.
CrossRef - Kresnamurti A, Hardiyono, Siswulandari F, Hamid IS. The Anti-inflamation activity of Ethanolic Extract of Echinometra mathaei on White Male Rat with Carrageenan Induced Paw Oedema. Pharmacon:Jurnal Pharmacy Indonesia. 2021;18(2): 141-7.
CrossRef - Ihsan M, Rahmadian R, Raymond B. Comparison of Burn Wound Histopathology Imaging between Epidermal Growth Factor Spray and Silver Sulfadiazine Application: An Invivo Study. Biosci Med J Biomed Transl Res. 2022;6(5):1749–56.
CrossRef - Karmilah, Musdalipah, Daud NS, Reymon, Fauziah Y. Identification of sea urchin gonads chemical compounds using thin-layer chromatography from Bokory island, Southeast Sulawesi. J Phys Conf Ser. 2021;1899(1).
CrossRef - Salma WO, Asriati, Eso A, Kusnan A, Fristiohady A, Nurdin KDS, Bastaman HR, Abdilah MN, Mudjahidah NH. Gonad Extracts of Diadema setosum as Potential Antibacterial Agent Derived from Wakatobi District Sea Waters Southeast Sulawesi Province-Indonesia. International Journal of Science:Basic and Apllied Research. 2020;49(1):125-132
- Olivia Akaerina F, Nurhayati T, Suwandi R. Isolation and Characterization of Antibacterial compound from Sea Urchin. Jurnal Pengolah Hasil Perikanan Indonesia. 2015;18(1):61–73.
CrossRef - Salma WO, Wahyuni S, Kadir N, As’ad S, Yusuf I. Effects of sea urchin (Diadema setosum) gonad extracts on gene expression of FOXP3 and the production of cytokine on Salmonella typhi-induced mice. J Appl Pharm Sci. 2018;8(12):140–6.
CrossRef - Sulistiyo J, Ulfa DM, Tulandi SM. Antioxidant activity of Diadema setosum gonads from Kelapa island, district of Seribu island, Indonesia. Trop J Nat Prod Res. 2021;5(5):809–13.
CrossRef - Ode Salma W. Immune Nutrient Content of Sea Urchin (Diadema setosum;) Gonads. Int J Nutr Food Sci. 2016;5(5):330.
CrossRef - Sabilu Y, Jafriati, Zainuddin A. Testing the Toxicity, Protein Content, and Anticholesterol of the Ethanol Extract of the Sea Urchin (Diadema Setosum) Gonad as Marine Biodiversity-Based Medicinal Ingredients. Hunan Daxue Xuebao/Journal Hunan Univ Nat Sci. 2022;49(9):173–8.
CrossRef - El-Sayeed WMM, Elshaer MM, Ibrahin HAH, El-Metwaly MEA. Antimicrobial Agents from Sea Urchin (Diadema setosum) Collected from Red Sea. Egyptian Journal of Aquatic Biology and Fisheries. 2020;24(5):33-51.
CrossRef - Khalil EA, Swelim H, El-Tantawi H, Bakr AF, Abdellatif A. Characterization, cytotoxicity and antioxidant activity of sea urchins (Diadema savignyi) and jellyfish (Aurelia aurita) extracts. Egypt J Aquat Res. 2022;48(4):343–8. Available from: https://doi.org/10.1016/j.ejar.2022.05.005
CrossRef - Kresnamurti A, Izazi F, Praditapuspa E, Siswandono. In Silico Analysis of Bioactive Compounds from Sea Urchin (Echinometra mathaei) against SARS-COV-2. Biomedical and Pharmacology Journal. 2023;16(March):329–37.
CrossRef - Shaikh JR. and Patil, M. “Qualitative tests for preliminary phytochemical screening: An overview.” International Journal of Chemical Studies. 2020;8(2):603-608.
CrossRef







