Manuscript accepted on :06-12-2023
Published online on: 01-01-2024
Plagiarism Check: Yes
Reviewed by: Dr. Md.Sarwar Hossain and Dr. Ramdas Bhat
Second Review by: Dr. Francisco Solano and Dr. Niharika Kondepudi
Final Approval by: Dr. Luis Jesús Villarreal-Gómez
Ali Abd Sulaiman 1 , Hawraa kadhim abbas2
, Hawraa kadhim abbas2 , Ali Mahmoud Al-Samydai3, Hussein K. Alkufi4
, Ali Mahmoud Al-Samydai3, Hussein K. Alkufi4 , Haneen abdul hadi kharaba5
, Haneen abdul hadi kharaba5 and Hany A. Al-hussaniy*6
and Hany A. Al-hussaniy*6
1 Radiology Department, Al-Karama Hospital, Baghdad, Iraq.
2 Department of Pharmacy, Alzahraa teaching hospital at Al-Najaf city. Iraq.
3College of Pharmacy, Al-Ahliyya Amman University, Amman, Jordan.
4Department of Pharmacognosy, College of Pharmacy, Thi-Qar University, Iraq.
5 Department of pharmaceutics, college of pharmacy, University of Baghdad, Baghdad, Iraq.
6 Dr Hany Akeel Institute, Iraqi Medical Research Center, Baghdad, Iraq.
Corresponding Author E-mail: Hany_akeel2000@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2827
Abstract
Background: Multiple sclerosis (MS) is a neuropathological disease diagnosed by a magnetic resonance imaging device. Early patients affected with MS are named active. Objectives: This study assessed the difference between active and non-active MS using the region of interest value. Methods: Twenty patients with MS included in this study were examined with T1 weighted image (T1W) with and without contrast agent injection to increase the brightness of darker regions after only 10 minutes. Also, T2 weighted images (T2W) and Fluid-attenuated inversion recovery (FLAIR) were scanned. The area of interest option was calculated for all cases. Results: The result shows that the region of interest (ROI) value was significantly higher for T1 weighted image (T1W) with contrast than without. Furthermore, the range of FLAIR values was higher than the (T2W). Conclusion: The ROI is an effective parameter for diagnosing active MS early by values instead of the radiographic picture.
Keywords
Fluid-attenuated inversion recovery; Lymphatic System; Multiple Sclerosis; Magnetic Resonance Imaging; Nervous System Diseases
Download this article as:| Copy the following to cite this article: Sulaiman A. A, Abbas H. K, Al-Samydai A. M, Alkufi H. K, Kharaba H. A. H, Al-hussaniy H. A. A Comparative Diagnostic Study for Using the Contrast Agent in Active and Non-Active Multiple Sclerosis by Region of Interest Parameter. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Sulaiman A. A, Abbas H. K, Al-Samydai A. M, Alkufi H. K, Kharaba H. A. H, Al-hussaniy H. A. A Comparative Diagnostic Study for Using the Contrast Agent in Active and Non-Active Multiple Sclerosis by Region of Interest Parameter. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/47rxTja |
Introduction
The basic principle of MRI lies in the magnetization of the body protons, which are positively charged particles. MRI in Neurological examination uses a strong magnetic field and radio waves to produce detailed pictures of the inside of the body1,2. The most common scans in MRI are Longitudinal Relaxation (T1), weighted Longitudinal Relaxation (T1W), Transverse Relaxation (T2), and Fluid Attenuated Inversion Recovery (FLAIR). The T1W used when a contrast agent is injected into the patient represents the differences in the T1 times of the tissues. The region of interest (ROI) is a programming method used in radiology. It is considered an essential parameter for diagnostic imaging. It aims to increase the image resolution depending on the shape and size of the selected part of the image to acquire the best accuracy and information. The ROI statisticas obtained from the signal that reached the voxel of the image 3. The ROI is a magnetic resonance imaging (MRI) device parameter set.
In MRI, the intensity and appearance of two common brain diseases are approximately the same. These diseases are multiple sclerosis (MS) and ischemia. Multiple sclerosis (MS) is an inflammatory demyelinating central nervous system disease (CNS). It occurs when the immune system of the body attacks the CNS. So, the neural signal transmission from the brain to the spinal cord will be disturbed4. Multiple sclerosis (MS) is identified by the presence of demyelination patches in the white matter of the central nervous system, typically initiating in the optic nerve, spinal cord, or cerebellum. The degradation of myelin sheaths and subsequent removal by microglial cells are characteristic features of this condition. Despite its prevalence, the precise cause of MS remains unclear. However, some researchers suggest a potential link between a viral infection and the host’s immune response as a contributing factor 5,6. The progress time of transforming from active to non-active depends on the patient 7. The Better diagnostic device for MS suggested using the MRI with T1, T2, T2W, and FLAIR 8,9.
This study aimed to distinguish between active and non-active multiple sclerosis by measuring the region for the main MRI sequences T1W without or with a double dose of contrast after 10 minutes only and T2W and FLAIR to acquire a better diagnosis of the new injury to use it as an alternative to the contrast.
Patient and Method
This is a prospective clinical study performed in the MRI unit of Saad Alwitry, a neuroscience teaching hospital. Neurologists and radiologists diagnosed all the patients with multiple sclerosis. The patients were scanned using the magnetic resonance imaging of 3.0 Tesla. Table 1 provides a comprehensive overview of the patients’ attributes considered for this research. The signal intensity ranges of the three sequences, namely T1W, T2W, and FLAIR, were assessed using the ROI option and recorded individually for each patient. Our study specifically focused on patients exhibiting early MS symptoms to discern between active and non-active cases of brain MS. Then, the patient was injected with the contrast agent (OmniscanTM0.5 mmol/ mL) and scanned with the TIW sequence.
Table 1: The Characteristics of Multiple Sclerosis Patients.
|
Characteristics |
|
|
Gender Male Female |
9 11 |
|
Age Range (years) |
27.5 (20 – 37) |
|
Weight (kg) |
70 (58 – 91) |
Statistical Analysis
The data analysis was conducted using SPSS-24 (Statistical Packages for Social Sciences, version 24), a commonly available statistical package. The presentation of data involved simple measures such as mean, standard deviation, and range (minimum-maximum values). Furthermore, appropriate statistical tests were employed to assess the significance of differences between various means (quantitative data). Specifically, the Student’s t-test was used to compare two independent means, the Paired t-test to compare paired observations (or two dependent means), and the ANOVA test to compare more than two independent means. Any result with a P-value equal to or less than 0.05 was considered statistically significant.
Results
The ROI signal value of the T1W sequence compared with and without using contrast and presented in Table (2) and Figure (1). The MS patients were injected with a contrast agent to enhance the T1W signal. The value includes the minimum. Maximum, mean, and range of the value showed significantly higher with contrast than without.
Table 2: A comparison of T1W sequence parameters with and without contrast.
|
T1W |
Without contrast |
With contrast |
P value |
|
T1W Min |
715.3±91.2 (510-913) |
1019.2±65.3 (891-1098) |
<0.0001* |
|
T1W Max |
781.1±80.9 (635-971) |
1407.5±69.1 (1167-1441) |
0.001* |
|
T1W Mean |
724.7±87.1 (541-940.8) |
1176.9±66.6 (1033.5-1321.7) |
0.002* |
*Significant difference between two dependent means using Paired t-test at 0.05 level.
The ROI boundary ranges of the TW2 and FLAIR sequences are important to investigate to estimate early MS’s predicting value. Therefore, these resulting values of ROI are shown in the Table. Also, figures 2 and 3 presented the values of T2W and FLAIR, respectively.
Table 3: A comparison of T2W sequence parameters with and without contrast.
|
T2W |
FLAIR |
|
|
Min |
855.4 ± 52.1 (763 – 941) |
1121.2 ± 117.2 (944-1438) |
|
Max |
1139.4 ± 67.8 (975 – 1219) |
2439.2 ± 301.08 (1094-13021) |
|
T2W: T2 weighted images FLAIR: Fluid-attenuated inversion recovery |
||
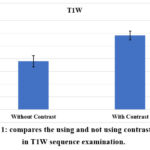 |
Figure 1: compares the using and not using contrast agents in T1W sequence examination. |
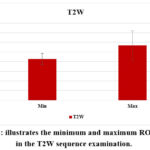 |
Figure 2: illustrates the minimum and maximum ROI values in the T2W sequence examination. |
 |
Figure 3: illustrates minimum and maximum ROI values in the FLAIR sequence examination. |
Discussion
The ROI parameter is an image processing added in the magnetic resonance imaging device to read the range of several voxels for the radiographic image precisely10-15. The signal intensity was enhanced for MS protocol when the patient was injected with contrast after and reexamined with T1W-MSE sequence after 30 min. In this study, we used the double dose of contrast and examined the patient after 10 minutes only. Not all the MS cells of the brain uptake the contrast, only the new injury because the neural cells will be destroyed and converted to non-active cells16,17. However, the high uptake of the newer injury was observed in the ROI value after the contrast agent injection. Therefore, this technique shows a significantly more effective method of distinguishing the recent sclerosis cells than the T1W sequence.
After a period depending on the patient’s status, the axonal loss is a function that plays a major role in determining permanent neurologic dysfunction in patients with MS18.
The present study’s findings are consistent with Pretorius PM and Quaghebeur G 13, who reported a 15-minute delay in scanning after receiving a double dose of intravenous contrast. Trip S. and Miller D. state that a 5-minute delay scanning after IV contrast injection improves T1 imaging scanning. Newly active enhancing lesions often remain for one month, making them an excellent marker for monitoring disease activity. Both triple-dose gadolinium and magnetization transfer imaging may help identify active lesions 19,20.
From the statistical observation, the ranges of T2W and the FLAIR have overlapped ranges of signals, iso their values are not precisely dependable and cannot give a predictable value. Severe, Highly Active, or Aggressive Multiple Sclerosis 21,22.
A study reported that the FLAIR sequence is better than others for detecting MS lesions. Periventricular lesions are often indistinguishable from the surrounding cerebrospinal fluid, which has a strong signal with T2 weighting 23-28.
According to Ma S . study.20 and Sweeney RH. study. 21, T2W.FLAIR is the optimal MRI imaging sequence for multiple sclerosis when utilizing a 3 Tesla MRI with a TE of 120 ms, comparable to the parameters utilized in the present research. By using a TE of 120ms, the contrast-to-noise ratio of the white matter lesion is significantly increased, thus improving the detectability of MRI and the spatial resolution of the picture 29-35. Additionally, the present study findings corroborate Ge. Y 28, who claimed that T2W.Flair is a more sensitive detector of lesions in the white and gray matter of the brain in MS patients.
Furthermore, image contrast enhancement and accurate disease state categorization can be influenced by various factors, such as the size of MS lesions, the type of disease-modifying therapy, and the stage of disease activity involving inflammation, demyelination, axonal loss, and gliosis. Gaj and his colleagues have supported the impact of lesion size on image contrast35-39. Their study utilized automatic segmentation analysis for gadolinium-enhanced MS lesions, similar to the technique used in our current research, albeit focusing on covering and identifying the MS lesion. In contrast, our study implemented this technique to cover and identify the MS lesion, thereby investigating its effects 39-45. Our work has shown that lesion location and form, in addition to lesion size, are important determinants. Particular brain areas, including the periventricular zone, might develop lesions that have a more noticeable impact on neurological processes. Our results, which are consistent with those of recent research by Alhussaniy research. and DeLuca, suggest that the form and perimeter of the lesions may also reflect the rate of disease development and severity46-49. Furthermore, we also investigate patient-specific characteristics that impact the effectiveness of MRI imaging in multiple sclerosis. Age, gender, and genetic predispositions have all been demonstrated to have differing effects on how the disease manifests itself and reacts to imaging methods.
Conclusion
The results of this comparative diagnostic investigation highlight the important developments in Multiple Sclerosis (MS) detection and monitoring magnetic resonance imaging (MRI) approaches. Utilizing Region of Interest (ROI) characteristics has shown to be an essential technique for improving MRI reading accuracy. This study demonstrated how well contrast agents work to identify active MS lesions in particular. Signal intensity and lesion detectability were significantly improved in active MS cases by using a twofold dose of contrast and shortening the test period to 10 minutes.
Finally, our research emphasizes how crucial it is to take into account a number of variables for precise image contrast enhancement and disease state classification, including lesion size, disease-modifying medication, and disease stage. The results of Gaj research. about how lesion size affects picture contrast are especially important since they support our approach of using automated segmentation analysis to improve lesion detection.
We conclude that the ROI readings are an effective parameter to diagnose early active multiple sclerosis by values instead of a radiographic picture and can predict the disease from the T1W sequence levels.
Acknowledgment
This research received ethical approval number 928-12-2017 from the ethical committee of the College of Medicine – almustansria University and followed the decoration of Helsinki and the recommendation of the Iraqi Medical research center.
Conflict of Interest
There are no conflict of interest
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or notfor- profit sectors
References
- Spratt J, Salkowski L, Loukas M, Turmezei T, Weir J, Abrahams PH. Weir & Abrahams’ Imaging Atlas of Human Anatomy. ElSevier. Elsevier; 2020. 272 p.
- Alabedi HH, Al Musawi MS, Mohammed Ali N. Dosimetric effects and impacts caused by a carbon fiber table and its accessories in a linear accelerator. J Contemp Med Sci. 2023;9(3 SE-Articles).
CrossRef - Abu Hajleh MN, AL-Samydai AM, Mare’i MN, Abd MM, Sibai OA, Mohammed AH, Al-Sharbatee SM, Yousif RO. The role of pharmacist in guiding the pharmacy clients towards pharmaceutical preparations use. Journal of Pharmaceutical Health Services Research. 2023;14(1):28-34.
CrossRef - Hauser SL, Cree BAC. Treatment of multiple sclerosis: A review. Am J Med. 2020;133(12):1380-1390.e2. doi:10.1016/j.amjmed.2020.05.049doi: 10.1016/j.amjmed.2020.05.049.
CrossRef - Oger J, Adnan AA. Multiple Sclerosis for the Practicing Neurologist. Seminar in Clinical Neurology. 2007. 1–107 p.
- Kuhlmann T, Moccia M, Coetzee T, Cohen JA, Correale J, Graves J, Marrie RA, Montalban X, Yong VW, Thompson AJ, Reich DS. Multiple sclerosis progression: time for a new mechanism-driven framework. The Lancet Neurology. 2023;22(1):78-88.
CrossRef - C D, LA Z, DM R. Highly active multiple sclerosis: An update. Mult Scler Relat Disord. 2019 ;30:215–24.
CrossRef - Sahraian MA, Radue EW. MRI Atlas of MS Lesions. MRI Atlas of MS Lesions. Springer-Verlag Berlin Heidelberg; 2008.
- Searson PC, Zhao N, Chung TD, Guo Z, Jamieson JJ, Liang L, Linville RM, Pessel A, Wang L. The influence of physiological and pathological perturbations on blood-brain barrier function. Frontiers in Neuroscience. 2023 Oct 23;17:1289894.
CrossRef - Galea RR, Diosan L, Andreica A, Popa L, Manole S, Bálint Z. Region-of-interest-based cardiac image segmentation with deep learning. Applied Sciences (Switzerland). 2021;11(4):1–11.
CrossRef - Freedman MS, Rush CA. Severe, Highly Active, or Aggressive Multiple Sclerosis. CONTINUUM Lifelong Learning in Neurology. 2016;22(3):761–84.
CrossRef - J L, YM Z. Magnetic Resonance Imaging and Clinical Features of the Demyelinating Degeneration of White Matter in Young Patients. Int J Gen Med. 2021;14:3177–86.
CrossRef - Tomassini V, Sinclair A, Sawlani V, Overell J, Pearson OR, Hall J, Guadagno J. Diagnosis and management of multiple sclerosis: MRI in clinical practice. Journal of Neurology. 2020;267:2917-25.
CrossRef - Capizzano AA, Moritani T, Romeo A. Demyelinating Diseases. Diffusion-Weighted MR Imaging of the Brain, Head and Neck, and Spine. 2021:313-51.
CrossRef - Cortese R, Collorone S, Ciccarelli O, Toosy AT. Advances in brain imaging in multiple sclerosis. Therapeutic advances in neurological disorders. 2019;12:1756286419859722.
CrossRef - Salim Mahmood A, Ammoo AM, Ali MH, Hameed TM, Al-Hussaniy HA, Aljumaili AA, Al-Fallooji MH, Kadhim AH. Antiepileptic Effect of Neuroaid® on Strychnine-Induced Convulsions in Mice. Pharmaceuticals. 2022;15(12):1468.
CrossRef - Al-kuraishy AA, Jalil HJ, Mahdi AS, Al-hussaniy H. General anesthesia in patient with Brain Injury. Medical and Pharmaceutical Journal. 2022;1(1):25-34.
CrossRef - Al-hussainy HA, AL-Biati HA, Ali IS. The Effect of Nefopam Hydrochloride on the Liver, Heart, and Brain of Rats: Acute Toxicity and Mechanisms of Nefopam Toxicity. J. Pharm. Negat. Results 2022;13(3):393-400.
CrossRef - Tawfeeq KT, Al-Khalidi GZ, Al-hussaniy HA. Neurodevelopmental Abnormalities in Children Associated with Maternal Use of Psychoactive Medication: Maternal Use of Psychoactive Medication. Medical and Pharmaceutical Journal. 2022;1(2):64-73.
CrossRef - Ma S, Wang N, Fan Z, Kaisey M, Sicotte NL, Christodoulou AG, Li D. Three‐dimensional whole‐brain simultaneous T1, T2, and T1ρ quantification using MR multitasking: method and initial clinical experience in tissue characterization of multiple sclerosis. Magn Reson Med . 2021;85(4):1938-52
CrossRef - Sweeney EM, Nguyen TD, Kuceyeski A, Ryan SM, Zhang S, Zexter L, Wang Y, Gauthier SA. Estimation of Multiple Sclerosis lesion age on magnetic resonance imaging. Neuroimage. 2021;225:117451.
CrossRef - Al-hussaniy HA, AL-Biati HA. The Role of Leptin Hormone, Neuropeptide Y, Ghrelin and Leptin/Ghrelin ratio in Obesogenesis. Medical and Pharmaceutical Journal. 2022 Dec 2;1(2):52-63.
CrossRef - Kumar KV, Sree SL, Sree SJ. Cabergoline-Dopamine Receptor Agonist. Med. pharm. j 2023;2(2):90-8.
CrossRef - Al_hussaniy HA. Medical Scientific Research Challenges in Iraq. Medical and Pharmaceutical Journal. 2023;2(1):1-3.
CrossRef - Gong NJ, Dibb R, Bulk M, van der Weerd L, Liu C. Imaging beta amyloid aggregation and iron accumulation in Alzheimer’s disease using quantitative susceptibility mapping MRI. Neuroimage. 2019;191:176-85.
CrossRef - Goyal B, Agrawal S, Sohi BS. Noise issues prevailing in various types of medical images. Biomed Pharmacol J. 2018;11(3):1227.
CrossRef - Beshna E, Alwafi SA, Lazrak RR. Evaluation of the Quality of life of Zawia (Libya) patients undergoing hemodialysis. Medical and Pharmaceutical Journal. 2023;2(1):4-16.
CrossRef - Ge Y. Multiple Sclerosis: The Role of MR Imaging. AJNR Am J Neuroradiol. 2006;27(6):1165.
- Faisal F, Nishat MM. An Investigation for Enhancing Registration Performance with Brain Atlas by Novel Image Inpainting Technique using Dice and Jaccard Score on Multiple Sclerosis (MS) Tissue. Biomed Pharmacol J. 2019;12(3):1249-62.
CrossRef - Al-Kelaby WJ, Al Kaabi ZS, Alhussaniy HA. Histological and Histochemical Studies of the Eye Structure of Anas Platyrhynchus (Mallard) Duck Species. Indian Vet. J. 2023;100(3):07-15.
- Pacheco-Barrios K, Cardenas-Rojas A, Thibaut A, Costa B, Ferreira I, Caumo W, Fregni F. Methods and strategies of tDCS for the treatment of pain: current status and future directions. Expert Rev Med Devices. 2020;17(9):879-98.
CrossRef - Al-hussaniy HA, Al-Shammari AH, Sameer HN, Oraibi AI. The relationship between statin therapy and adipocytokine/inflammatory mediators in dyslipidemic nondiabetic patients: A comparative study. Pharmacia. 2023;70(3):581-5.
CrossRef - Al-Hussaniy HA, Hassan AF, Oraibi AI, Al-Juhaishi AM, Naji FA, Al-Tameemi ZS. Clinical Pharmacogenetics of Angiotensin II Receptor Blockers in Iraq. J Pharm Bioallied Sci 2023;15(3):101-6.
CrossRef - Cho Y, Jeong S, Kim H, Kang D, Lee J, Kang SB, Kim JH. Disease-modifying therapeutic strategies in osteoarthritis: Current status and future directions. Exp Mol Med. 2021;53(11):1689-96.
CrossRef - AL-HUSSANIY HA, AL-TAMEEMI ZS, AL-ZUBAIDI BA, ORAIBI AI, NAJI FA, KILANI S. PHARMACOLOGICAL PROPERTIES OF SPIRULINA SPECIES: HEPATOPROTECTIVE, ANTIOXIDANT AND ANTICANCER EFFECTS. Farmacia. 2023;71(4).670-678.
CrossRef - Maguire R, Maguire P. Caregiver burden in multiple sclerosis: recent trends and future directions. Curr Neurol Neurosci Rep. 2020;20:1-9.
CrossRef - Al-Hussaniy HA, Аlmajidi YQ, Oraibi AI, Alkarawi AН. Nanoemulsions as medicinal components in insoluble medicines. Pharmacia. 2023;70(3):537-47.
CrossRef - DeLuca J, Chiaravalloti ND, Sandroff BM. Treatment and management of cognitive dysfunction in patients with multiple sclerosis. Nat Rev Neurol. 2020;16(6):319-32.
CrossRef







