Manuscript accepted on :09-02-2023
Published online on: 28-07-2023
Plagiarism Check: Yes
Reviewed by: Dr. Aktsar Roskiana Ahmad
Second Review by: Dr. Akhtar Ali
Final Approval by: Dr. H Fai Poon
Himangshu Baruah , Harmonjit Boro and Ananta Swargiary*
, Harmonjit Boro and Ananta Swargiary*
Pharmacology and Bioinformatics Laboratory, Department of Zoology, Bodoland University, Kokrajhar, Assam, India.
Corresponding Author E-mail: ananbuzoo101@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2753
Abstract
Background: Mosquito-borne diseases are among the major ailments of world affecting billions of people living in economically poor and developing countries. The development of insecticide resistance in mosquito vectors has forced the global community to look into alternative sources of medicines with better efficacy and less side effects. Plants with rich sources of metabolites have been explored extensively for mosquitocidal activity. The present study explored the antioxidant and larvicidal activities of five important plants traditionally used as mosquito repellent by tribal communities of fringe villages of Manas National Park of Assam. Methods: Methanolic crude extracts were prepared for all the plants following standard protocols. Phytochemical and antioxidant study was performed following the protocol published in recent publications. Larvicidal bioassay was carried out as per WHO protocol. Results: The study observed considerable phytochemical and antioxidant activity. Phenolics, flavonoid and antioxidant activity, were found to be highest in Cinnamomum tamala. The phenolic and flavonoid value ranged from 9.89 to 147.15µgGAE/mg and 4.32 to 28.43µgQE/mg plant extract, respectively. The IC50 for various antioxidant activities ranged from 27.94 to 114.15µg/mL (DPPH), 15.05 to 707.74µg/mL and 40.23 to 338.91µg/mL (TBARS). Similarly, C. tamala showed the strongest larvicidal activity with LC50 value of 3.11mg/mL in Aedes aegypti larvae. Conclusion: The present study observed that C. tamala leaves could be a good source of phytochemicals and antioxidant and larvicidal activity.
Keywords
Antioxidants; Ethnomedicines; Larvicide; Manas National Park; Phytochemicals
Download this article as:| Copy the following to cite this article: Baruah H, Boro H, Swargiary A. Study of Antioxidant and Larvicidal Properties of Selected Medicinal Plants of Fringe Villages of Manas National Park, Assam, India. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Baruah H, Boro H, Swargiary A. Study of Antioxidant and Larvicidal Properties of Selected Medicinal Plants of Fringe Villages of Manas National Park, Assam, India. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/455mLYs |
Introduction
Vector-borne diseases are one of the major diseases that kill nearly one million people every year. Globally, malaria alone results in about 400,000 people death out of about 219 million cases. Dengue is another mosquito-borne disease transmitted by Aedes mosquitoes, affecting nearly four billion people worldwide and causing fatalities of about 40,000 people annually.1 Though a good number of commercial insecticides are available, the development of drug resistance presents a major challenge to controlling vector-borne diseases.2 Plants can be an alternative to commercial insecticides with their rich secondary metabolites. Worldwide several plants are being investigated to see the bio-efficacy against many diseases, including larvicidal and mosquitocidal activities.3 India is among the wealthiest countries with diverse cultural traditions associated with using plants and herbs for human ailments.4 The use of plants and herbs to cure diseases has been practised in this part of the world since ancient times. Many studies have explored the medicinal properties of traditionally used plants because of their rich and essential secondary metabolites.5,6 With rich antioxidant phytocompounds, plants can counteract the toxic and cell-damaging biomolecules, the free radicals.7-9 In northeast India, people use herbal medicinal formulations to cure common diseases, especially in rural areas. Since ancient times, tribal groups of Assam have been practicing several plant-based preparations to repel insects and mosquitoes from edible items. Based on ethnomedicinal knowledge, the present study investigates the larvicidal activity of five plants commonly used by villages as insect repellents, namely Azadirachta indica, Brassica rapa, Cinnamomum tamala, Nicotiana tabacum and Ocimum sanctum.
A. indica belonging to the family Meliaceae is an important plant having rich medicinal values. The plant possesses several bioactive properties such as larvicidal, anthelmintic, insecticidal, pesticidal, cancer, hypertension, diabetes, antiviral, anti-inflammatory, anti-dermatic, anti-ulcer, etc.10-12 B. rapa is a herbaceous plant belonging to the family Brassicaceae. Traditionally, the plant parts are used for numerous ailments such as rheumatism, neuralgia, alopecia, snakebite, toothache, carcinoma, throat tumours, bronchitis, cardiovascular disease, and diabetes.13,14 C. tamala (Family Lauraceae), also known as Tejpatt in Assam, is an evergreen, aromatic tree distributed widely in north-eastern Himalayas in Assam, Mizoram, and Meghalaya. Traditionally, different parts of C. tamala are used to treat cough, asthma, wound healing, food preservatives, and spices.15,16 Similarly, N. tabacum is a herbaceous plant (Family Solanaceae) known to enhance digestive system, urinary tract disorders, cough, itching, helminth-related problems, toothache, pain in the eye, scorpion bite, relaxant, and antispasmodic.17 O. sanctum (Family Lamiaceae) is a herbaceous plant used to treat snake bites, scorpion stings, skin infections, cough, respiratory disorders, malarial, wound healing, and ulcers.[18,19] Because of its medicinal importance, the present study investigated the antioxidant and larvicidal properties of five traditionally used medicinal plants, Azadirachta indica, Brassica rapa, Ocimum sanctum, Cinnamomum tamala, and Nicotiana tabacum.
Method and Materials
Collection and identification of plant materials
Five medicinal plants, namely Azadirachta indica A. Juss.(identification no. BUBH2118051), Brassica rapa L. (BUBH0000849), Ocimum sanctum L.(BUBH28045), Cinnamomum tamala (Buch-Ham) T.Nees & C.H. Ebern (BUBH0000860), and Nicotiana tabacum L. (BUBH0000861) were collected from fringe villages of Manas National Park (N-26°45´17.3´´ E-091°13´57.3´´), Assam, India. The scientific identification of plants was carried out in the Department of Botany, Bodoland University, Kokrajhar.
Preparation of alcoholic crude extract
Sample plants were collected from the fringe villages of Manas National Park, Assam. The leaves were processed for alcoholic crude extract preparation following the process described in earlier publications.20,21 Dry or semi-solid extracts of plants were collected and kept in a vial at -20ºC for further experimental uses.
Phytochemical Study
Qualitative study
The qualitative phytochemical of methanolic plant extract was determined by the following latest publications.22,23 The various qualitative tests performed were as follows: Tannins (Braymer’s test), Saponins (Frothing test), Terpenoids (Salkowski test), Reducing Sugar (Fehling’s test), Coumarins (NaOH test), Quinones (Sulphuric acid test), Alkaloids (Dragendorff test), Phlobatannins (HCl test), Anthraquinones (Borntrager’ test), Anthocyanin (HCl test), and Cardiac Glycosides (Keller-Kiliani test).
Quantitative study
Carbohydrates
Anthrone method was used for the estimation of crude carbohydrate content of crude plant extracts.24 The value (µg/mg plant extract) was correlated with the standard curve prepared using glucose (R2 = 0.9741).
Protein
Lowry method was used for the estimation of protein using Folin-Ciocalteu reagent.25 Values were expressed as µg protein/mg plant extract using the calibration curve of BSA (R2 = 0.999).
Total Phenolic Content (TPC)
The presence of total phenolic contained was evaluated by Folin-Ciocalteu reagent.26 TPC value was compared as gallic acid equivalent (R2 = 0.995).
Total Flavonoid Content (TFC)
The total flavonoid content (TFC) was estimated following the method of Ordonez et al.27 Quercetin was used as a reference chemical for standard curve preparation (R2 = 0.9879).
Antioxidant Activities
Total antioxidant activity (TAA) assay
TAA of plant extract was estimated following phosphomolybdate method.28 TAA value was compared with ascorbic acid as the standard chemical.
Ferric-reducing antioxidant power (FRAP) assay
The procedure of Iloki-Assanga et al.29 was followed to estimate FRAP activity of plant extracts. FRAP activity of the plant extract was compared with the ascorbic acid (as standard).
1,1-Diphenyl-2-Picryl-hydrazyl (DPPH) assay
The antioxidant activity of plant extracts was estimated by the scavenging activity of DPPH free radicals following Mamta et al.30 The scavenging activity was measured as percentage inhibition using the following formula:
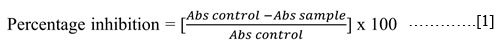
Abs control = absorbance of DPPH and methanol.
Abs sample = absorbance of DPPH and plant extract or ascorbic acid.
2-2’-Azinobis- (3-ethylbenzothiazoline-6-sulfonate) (ABTS) assay
The free radical scavenging activity of plant extracts was also estimated using ABTS as free radicals.31 The ABTS radical scavenging activity of plant extract was compared with gallic acid as a standard antioxidant molecule. The free radical scavenging potentials of the plant extracts were estimated following the formula of DPPH assay.
Lipid peroxidation inhibition or Thiobarbituric acid reactive species (TBARS) Assay
Lipid peroxidation inhibition property of plant extracts was studied in egg yolk as a lipid-rich medium following the modified protocol of Thiobarbituric acid reactive species.32 Ascorbic acid was used as a reference chemical. The formula for the calculation of TBARS was similar to DPPH assay.
The larvicidal bioassay
Larval bioassay was carried out as per WHO protocol. Larvae were exposed to different test doses of plants as per the efficacy of the plants. The overall test doses ranged from 3.0 – 12 mg/mL for different plant extracts. A total of 20 – 25 numbers of 3rd or 4th instar A. aegypti larvae were exposed to different plant extract concentrations for 24h.33 The experiment was replicated three times.
Statistical Analysis
Statistical calculations were performed in Mis. excel and OriginPro-8.5. Correlation study was carried out in IBM-SPSS-Ver.21. All experiments were carried out in triplicate (n=3), and values were expressed as mean ± standard deviation (SD).
Results
Plants have been used as emerging sources of medicines in the modern-day scenario as they are rich in phytochemicals and secondary metabolites. Table 1 shows the qualitative analysis of phytocompounds. Fig. 1 shows the protein, carbohydrate and phytochemical contents of all five plants. The study observed a considerable quantity of protein content in the methanolic extracts of plants, while the carbohydrate content was much higher (almost five times) than protein. The average protein and carbohydrate content were found to be 78.68±8.26µg/mg and 355.80±115.57µg/mg plant extract, respectively. Highest protein content was revealed in O. sanctum (86.67±4.64 µg/mg extract) and lowest in A. indica (67.07±6.01µg/mg extract). On the contrary, A. indicum and O. sanctum showed highest (564.52±8.76µg/mg extract) and lowest (222.04±3.98µg/mg extract) carbohydrate content, respectively (Fig. 1a). The crude extracts of plants showed significant variations in phenolic and flavonoid contents among the five plants. Highest TPC and TFC was found in C. tamala. Lowest TPC and TFC were found in B. rapa and A. indicum, respectively. The TPC ranged from 9.89 (B. rapa) to 147.15µgGAE/mg extract (C. tamala). Overall, the phenolic content was significantly higher (average, 55.25µgGAE/mg extract) compared to flavonoid content (average, 16.78 µgQE/mg extract). TFC value ranged from 4.32 to 28.43µgQE/mg extract (Fig. 1b). Similarly, C. tamala showed strongest total antioxidant and ferric-reducing properties among the five plants, while B. rapa and N. tabacum showed weakest total antioxidant and FRAP activity, respectively. Among the five plants, the total antioxidant activity ranged from 210.47±2.06 to 397.61±4.23µgAAE/mg extract, respectively. Similarly, C. tamala showed highest FRAP activity, while N. tabacum showed the lowest ferric-reducing activity (Fig. 1c).
Table 1: Qualitative analysis of phytocompounds from five medicinal plants
|
Phytochemicals |
Test performed |
Name of the plants |
||||
|
|
|
O. sanctum |
C. tamala |
N. tabacum |
B. rapa |
A. indica |
|
Tannins |
|
+ |
+ |
– |
– |
+ |
|
Saponins |
|
+ |
+ |
– |
– |
+ |
|
Coumarins |
|
+ |
– |
+ |
+ |
+ |
|
Quinine |
|
+ |
+ |
+ |
+ |
– |
|
Alkaloids |
|
– |
+ |
+ |
+ |
– |
|
Terpenoid |
|
+ |
+ |
+ |
+ |
+ |
|
Phlabotannins |
|
– |
+ |
+ |
+ |
– |
|
Reducing sugar |
|
– |
– |
– |
+ |
– |
|
Anthracyanine |
|
– |
– |
– |
– |
– |
|
Anthraquinones |
|
– |
– |
– |
– |
– |
|
Glycosides |
|
+ |
– |
– |
+ |
+ |
“+” indicate present, “−” indicate absent
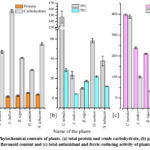 |
Figure 1: Phytochemical contents of plants. (a) total protein and crude carbohydrate, (b) phenolic and flavonoid content and (c) total antioxidant and ferric-reducing activity of plants |
The present study explored the antioxidant properties of plant extracts. All the plants showed concentration-dependent antioxidant activity. Fig. 2 shows the DPPH free radical scavenging activity of all the plants. Overall, C. tamala showed the most active scavenging activity of DPPH free radicals. Increased concentration of plant extracts showed increased DPPH scavenging activity. Highest DPPH radical scavenging activity was found in C. tamala (IC50, 27.94µg/mL), followed by O. sanctum (IC50, 88.90µg/mL) and N. tabacum (IC50, 174.78µg/mL). About 94% inhibition was observed in 100µg/mL of C. tamala crude extract. B. rapa (IC50, 1114.36µg/mL) showed the lowest DPPH free radical scavenging activity, while the reference chemical, ascorbic acid, showed strongest antioxidant activity with an IC50 value of 1.07 µg/mL. The average IC50 value for all five plants was found to be 413.57µg/mL.
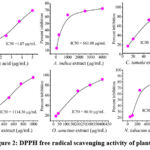 |
Figure 2: DPPH free radical scavenging activity of plants |
For ABTS assay, both C. tamala and A. indica showed the strongest ABTS free radical scavenging activity with almost similar IC50 values of 15.05 and 15.96µg/mL, respectively (Fig. 3). Unlike DPPH assay, N. tabacum showed the weakest ABTS radical scavenging property (IC50, 707.74µg/mL). All the tested plants showed much stronger ABTS radical scavenging activity than DPPH. The mean IC50 value for ABTS assay was 192.96µg/mL, almost two times smaller than the DPPH IC50 value. The reference chemical, ascorbic acid, showed the most potent ABTS scavenging activity with an IC50 value of 0.77µg/mL.
 |
Figure 3: ABTS free radical scavenging activity of plants |
Plants are also known to possess chemicals that exhibit inhibition properties of lipid peroxidation inhibition. The present study also observed dose-dependent lipid peroxidation inhibition properties in all the plants. N. tabacum showed the most potent peroxidation inhibition property among the five plants with IC50 value of 40.23µg/mL, followed by C. tamala and A. indica. Alternatively, B. rapa showed the weakest inhibition property of lipid peroxidation (IC50, 338.91µg/mL). The average IC50 value for lipid peroxidation inhibition of plant extracts was found to be 184.68µg/mL (Fig. 4).
 |
Figure 4: Lipid peroxidation inhibition activity of medicinal plants |
Fig. 5 shows the larvicidal activity of all five plants. Like antioxidant activity, the study observed dose-dependent mortality of the larvae after 24 h treatment. Of five plants, C. tamala showed the most potent larvicidal activity. A. indica and N. tabacum also showed almost similar larvicidal activity. The LC50 value ranged from 3.11 mg/mL to 8.19 mg/mL, with a mean value of 5.26 mg/mL.
 |
Figure 5: Larvicidal activity of medicinal plants against Aedes aegypti mosquito |
Discussion
Plants have been the source of phytocompounds and secondary metabolites having rich medicinal values and are investigated for several medicinal values. They are preferably the second most chosen treatment system against synthetic drugs and have attracted scientific attention since the 19th century. Based on ethnomedicinal values, the present study investigated the phytochemical, antioxidant, and larvicidal properties of five ethnomedicines, namely – C. tamala, A. indica, N. tabacum, B. rapa and O. sanctum. The present study observed that the plant extracts contain substantial phytochemical content and antioxidant properties. Many studies have reported similar results having rich phenolic and flavonoid content in several plants.34,35 Phenolics are widely distributed secondary metabolites in plants with tremendous medicinal values and health benefits. The present study observed considerable quantities of phenolics and flavonoids in all the plants. Highest quantity was reported in C. tamala (147.15 µgGAE/mg extract). In the same way, Bernard et al.36 showed similar results in Cinnamomum zeylanicum and C. osmophloeum leaf extracts.On the contrary, Rahman et al.37 showed a significant difference in phenolic content (2.76mgGAE/g) compared to our result. Silva et al.38 also revealed a considerably lower quantity of phenolic content (8.08±1.83mgGAE/g) in the ethanolic extract of C. triplinerve leaves.38 Presence of phenolics and flavonoid contents is linked to the antioxidant property of plants. In the present study, we observed a significant relationship between phenolics and the antioxidant property of plants. Our study showed highest antioxidant activity in C. tamala among the five plants. Following our study, Prasad et al.39 also reported a very relevant result in C. zeylanicum. Abeysekera et al.40 also showed the leaf and barks of C. zeylanium to possess strong antioxidant activity comparable to our result. The IC50 values of antioxidant study ranged from 27.94 µg/mL to 1114.36 µg/mL for DPPH study averaging 413.57 µg/mL for all five plants. In a similar study, several researchers have revealed almost similar antioxidant properties of C. tamala and C. verum, as reported in the present study.41,42
Like the antioxidant study, our study also revealed potential larvicidal properties of all five plants. In the same way, Das et al.43 also showed that the plant roots of Andrena saccata and leaves of A. squamosa had the most larvicidal activity against Culex quinquefasciatus and Ae. aegypti larvae with methanol and ethanol extract. A very similar kind of study and findings were seen in Iqbal et al.44 with C. tamala and O. basilicum leaves, where LC50 value of C. tamala was 1.48mg/mL, and O. basilicum was found to be 5.32mg/mL on Cu. quinquefasciatus.44 Ullah et al.45 also showed the larvicidal efficacy of Nicotiana tabacum with LC50 value of 17.77ppm in Cu. quinquefasciatus of 3rd instar larvae were observed in their study. Similarly, the aqueous extract of A. indica seed was investigated, and it found that doses up to 1.0 – 5.0mg/mL have strong larvicidal activity with almost 77 – 99% mortality of Cu. quinquefasciatus.46 Komolamisra et al.47 tested 84 ethnomedicinal plants of Thiland, where C. rhyncophyllum showed considerably better larvicidal activity with an LC50 value of 188.64 mg/mL. The presence of high phenolic content and strong antioxidant molecules might have improved the larvicidal property of Cinnamomum tamala on Aedes aegypti larvae showing the most effective larvicidal activity compared to other plants. However, further study needs to be carried out to explore the bioactive phytocompounds responsible for larvicidal activity and the molecular mode of action.
Conclusion
Traditional uses and ethnomedicinal values of the fringe villages of Manas National Park of Assam, India, were the primary basis of the present study. The study observed that traditional ethnomedicinal practices have a scientific background and merit. The present study approves the traditional faiths behind the insecticidal properties of A. indica, B. rapa, O. sanctum, C. tamala, and N. tabacum. With its potent larvicidal activity supported by the presence of rich phenolics, flavonoids, and antioxidant properties, the present study opined that the leaves of C. tamala could be a potential source of insecticidal agents, and further studies may be carried out to explore the molecular mode of action.
Acknowledgment
Authors would like to thank the Department of Zoology, Bodoland University, for providing the necessary infrastructural facilities for carrying out this work. Authors also thank Dr. Sanjib Baruah of Department of Botany Bodoland University for scientifically identifying the plants.
Conflict of Interest
The authors declare no conflicts of interest among the authors.
Funding Sources
There is no financial support from any external sources for this study.
References
- WHO. Vector-borne diseases: Key facts. WHO, Avenue Appia 20
1211, Geneva, Switzerland, 2020. - Neff E, Evans CC, Jimenez Castro PD, Kaplan RM, Dharmarajan G. Drug resistance in filarial parasites does not affect mosquito vectorial capacity. Pathogens, 2020; 10(1): 2.
CrossRef - Souto AL, Sylvestre M, Tölke ED, Tavares JF, Barbosa-Filho JM, Cebrián-Torrejón G. Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: Prospects, applications and challenges. Molecules, 2021; 26(16): 4835.
CrossRef - Chakraborty U, Das H. Antidiabetic and antioxidant activities of Cinnamomum tamala leaf extracts in STZ-treated diabetic rats. Glob J Biotechnol Biochem., 2010; 5(1): 12-18.
- Saxena M, Saxena J, Neema R, Singh D, Gupta A. Phytochemical of medicinal plants. J Pharmacogn Phytochem., 2013; 1(6): 168-182.
- Sharifi-Rad J, Dey A, Koirala N, Shaheen S, El Omari N, Salehi B, Goloshvili T, et al. Cinnamomum species: bridging phytochemistry knowledge, pharmacological properties and toxicological safety for health benefits. Front Pharmacol., 2021; 12: 600139.
CrossRef - Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci., 2008; 4(2): 89-96.
- Swargiary A, Nath P, Basumatary B, Brahma D. Phytochemical, antioxidant, and trace element analysis of anthelmintic plants of North-East India. Int J Pharm Sci., 2017; 9(9): 228-232.
CrossRef - Swargiary A, Daimary D, Roy MK, Haloi D, Ramchiary B. Evaluation of phytochemical properties and larvicidal activities of Cynodon dactylon, Clerodendrum viscosum, Spilanthes acmella and Terminalia chebula against Aedes aegypti. Asian Pac J Trop Med., 2019; 12(5): 224-231.
CrossRef - Maithani A, Parcha V, Pant G, Dhulia I, Kumar D. Azadirachta inidica (neem) leaf: a review. J Pharm Res., 2011; 4(6): 1824-1827.
- Ogebuewu IP, Odoemenam VU, Obikaonu HO, Opara MN, Emenalom OO, Uchegbu MC, et al. The growing importance of neem (Azadirachta indica A. Juss) in agriculture, industry, medicine and environment: a review. Research J Med Plant., 2011; 5(3): 230-245.
CrossRef - Islas JF, Acosta E, Zuca G, Delgado-Gallegos JL, Moreno-Treviño MG, Escalante B, Moreno-Cuevas JE. An overview of neem (Azadirachta indica) and its potential impact on health. J Funct Foods., 2020; 74: 104171.
CrossRef - Lee YH, Choo C, Waisundara VY. Determination of the total antioxidant capacity and quantification of phenolic compounds of different solvent extracts of black mustard seeds (Brassica nigra). Int J Food Prop., 2015; 18(11): 2500-2507.
CrossRef - Al-Snafi AE. The pharmacological importance of Brassica nigra and Brassica rapa grown in IRAQ. J Pharm Biol., 2015; 5(4): 240-253.
- Brandis, D. Indian Trees: an account of trees, shrubs, woody climbers, bamboos and palms indigenous or commonly cultivated in the British Indian Empire. Bishen Singh Mahendra Pal Singh Dehra Dun India. 1998. pp. 533.
- Showkat RM, Mohammed A, Kapoor R. Chemical composition of essential oil of Cinnamomum tamala Nees and Eberm leaves. Flavour Fragr J., 2004; 19(2): 112–114.
CrossRef - Rawat A, Mali RR, Saini AK, Chauhan PK, Singh V, Sharma P. Phytochemical properties and pharmacological activities of Nicotiana tabacum: A review. Indian J Pharm Biol Res., 2013; 1(2): 74-82.
CrossRef - Mondal S, Mirdha BR, Mahapatra SC. The science behind sacredness of tulsi (Ocimum sanctum Linn.) Indian J Physiol Pharmacol., 2009; 53(4): 291–306.
- Pattanayak P, Behera P, Das D, Panda SK. Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharmacogn Rev., 2010; 4(7): 95–105.
CrossRef - Seidel V. Initial and bulk extract. In: Satyajit D, Sarker SD, Latif Z, Gray AI (Ed), Natural product research, 2nd Ed, Humana Press, Totowa, New Jersey, (2005).
- Swargiary A, Brahma K, Boro T, Daimari M, Roy MK. Study of phytochemical content, antioxidant and larvicidal property of different solvent extracts of Clerodendrum infortunatum and Citrus grandis. Ind J Tradit Know., 2021; 20(2): 329-334.
CrossRef - Mujeeb F, Bajpai P, Pathak N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. BioMed Res Int., 2014; 11: 1-11.
CrossRef - Roghini R, Vijayalakshmi K. Phytochemical screening, quantitative analysis of flavonoids and minerals in ethanolic extract of Citrus paradisi. Int J Pharm Sci., 2018; 9(11): 4859-4864.
- Sadasivam S, Manickam A. Biochemical Methods. 3rd ed. New Age International: New Delhi. 2008.p.8.
- Lowry OH, Rosebrough NJ, Farr Al, Randall RJ. Protein Measurement with the Folin phenol reagent. J Biol Chem., 1951; 193(1): 265-275.
CrossRef - Iloki S, Lewis L, Rivera G, Gil A, Acosta A, Meza C, et al. Effect of maturity and harvest season on antioxidant activity, phenolic compounds and ascorbic acid of Morinda citrifolia L. (Noni) grown in Mexico. Afr J Biotechnol., 2013; 12(29): 4630-4639.
- Ordonez AAL, Gomez JD, Vattuone MA, Isla MI. Antioxidant activities of Sechium edule (Jacq) Swartz extract. Food Chem., 2006; 97(3): 452-458
CrossRef - Huda-Faujan N, Norrakiah AS, Babji AS. Antioxidant activity of plants methanolic extract containing phenolic compounds. Afr J Biotechnol., 2009; 8(3): 484-489.
- IIoki-Assanga SB, Lewis-Lujan LM, Lara-Espinoza CL, Gil-Salido AA, Fernandez-Angulo D, Rubio-Pino JL, et al. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron colifornicum. BMC Res Notes. 2015; 8(1): 396.
CrossRef - Mamta, Mehrotra S, Amitabh, Kirar V, Vats P, Nandi SP, et al. Phytochemical and antimicrobial activities of Himalayan Cordyceps sinensis (Berk.) Sacc. Indian J Exp Biol., 2015; 53(1): 36-43.
- Re R, Pellergini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med., 1999; 26(9-10): 1231-1237.
CrossRef - Ohkawa H, Ohsini N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem., 1979; 95(2): 351-358.
CrossRef - WHO. Guidelines for laboratory and field testing of mosquito larvicides, WHO, Avenue Appia 201211, Geneva, Switzerland, 2005.
- Martín C, Moure A, Martin G, Carrillo E, Domínguez H, Parajó JC. Fractional characterization of jatropha, neem, moringa, trisperma, castor and candlenut seeds as potential feedstocks for biodiesel production in Cuba. Biomass Bioenerg., 2010; 34(4): 533-538.
CrossRef - Banik B, Kakoti S, Saikia M, Das J. Evaluation of nutritional parameters in medicinal plants of Assam. Curr Trends Biotechnol Pharm., 2018; 5(1): 34-43.
- Bernard D, Asare IK, Ofosu DO, Daniel GA, Elom SA, Sandra A. The effect of different drying methods on the phytochemicals and radical scavenging activity of Ceylon cinnamon (Cinnamomum zeylanicum) plant parts. European J Med Plants., 2014; 4(11): 1324-1335.
CrossRef - Rahman M, Khatun A, Islam MM, Akter MN, Chowdhury SA, Khan MA et al. Evaluation of antimicrobial, cytotoxic, thrombolytic, diuretic properties and total phenolic content of Cinnamomum tamala. Int J Green Pharm., 2013; 7(3): 236-2343.
CrossRef - Silva AF, Pezenti L, Abel MC, Yunes RV. Antioxidant activity and quantification of phenols, flavonoids and total tannins of Cinnamomum triplinerve (Lauraceae). Ciência e Natura., 2019; 11(41): e34.
CrossRef - Prasad KN, Yang B, Dong X, Jiang G, Zhang H, Xie H, Jiang Y. Flavonoid contents and antioxidant activities from Cinnamomum species. Innov Food Sci Emerg Technol., 2009; 10(s4): 627-632.
CrossRef - Abeysekera WP, Premakumara GA, Ratnasooriya WD. In vitro antioxidant properties of leaf and bark extracts of Ceylon cinnamon (Cinnamomum zeylanicum Blume). Trop Agric Res., 2013; 24(2): 128–138.
- Kalauni SK, Maharjan R, Pathak I, Khadayat K, Niraula M, Thapa P. Different Crude Extracts of Cinnamomum tamala with antioxidant and antibacterial capabilities. Amrit Research Journal, 202; 2(01): 68-74.
CrossRef - Mathew S, Abraham TE. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem Toxicol., 2006; 44(2): 198-206.
CrossRef - Das NG, Goswami D, Rabha B. Preliminary evaluation of mosquito larvicidal efficacy of plant extracts. J Vector Borne Dis., 2007; 44(2): 145-148.
- Iqbal J, Ishtiaq F, Alqarni AS, Owayss AA. Evaluation of larvicidal efficacy of indigenous plant extracts against Culex quinquefasciatus (Say) under laboratory conditions. Turk J Agric For., 2018; 42(3): 207-215.
CrossRef - Ullah Z, Ijaz A, Mughal TK, Zia K. Larvicidal activity of medicinal plant extracts against Culex quinquefasciatus Say. (Culicidae, Diptera). Int J Mosq Res., 2018; 5: 47-51.
- Tandon P, Sirohi A. Assessment of larvicidal properties of aqueous extracts of four plants against Culex quinquefasciatus larvae. Jordan J Biol Sci., 2010; 3(1): 1-6.
- Komalamisra N, Trongtokit Y, Rongsriyam Y, Apiwathnasorn C. Screening for larvicidal activity in some Thai plants against four mosquito vector species. Southeast Asian J Trop Med Public Health, 2005; 36(6): 1412.







