Manuscript accepted on :24-08-2023
Published online on: 18-09-2023
Plagiarism Check: Yes
Reviewed by: Dr. Shiv Gunjegaonkar
Second Review by: Dr. Nicolas Padilla
Final Approval by: Dr. Anton R Kiselev
Eman M. El-Sayed1 , Khadiga S Ibrahim2
, Khadiga S Ibrahim2  and Eman Refaat Youness3*
and Eman Refaat Youness3*
1Nutrition and Food Sciences Department, National Research Centre, Dokki, Giza, Egypt
2Environmental and Occupational Medicine Department, National Research Centre, Dokki, Giza, Egypt
3Medical Biochemistry Department, Medical Researches and, Clinical Studies National Research Centre, Dokki, Giza, Egypt.
Corresponding Author E-mail: hoctober2000@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2708
Abstract
Patients with severe Coronavirus disease 2019 (COVID-19) experience thrombotic complications, cytokine storm, immune disorder, hypoxia, numerous disturbances in iron homeostasis, and increased oxidative stress. In addition to the appearance of the classic onset symptoms of COVID-19 which are cough fever and chest pain. Dietary supplements or nutraceuticals can be used as an adjunct treatment to improve patients' recovery. Omega 3-polyunsaturated fatty acids (ω-3PUFAs) in particular, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) exhibit anti-inflammatory, anticoagulant, and immunomodulatory properties that, when combined with the appropriate therapeutic intervention, may improve patient outcomes. Upon oxidation, EPA and DHA produce specialized pro-resolving lipid mediators (SPMs) that induce resolution of inflammation through inhibiting neutrophil migration, enhancing macrophage phagocytosis, and decreasing proinflammatory mediators which are risk factors for COVID-19 and increasing its severity. Moreover, ω-3PUFAs have many pathways to ameliorate various metabolic changes induced by viral infection. In this review, we attempted to summarize the available literature to understand the actual role of ω-3PUFAs that might improve or protect against COVID-19 and to determine whether it is possible to administer ω-3PUFAs as a co-therapy with conventional COVID-19 treatments.
Keywords
COVID-19, inflammation; Immunity; Iron homeostasis; Pro-resolvins ω-3 PUFAs
Download this article as:| Copy the following to cite this article: El-Sayed E. M, Ibrahim K. S, Youness E. R. Omega-3 Polyunsaturated Fatty Acids as Adjunctive Therapy for COVID-19 Management: Review. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: El-Sayed E. M, Ibrahim K. S, Youness E. R. Omega-3 Polyunsaturated Fatty Acids as Adjunctive Therapy for COVID-19 Management: Review. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/3PF0yeG |
Introduction
The coronavirus disease 2019 (COVID-19) originated in Wuhan, China in 2019. A novel member of the coronavirus family was identified and caused this pandemic which is severe acute respiratory syndrome coronavirus-2 (SARS-COV2). It is worth noting that person-to-person transmission is the main cause of the rapid spread over the world. Until recently, the number of positive and death-related cases is still growing all over the world 1. COVID-19 common symptoms at the onset of infection include cough, fever, dyspnea, and myalgia. Besides, intestinal, hepatic, dermatological, and neurological manifestations may also present in COVID-19 patients 2. COVID-19 endangers elderly people, patients with chronic diseases such as diabetes, hypertension, or cardiovascular diseases, and individuals with compromised immunity. COVID-19 could cause sepsis, septic shock, and multiple organ dysfunctions. Multiple disturbances in blood tests such as leukopenia, lymphopenia, raised hepatic enzyme activities, D-dimer level, and many abnormalities in tomography of the chest 3. There is a strong correlation between our immune response and the progression of COVID-19. Various types of immune cells and inflammatory markers have been implicated in the disease process 4,5. In this context, Wongpostulated that inflammation is a crucial feature of COVID-19 outcomes where an overwhelming of cytokines (cytokine storm) may lead to mortality 6. Moreover, many investigators have shown that alterations in iron metabolism like hypoferremia, hyperferritinemia, and hepcidin dysregulation may contribute to multiple organ failures in COVID-19 7, 8. On the other hand, some researchers have postulated that the presence of iron deficiency anemia (IDA) may increase both the severity of COVID-19 and the mortality rate 9, 10. But in fact, it is still a matter of debate if iron deficiency contributes to COVID-19 pathophysiology and its outcomes 11.
Omega-3 polyunsaturated fatty acids (ω-3PUFAs) are necessary nutrients. They include EPA DHA and α- linolenic acid (ALA). EPA and DHA are the most important for human health. They should be obtained from foods because the body can only produce a limited amount of them by converting ALA. EPA and DHA can be found in fish, microalgae, and fungi. Meanwhile, soybeans and flaxseed are good sources of ALA. ω-3PUFAs have several health benefits. They can reduce inflammation in inflammatory conditions and chronic diseases like cardiovascular and neurological ones 12. They have immunosuppressive effects in critical illnesses 13. Chang 14 added that ω-3PUFAs have a marked potential for modulating the various symptoms of COVID-19. The molecular mechanisms responsible for ω-3PUFAs health benefits effects may include the production of pro-resolving mediators that modulate and resolve inflammatory responses 15. Herein we tried to summarize the hypotheses and trials that explained how ω-3PUFAs could ameliorate the serious metabolic disorders that accompanied covid-19 especially, on iron homeostasis and inflammation.
Iron homeostasis and COVID-19
Iron (Fe) is an important transition metal that can participate in oxidation-reduction reactions by accepting and donating electrons. It has an essential role in many biological processes. Also, iron in heme is incorporated into multiple proteins such as hemoglobin, cytochrome proteins, nitric oxide synthases, and myoglobin. Iron complexes with sulfur are in respiratory complexes, coenzyme Q10, and DNA primase. The presence of iron-sulfur complex in DNA primase, an essential enzyme for DNA replication, contributes to enzyme activity 16. Also, iron-sulfur clusters in CoQ10 are responsible for its redox activity 17. Therefore, iron is essential for vital cellular functions like oxygen transport, nucleic acid metabolism, xenobiotic metabolism, and cell signaling 18. Red blood cells contain the majority of Fe in the form of hemoglobin. Iron is bound to transferrin and circulates in the bloodstream. It is stored mainly in macrophages and hepatocytes. Meanwhile, all other body cells contain smaller quantities of iron for their essential processes. The liver plays an important role in iron homeostasis through its important synthetic, regulatory, and storing functions 19. The bone marrow is the primary iron consumer in the body, as it is the site of erythropoiesis, whereas the reticuloendothelial system is responsible for iron recycling via erythrocyte phagocytosis. The circulating iron level is about 2-4 mg 20. Notably, the diet provides 1-2 mg of iron and its excretion is minimal. Figure (1) shows systemic iron homeostasis 21.
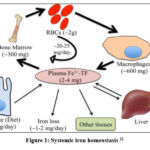 |
Figure 1: Systemic iron homeostasis 21 |
Dietary iron is absorbed as nonheme, heme, and ferritin. Excess iron is stored in the form of ferritin in an inert form to control the production of reactive species. Systemic delivery of iron is performed by ferroportin in the ferrous form (Fe2+) which is oxidized to ferric (Fe3+) by three ferroxidases which are ceruloplasmin, hephaestin, and zyklopen to be loaded on transferrin, the main carrier for iron in the bloodstream 22. However, ferritin, heme, and hemoglobin also circulate in plasma. The iron hormone hepcidin is the major regulatory factor at the systemic level. Hepcidin limits the entry of iron into circulation by binding to ferroportin and inducing its degradation. It was noted that stimulating hepcidin expression inhibits iron absorption 23. Consequently, iron deficiency anemia may be the result.
Anemia is a common global disorder. It is a public health problem that affects both developing and developed countries with significant clinical consequences for human health and economic development. The occurrence of anemia is the magnitude of various red blood cell (RBC) defects. For instance, RBC production defects result in aplastic anemia, maturation defects cause megaloblastic anemia and abnormal hemoglobin (Hb) synthesis leads to IDA.
Malnutrition affects one-half of the world’s population and leads to diseases especially related to iron. Women, infants, and children are the most vulnerable people affected by such deficiencies, especially in developing countries like Egypt. IDA is the major anemic type that affects about one-fourth of the world population and accounts for one-half of anemic cases. The World Health Organization (WHO) defines anemia in non-pregnant women as having Hb concentration of less than 12g/dl. Mild to moderate anemia may be asymptomatic. Meanwhile, some anemic individuals have chronic fatigue, weakness, shortness of breath, headache, pica, hair loss, brittle nails, cold insensitivity, and restless leg syndrome 24. Besides, iron deficiency significantly impairs many immune responses like those regulated by interferon-γ (IFγ) such as T-cell proliferation, natural killer cell activity, and those responses that are down-regulated by interleukin 10 (IL10) like respiratory bursts 25. Moreover, it is documented that iron deficiency disrupts the balance between pro-inflammatory and anti-inflammatory cytokines with concomitant effects on both innate and cell-mediated immunity 26. In addition, IDA markedly influences and decreases cognition, motor, and social-emotional activity in the animal model and human 27-28. Interestingly, iron deficiency during pregnancy affects the genomic profile of the developing hippocampus of the fetus which persists despite iron supplementation 29. Iron induces these changes by affecting neurochemistry, neurometabolic, and neuroanatomy during development. Therefore, proper management of IDA improves the quality of life and significantly reduces symptoms and complications of anemia.
Oral iron is frequently poorly tolerated, with up to 70% of patients reporting gastrointestinal problems; this can make it difficult to stick to a treatment regimen. Furthermore, due to their underlying condition, many individuals will not respond to oral iron. Intravenous iron is increasingly being utilized to replenish iron reserves 30-31. However, IV iron may be undesirable and unsafe in very high doses or when ferritin concentrations are high. IV iron administration may expose patients to toxicity due to increased oxidative stress and ferroptosis. However, the guidelines for treating IDA lack consensus 32. Consequently, a recent strategy for managing anemia is to use functional foods as iron adjuvants to enhance their bioavailability and reduce their side effects 33.
The prevalence of anemia was strongly associated with the severity of respiratory diseases, poor outcomes, and increased mortality in patients with pneumonia 34-35. Other studies indicate that IDA induces a 2-6 times greater risk of mortality in chronic pulmonary diseases 36-37. A recent meta-analysis demonstrated that anemia is closely associated with a 41% and 33% elevated risk of general mortality, and cardiovascular mortality 38. The same results were obtained when comparing heart failure patients with and without anemia, or stroke patients with and without anemia, anemic patients usually have a higher mortality risk than non-anemic 39. In this context lower hemoglobin levels in patients with heart failure are associated with increased mortality rates 40.
COVID-19 patients have decreased hemoglobin levels, indicating anemia and increased ferritin values 41. Each anemia and hyperferritinemia are strong predictors of mortality as mentioned above 38 especially in pregnant patients 42. Anemia reduces the oxygen delivery to the tissue and therefore it may cause hypoxia and mortality in COVID-19 patients. It should be noted that increased anemia severity is directly proportional to the severity of COVID-19. The respiratory symptoms of COVID-19 in mild (Hb11.6 g/L) and moderate (Hb10.3 g/L) anemic patients are less than in those with severe anemia (Hb7.2 g/L) 10. Ferritin levels are increased in inflammatory cases as an acute phase reactant and as a contributor to the development of a cytokine storm 43. Where the H-chain of ferritin activates macrophages to increase the secretion of inflammatory cytokines; interleukin 6 (IL-6) and tumor necrosis factor (TNFα). The persistence of hyperferritinemia in patients with COVID-19 is accompanied by increased disease severity, poor patient performance, and severe pulmonary deterioration 9. These are the cases that could not overcome the virus. On the other hand, in those patients who recovered from the viral infection, ferritin as an acute phase reactant induces IL-6 production which increases the concentration of hepcidin, the master regulator of iron homeostasis. Hepcidin reduces cellular iron efflux via binding to ferroportin-1 with concomitant iron retention in the macrophages and a reduction in duodenal iron absorption 43. The increase of macrophage iron supports the innate immune system to fight viral invasion by decreasing the bioavailability of iron required for viral replication 44. Meanwhile, other cytosolic iron overload leads to cell death and ferroptosis. Figure (2) demonstrates the interrelationship between anemia and COVID-19.
 |
Figure 2: Anemia and COVID-19 Interrelationship. |
ω -3PUFAs and COVID-19
Functional foods are defined as any food or food ingredients that may provide health benefits beyond their nutritional values. They are similar in appearance to conventional foods, but they demonstrate physiological benefits and may reduce the risk of various diseases. Also, they provide the body with its requirements from different nutrients 25. Some vitamins, minerals, carbohydrates, proteins, fibres, certain fats, edible herbs, and phytochemicals are examples of functional foods.
ω-3PUFAs are derived from essential polyunsaturated fatty acids (EFAs) such as linolenic acid. EFAs play a vital role in promoting human health. Alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) are the three major types of ω-3PUFAs. They are documented to be effective in cases of depression, anxiety, and many mental health disorders 46. Also, they are anti-inflammatory, antithrombotic, immunomodulator, antiarrhythmic, and anti-tumour agents. Recently, they are put in nanoforms using nanotechnology to increase bioactivity and bioavailability and reduce their oxidative deterioration 47. ω-3PUFAs are precursors of some bioactive compounds and exert multifaceted roles in enhancing membrane fluidity, cell signalling, receptor functioning, and preventing oxidative damage and inflammation 48. Fish oil contains DHA and EPA which may help to reduce cardiovascular mortality, the prevalence of some cancers, and inflammatory diseases 49. In addition, flaxseed oil is a natural source of ALA (about 56%) which is a precursor to EPA and DHA and has potential anti-inflammatory 50,51 and antiviral activities 52.
ω-3PUFAs and anaemic COVID-19 patients
It is noted that iron affects ω -3PUFAs metabolism via iron-dependent hepatic desaturases, which convert essential fatty acids to long-chain polyunsaturated fatty acids (LCPUFAS), as well as iron-dependent cyclooxygenase (COX) and lipoxygenase (LOX), which participate in the synthesis of eicosanoids from LCPUFAS 53-54. On the other side, ω-3PUFAs can affect iron metabolism. They are extensively used as adjunctive therapy for anaemia treatment 48, 55. Therefore, ω-3PUFAs can be used to manage anemia associated with COVID -19 (9, 10). It is worth noting that ω-3PUFAs supplementation can change the n6 to n3 ratio which may modulate inflammatory mediators. Furthermore, they participate in the production of anti-inflammatory eicosanoids, which inhibit the production of pro-inflammatory eicosanoids through competitive inhibition. Consequently, prostaglandinE2 (PGE2) concentration is limited. Inhibiting PGE2 reduces the production of IL-6, IL-1β, and TNF-α, which may mediate the inflammation associated with anaemia 56. Okpala 57 and Khan 58 demonstrated that ω-3PUFAs could reduce the number of crises and haemolysis in sickle anaemia by reducing inflammation. Moreover, it was noticed that ω-3PUFAs reduced ferritin, an acute-phase protein, in anaemic haemodialysis patients. In this case, ferritin was more indicative of inflammation than iron stores 48. Besides, the anti-inflammatory properties of ω-3PUFAs, they resemble a structural cornerstone in RBCs. Premenopausal women having IDA were found to have a lower ω-3PUFAs level in the erythrocyte membrane than premenopausal women without anaemia 59-60. As a consequence, IDA is characterized by decreased RBCs deformability due to anomalies in the structure and function of the erythrocyte membrane and hypochromia 61. Also, ω-3PUFAs membrane deficiency exposes RBCs to oxidative stress. RBCs of rats supplemented with fish oil (0.4g/kg/day) had increased catalase activity and reduced malondialdehyde (MDA) and nitric oxide (NO) levels 62.
Pregnant women were given 400ml of DHA-fortified milk per day in two doses of 200ml each.
The duration of the intervention is from the 28th week of pregnancy to the delivery. The iron concentration in this woman’s plasma and umbilical cord vessels was significantly higher. Besides, the expression of key genes (divalent metal transporter 1 (DMT1), Ferroportin 1 (FPN1), Transferrin 1 Receptor (TfR1), and hepcidin antimicrobial peptide 1 (Hamp1)) and proteins involved in iron metabolism were up-regulated. ω-3PUFAs supplementation, also enhanced iron transfer and boosted neonates’ iron stores 63. The increase of RBCs iron by ω-3PUFAs in those women may be attributed to the up-regulation of DMT-1(divalent metal transporter 1) mRNA expression which enhances the uptake of iron by erythrocytes. This effect was also seen in inflamed rats and was attributed to high erythrocyte turnover 64. Figure (3) shows the pathways of the anti-anaemic effects of ω-3PUFAs
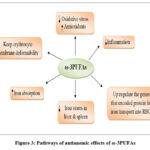 |
Figure 3: Pathways of antianemic effects of ω-3PUFAs |
Anti-inflammatory, anticoagulant, and antidepressant activities of ω-3PUFAs in COVID-19 patients
COVID-19 patients had various complications after two weeks of infection. They may have acute respiratory distress syndrome (ARDS) and kidney, liver, or other organ failure depending on host immunity and the viral load. In addition, if the virus reaches the alveoli, it will strongly replicate and induces the adaptive immune response for cytokine storm. Moreover, the virus increases platelet aggregation with a concomitant increase in blood coagulation 65-66. On the other hand, mood disturbance was documented to be associated with COVID-19 15.
There are global efforts underway to develop and implement a variety of medications and vaccines to combat COVID-19. However, the consumption of functional foods may have the potential to relieve symptoms or aid in the treatment of COVID-19 patients. Vitamins D, C, and E, selenium, zinc, and ω-PUFAs act against COVID-19 by boosting immunity 67. The higher levels of circulating PUFAs, which protect against severe COVID-19, omega-3 PUFAs, particularly DHA and EPA, were linked to lower COVID-19 susceptibility 68, 69. Potential evidence has shown that ω-3PUFAs could counteract COVID-19 through their antidepressant, anti-inflammatory, and anticoagulant, activities 15, 70. Both EPA and DHA are oxidized via COX, LOX, or cytochrome P450, and specialized pro-resolving mediators (SPMs) like resolvins, protectins, and maresins are produced. These metabolites have an important immune-regulating function 71. SPMs play an essential direct role in inflammation resolution via inhibition of the neutrophil migration, activation of macrophage phagocytosis of apoptotic neutrophils, and also via suppression of pro-inflammatory chemokines and cytokines 72-73. In general, EPA and DHA and their metabolites can modulate the immune response and its function in many ways. They could inhibit leukocyte chemotaxis, disruption of lipid rafts, reduction of adhesion molecule expression and leukocyte-endothelial adhesive interactions, inhibition of nuclear factor- kappa B (NK-κB), activation of anti-inflammatory transcription factors like peroxisome proliferator-activated receptor gamma (PPARγ), and also binding to G-protein-coupled receptor(GPCR) 74. Furthermore, they and their metabolites can modulate migration, increase phagocytotic ability, and decrease both ROS and cytokine production of macrophages and neutrophils. Also, they ameliorate the T cell activation by altering the activation of antigen-presenting cells (APCs) such as macrophages and dendritic cells. Stimulation of immunoglobulin M (IgM) is significantly increased by ω-3PUFAs 75.
It is worth noting that the inhibition of cytokine storm especially of IL-6, the main cause of mood disorders in COVID-19 patients will lead to mood improvement by ω-PUFAs and their metabolites. Also, the alteration of the hypothalamus-pituitary-adrenal axis and the modulation of neurotransmission via changing lipid rafts by ω-3PUFAs may improve mood disturbances accompanied by COVID-19 70.
Coagulopathy is usually found in severe cases of COVID-19 66. Recent research indicated that SARS- COV infected patients, those non-survivors had markedly higher levels of D-dimer and fibrin degradation products, as well as a longer prothrombin time compared to survivors 76. Dietary administration of DHA and EPA may alter platelet aggregation and their lipid membrane phospholipids composition; consequently, fish oil may alter the progression and thrombotic complications in COVID-19 77. Wander and Patton 78 hypothesized that a moderate fish diet could have a moderately positive effect on platelet aggregation, increasing bleeding time and elevating the EPA content of the platelet fatty acids. This could be explained by the action of EPA and DHA on COX-1 and LOX-12 with concomitant reduction of both thromboxane release and platelet aggregation 79.
It should be noted that the dysregulation of human lipid metabolism is associated with the increased spread of coronavirus. Linolenic acid supplementation significantly reduced viral replication by correcting the linolenic to arachidonic acid ratio 52 and by inhibiting virus entry through the Angiotensin-Converting Enzyme 2 (ACE2) receptor 80, 81. Based on the above-mentioned effects of ω-3PUFAs, Arnardottir 82 tried intravenous ω-3PUFAs for elderly hospitalized COVID-19 patients and reported that this intervention seems to possess positive effects on the immune response to cope with the virus. Moreover, Doaei 81 agreed with Arnardottir 82, they used a fortified formula with ω-3PUFAs for critically ill patients infected with COVID-19. The survival rate of patients was increased by one month and their biochemical parameters were improved as a control. Also, Asher 83 postulated that the levels of EPA and DHA in the RBCs may be inversely associated with the risk of death from COVID-19. Figure (4) depicts the various pathways through which ω -3PUFAs may improve COVID-19 prognosis.
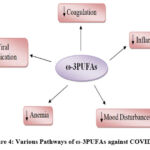 |
Figure 4: Various Pathways of ω-3PUFAs against COVID-19 |
Adverse effects of ω 3PUFAs
However, some studies documented that high doses of EPA and DHA can increase susceptibility to viral infection 84. Also, both of them could replace arachidonic acid in phospholipids of the cell membrane that are prone to oxidative damage when there is an increase in ROS 85. Besides, some evidence suggests that chronic ω-3PUFAs administration may increase the risk of some cancers but the obtained results are conflicting 86. Therefore, the guideline emphasized that patients administered, ω3 fatty acids (especially high doses), should be closely monitored for gastrointestinal and dermatological adverse effects. Also, the guideline further suggested a clinical examination before ω-3PUFAs prescription, EPA/DHA ratio should be more than 2 and one dosage should be 1-2g of net EPA. On the other hand, the US Department of Health and Human Services National Institutes of Health Office of Dietary Supplements (USdhhs) recommended that the daily intake of EPA + DHA dose of up to 3g/day is potentially safe. Moreover, the European Food Safety Authority (EFSA) permitted that chronic consumption of ω-3PUFAs at doses of up to 5g/day is safe 87.
Conclusion
In conclusion, we hypothesized here that alterations in iron homeostasis have a critical potential role in the severity, progression, outcome, multiple organ dysfunction, and mortality of COVID-19 patients. To date, no conventional medicines have been developed to combat COVID-19 except for the recently developed vaccines.
ω- 3PUFAs may be useful as an adjuvant in the treatment of COVID-19 and its associated anemia. In anemic corona patients, PUFAs may increase iron bioavailability in a variety of mechanistic ways including increased absorption, up-regulating genes that help iron transport into RBCs, decreasing oxidative stress and inflammation, and maintaining erythrocyte membrane fluidity. Other ω-3PUFAs pathways for modulating COVID-19 symptoms are to decrease clotting, viral replication, and inflammation, and improve mood disorders. The effects of ω-3PUFAs mentioned above are achieved by ω-3PUFAs themselves as well as their important metabolites SPMs such as resolvins, protectins, and maresins. However, ω-3PUFAs supplementation should be done in controlled doses with constant monitoring of the patient’s condition. Excessive doses of ω-3PUFAs may have some adverse effects.
Conflict of interest
The authors declared no conflict of interest.
References
- Sanche S., Lin Y., Xu C., Romero-Severson E., Hengartner N., Ke R., High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerging Infectious Diseases, 2020, 26 (7), 1470-1477.
- Jiang F., Deng L., Zhang L., Cai Y., Cheung C.W., Xia Z., Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). General Internal Medicine, 2020, 35 (5), 1545-1549.
- Lake M.A., What we know so far: COVID-19 current clinical knowledge and research. Clinical Medicine, 2020, 20(2), 124-127.
- Tan M., Liu Y., Zhou R., Deng X., Li F., Liang K., Shi Y., Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology, 2020, 160(3), 261-268. doi: 10.1111/imm.13223.
- Qin C,, Zhou L,, Hu Z,, Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S., Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clinical Infectious Diseases, 2020, 71,762–768.
- Wong R.S.Y., Inflammation in COVID-19: from pathogenesis to treatment. International Journal of Clinical and Experimental Pathology, 2021, 14(7), 831-844.
- Edeas M., Saleh J., Peyssonnaux C., Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? International Journal of Infectious Diseases, 2020, 97, 303–305.
- Phua J., Weng L., Ling L., Egi M., Lim C.M., Divatia J.V., Shrestha B.R., et al.; Asian Critical Care Clinical Trials Group, Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respiratory Medicine, 2020, 8(5), 506–517.
- Sonnweber T., Boehm A., Sahanic S., Pizzini A., Aichner M., Sonnweber B., et al., Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: a prospective observational cohort study. Respiratory Research , 2020, 21(1), 276. doi: 10.1186/s12931-020-01546-2.
- Taneri P.E., Gómez-Ochoa S.A., Llanaj E., Raguindin P.F., Rojas L.Z., Roa-Díaz Z.M., et al., Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. European Journal of Epidemiology, 2020, 35(8), 763-773.
- Cavezzi A., Troiani E., Corrao S., COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clinical Practice, 2020, 10(2),1271.
- Molfino A., Amabile M.I., Monti M., Muscaritoli M., Omega-3 Polyunsaturated Fatty Acids in Critical Illness: Anti-Inflammatory, Proresolving, or Both? Oxid Med Cell Longev, 2017, 5987082 (2017).
- Manzanares W, Langlois PL, Hardy G., Intravenous lipid emulsions in the critically ill: an update. Current Opinion in Critical Care, 2016, 22(4), 308–315.
- Chang J.P., Pariante C.M., Su K.P., Omega-3 fatty acids in the psychological and physiological resilience against COVID-19. Leukot Essent Fatty Acids, 2020, 161,102177.
- Ishihara T., Yoshida M., Arita M., Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. International Immunology, 2019, 31(9),559–567.
- Weiner BE, Huang H, Dattilo BM et al., An iron-sulfur cluster in the C-terminal domain of the p58 subunit of human DNA primase. Journal of Biological Chemistry, 2007, 282(46), 33444-33451.
- Lippard S.J., Berg J.M., Principles of Bioinorganic Chemistry. University Science Books, Mill Valley, California., 1995, 23(2), 115.
- Evstatiev R., Gasche C., Iron sensing and signalling. Gut, 2012, 61(6), 933-952.
- Yiannikourides A., Latunde-Dada G.O., A short review of iron metabolism and Pathophysiology of iron disorders. Medicines,2019, 6, 85.
- Gao G, Li J, Zhang Y, Chang YZ., Cellular iron metabolism and regulation. Adv Exp Med Biol, 2019, 1173:21-32.
- Dev S., Babitt J.L., Overview of iron metabolism in health and disease. Hemodia International, 2017, 21 (1), S6-S20.
- Wierzbicka D., Gromadzka G., Ceruloplazmina, hefajstyna i cyklopen:trzy multimiedziowe oksydazy uczestniczące w metabolizmie żelaza u człowieka [Ceruloplasmin, hephaestin and zyklopen: the three multicopper oxidases important for human iron metabolism]. Postepy higieny i medycyny doswiadczalnej (Online), 2014, 68, 912–924.
- De Domenico I., Ward D.M., Kaplan J., Hepcidin regulation: ironing out the details. Journal of Clinical Investigation, 2007, 117(7), 1755-8.
- McLean E., Cogswell M., Egli I., Wojdyla D., de Benoist B., Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutrition, 2009, 12(4), 444-54.
- Nairz M., Haschka D., Demetz E., Weiss G., Iron at the interface of immunity and infection. Frontiers in Pharmacology, 2014, 5,152.
- Kuvibidila S., warrier RP., Differential effects of iron deficiency and underfeeding on serum levels of interleukin-10, interleukin-12p40, and interferon-gamma in mice. Cytokine, 2004, 26(2), 73-8.
- Beard J.L., Felt B., Schallert T., Burhans M., Connor J.R., Georgieff M.K. Moderate iron deficiency in infancy: biology and behavior in young rats. Behaviors Brain Research, 2006, 170 (2),224-232.
- Lozoff B., Georgieff M.K., Iron deficiency and brain development. Semin Pediatr Neurol, 2006, 13(3),158-165.
- Carlson E.S., Magid R., Petryk A., Georgieff M.K., Iron deficiency alters expression of genes implicated in Alzheimer disease pathogenesis. Brain Research, 2008, 1237, 75-83.
- Jimenez K., Kulnigg-Dabsch S., Gasche C., Management of iron deficiency anemia. Gastroenterology and Hepatology , 2015, 11(4), 241–250.
- DeLoughery T.G., Safety of oral and intravenous iron. Acta Haematology, 2019, 142, 8–12.
- Mansour D., Hofmann A., Gemzell-Danielsson K., A Review of Clinical Guidelines on the Management of Iron Deficiency and Iron-Deficiency Anemia in Women with Heavy Menstrual Bleeding. Advances in Therapy, 2020, 27,1–25.
- Verna G, Sila A, Liso M, Mastronardi M, Chieppa M, Cena H, Campiglia P Iron-Enriched Nutritional Supplements for the 2030 Pharmacy Shelves. Nutrients, 2021, 13(2):378.
- Fan B.E., Chong V.C.L,, Chan S.S.W., Lim G.H., Lim K.G.E., Tan G.B., et al., Hematologic parameters in patients with COVID-19 infection. American Journal of Hematology, 2020, 95(6), E131-E134.
- Wang L., He W., Yu X., Hu D., Bao M., Liu H., Zhou J,, Jiang H., Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. Journal of Infection, 80(6), 639–645(2020).
- Abassi Z., Knaney Y., Karram T., Heyman S.N., The lung macrophage in SARS-CoV-2 infection: A friend or a foe? Frontiers in Immunology, 2020, 11, 1312.
- Hoffmann M., Kleine-Weber H., Schroeder S., et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 2020, 181(2), 271–280.
- 38. Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., et al., Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy , 2020, 75(7), 1730–1741.
- Camaschella C, Nai A, Silvestri L., Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica, 2020, 105(2), 260-272.
- Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q., Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. International Journal of Antimicrobial Agents, 2020, 55(5),105954.
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al., DSC China Medical Treatment Expert Group For Covid 19. Clinical Characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine, 2020, 382,1708-20.
- Gromova O.A., Torshin I. Yu., Shapovalova Yu O., Kurtser M.A., Chuchalin A.G., COVID-19 and iron deficiency anemia: relationships of pathogenesis and therapy. Obstetrics, Gynecology & Reproduction, 2020, 14(5), 644-655.
- Ganz T., Nemeth E., Hepcidin and iron homeostasis.Biochimica et Biophysica Acta, 1823,1434–1443(2012).
- Menshawey R., Menshawey E., Alserr AHK, Abdelmassih A.F., Low iron mitigates viral survival: insights from evolution, genetics, and pandemics—a review of current hypothesis. Egyptian Journal of Medical Human Genetics, 2020, 21(1), 75.
- Boggia R., Zunin P., Turrini F., Functional foods and food supplements. Applied Sciences, 2020, 10, 8538.
- Sun G.Y., Simonyi A., Fritsche K.L., Chuang D.Y., Hannink M, Gu Z, et al., Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot Essent Fatty Acids, 2018, 136, 3-13.
- Ahmad M.Z., Ahmad J., Zafar S., Warsi M.H., Abdel-Wahab B.A., Akhter S., Alam M.A., Omega-3 fatty acids as adjunctive therapeutics: prospective of nanoparticles in its formulation development. Therapeutic delivery, 2020, 11(1), 851-868.
- Gharekhani A, Khatami MR, Dashti-Khavidaki S, Razeghi E, Abdollahi A, Hashemi-Nazari SS, Mansournia MA Potential effects of omega-3 fatty acids on anemia and inflammatory markers in maintenance hemodialysis patients. Daru, 2014, 22(1), 11.
- Goel A., Pothineni N.V., Singhal M., Paydak H., Saldeen T., Mehta J.L., Fish, fish oils and cardioprotection: Promise or Fish Tale? International Journal of Molecular Sciences, 2018, 19(12), 3703.
- Caughey G.E., Mantzioris E., Gibson R.A., Cleland L.G., James M.J., The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. American Journal of Clinical Nutrition, 1996, 63(1), 116-122.
- Parikh M., Maddaford T.G., Austria J.A., Aliani M., Netticadan T., Pierce G.N., Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients, 2019, 11(5), 1171.
- Yan B., Chu H., Yang D., Sze K.H., Lai P.M., Yuan S., et al., Characterization of the Lipidomic Profile of Human Coronavirus-Infected Cells: Implications for Lipid Metabolism Remodeling Upon Coronavirus Replication. Viruses, 2019, 11(1),73.
- Ward R.J., Zucca F.A., Duyn J.H., Crichton R.R., Zecca L., The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurology, 2014, 13(10), 1045–1060.
- Ndayisaba A., Kaindlstorfer C., Wenning G.K., Iron in Neurodegeneration – Cause or Consequence? Frontiers in Neuroscience, 2019, 13,180.
- Baumgartner J., Smuts C.M., Malan L., Kvalsvig J., van Stuijvenberg M.E., Hurrell R.F., Zimmermann MB Effects of iron and n-3 fatty acid supplementation, alone and in combination, on cognition in school children: a randomized, double-blind, placebo-controlled intervention in South Africa. American Journal of Clinical Nutrition, 2012, 96(6),1327-38.
- Watkins B.A., Li Y., Lippman H.E., Feng S., Modulatory effect of omega-3 polyunsaturated fatty acids on osteoblast function and bone metabolism. Prostaglandins Leukot Essent Fatty Acids, 2003, 68(6):387-398.
- Okpala I., Ibegbulam O., Duru A., Ocheni S., Emodi I., Ikefuna A., et al., Pilot study of omega-3 fatty acid supplements in sickle cell disease. APMIS, 2011, 119, 442–448.
- Khan S., Damanhouri G., Jameel T., Ali A., Makki A., Khan et al., Impact of omega-3 polyunsaturated fatty acids on calorie intake and certain anthropometric measurements in children with sickle cell disease in Saudi Arabia. Bioinformation, 2019, 15(3), 189-193.
- Sanghani S.P., Haldankar V.A., Comparative analysis of RBC membrane fatty acids, proteins and glycophorin in patients with heterozygous beta thalassemia and iron deficiency anemia. Indian Journal of Clinical Biochemistry, 2006, 21, 28–33.
- Aktas M., Elmastas M., Ozcicek F., Yilmaz N., Erythrocyte membrane fatty acid composition in premenopausal patients with iron deficiency anemia. Journal of Oleo Science. 2016, 65(3), 225-31.
- Yip R., Mohandas N., Clark M.R., Jain S., Shohet S.B., Dallman P.R., Red cell membrane stiffness in iron deficiency. Blood, 1983, 62(1):99-106.
- Iraz M., Erdogan H., Ozyurt B., Ozugurlu F., Ozgocmen S., Fadillioglu E., Brief communication: omega-3 essential fatty acid supplementation and erythrocyte oxidant/antioxidant status in rats. Annals of Clinical & Laboratory Science, 2005, 35(2), 169–173.
- Diaz-Castro J., Moreno-Fernández J., Hijano S., Kajarabille N., Pulido-Moran M., Latunde-Dada G.O., DHA supplementation: A nutritional strategy to improve prenatal Fe homeostasis and prevent birth outcomes related with Fe-deficiency. J Functional Foods, 2015, 19(A5), 385-393.
- Rodríguez M.C., Sáiz M.P., Mitjavila M.T., A sardine oil-rich diet increases iron absorption but does not compensate the hypoferremia associated with inflammation. Lipids, 2003, 38(8), 821–826.
- Iba T., Levy J.H., Levi M., Thachil J., Coagulopathy in COVID-19. Journal of Thrombosis and Haemostasis 2020, 18(9),2103-2109.
- Asakura H., Ogawa H., COVID-19-associated coagulopathy and disseminated intravascular coagulation. International Journal of Hematology , 2021, 113(1),45-57.
- Shakoor H.J., Feehan A.S, Al Dhaheri H.I., Ali C., Platat L.C., Ismail V., et al., Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturities, 2021, 143, 1–9.
- Sun Y., Chatterjee R., Ronanki A., Ye K., Circulating polyunsaturated fatty acids and COVID-19: a prospective cohort study and Mendelian randomization analysis. Preprint. medRxiv 2022. 02.06.22270562.
- Nursyifa Fadiyah N., Megawati G., Erlangga Luftimas D., Potential of Omega 3 Supplementation for Coronavirus Disease 2019 (COVID-19): A Scoping Review. International Journal of General Medicine, 2022, 15, 3915-3922.
- Yang C.P., Chang C.M., Yang C.C., Pariante C.M., Su K.P., Long COVID and long chain fatty acids (LCFAs): Psychoneuroimmunity implication of omega-3 LCFAs in delayed consequences of COVID-19. Brain Behav Immun., 2022, 103,19-27.
- Ferreira I., Falcato F., Bandarra N., Rauter AP., Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation. Molecules, 2022, 27(5):1677.
- Basil M.C., Levy B.D., Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nature Reviews Immunology, 2016, 16(1), 51-67.
- Lee C.H., Role of specialized pro-resolving lipid mediators and their receptors in virus infection: a promising therapeutic strategy for SARS-CoV-2 cytokine storm.Archives of Pharmacal Research, 2021, 44, 84–98.
- Calder P.C., Nutrition, immunity and COVID-19. BMJ Nutrition, Prevention & Health,2020, 3(1), 74–92.
- Gutiérrez S., Svahn S.L., Johansson M.E., Effects of omega-3 fatty acids on immune cells. International Journal of Molecular Sciences, 2019, 20(20), 5028.
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z., Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Journal of Thrombosis and Haemostasis, 2020, 18,1094–1099.
- Luo H.C., You C.Y., Lu S.W., Fu Y.Q., Characteristics of coagulation alteration in patients with COVID-19. Annals of Hematology, 2021, 100(1),45-52.
- Wander RC, Patton BD Comparison of three species of fish consumed as part of a Western diet: effects on platelet fatty acids and function, hemostasis, and production of thromboxane. American Journal of Clinical Nutrition, 1991, 54(2), 326–333.
- Adili R., Hawley M., Holinstat M., Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat, 2018, 139,10–18.
- Goc A., Niedzwiecki A., Rath M., Polyunsaturated Omega-3 Fatty Acids Inhibit Ace2-Controlled Sars-Cov-2 Binding and Cellular Entry. Scientific Reports, 2021, 11(1),5207.
- Doaei S., Gholami S., Rastgoo S., Gholamalizadeh M., Bourbour F., Bagheri S.E., et al The Effect of Omega-3 Fatty Acid Supplementation on Clinical and Biochemical Parameters of Critically Ill Patients with Covid-19: A Randomized Clinical Trial. Journal of Translational Medicine, 2021, 19(1), 128.
- Arnardottir H., Pawelzik S.C., Sarajlic P. et al., Immunomodulation by intravenous omega-3 fatty acid treatment in older subjects hospitalized for COVID-19: A single-blind randomized controlled trial. Clinical and Translational Medicine., 2022, 12(9), e895.
- Asher A., Tintle N.L., Myers M. et al., Blood omega-3 fatty acids and death from COVID-19: A pilot study. Prostaglandins Leukot Essent Fatty Acids, 2021, 166,102250.
- Mazidimoradi A., Alemzadeh E., Alemzadeh E., Salehiniya H., The effect of polyunsaturated fatty acids on the severity and mortality of COVID patients: A systematic review. Life Sciences, 2022, 299,120489.
- Grundt H., Nilsen D.W., Mansoor M.A., Nordøy A., Increased lipid peroxidation during long-term intervention with high doses of n-3 fatty acids (PUFAs) following an acute myocardial infarction. European Journal of Clinical Nutrition, 2003, 57(6), 793–800.
- Serini S., Calviello G., Long-chain omega-3 fatty acids and cancer: any cause for concern? Current Opinion in Clinical Nutrition and Metabolic Care, 2018, 21(2), 83–89.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Scientific Opinion related to the Tolerable Upper Intake Level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA Journal, 2012, 10 (7), 2815.







