Manuscript accepted on :21-09-2023
Published online on: 30-09-2023
Plagiarism Check: Yes
Reviewed by: Dr. Pranjal Gujarathi
Second Review by: Dr. M Mohan Varma
Final Approval by: Dr. Ian James Martin
Md. Mofazzal Hossain1 , Bidduth Kumar Sarkar1*
, Bidduth Kumar Sarkar1* , Arghya Prosun Sarkar2
, Arghya Prosun Sarkar2 , Maimuna Hasan3, Mst. Sarmin Afroz4
, Maimuna Hasan3, Mst. Sarmin Afroz4
1Department of Pharmacy, Comilla University,Cumilla-3506,Bangladesh
2Department of Pharmacy, Islamic University, Kushtia-7003 Bangladesh.
3Department of Pharmacy, R P Shaha University, Narayanganj-1400, Bangladesh
4Department of Pharmacy, Khulna University, Khulna, Bangladesh.
Corresponding Author E-mail: kumarbidduth@cou.ac.bd
DOI : https://dx.doi.org/10.13005/bpj/2712
Abstract
Monkeypox has recently garnered significant attention as a result of its rapid and simultaneous global dissemination. The objective of this study is to present a succinct overview of the existing literature, while also elucidating the development of the disease in respect to contemporary instances, possible therapeutic approaches, and strategies for preventing infection. To date, a total of 12,261 occurrences have been recorded over a wide range of 76 nations. Monkeypox is a zoonotic disease that has previously been limited to endemic areas in Western and Central Africa. However, there have been isolated outbreaks in other countries, including the United States, that have been linked to the importation of wild animals from Ghana and other affected areas. The current outbreak has seen a significant shift, with human-to-human transmission surpassing all other modes of transmission. This development has raised concerns regarding the potential extension of the outbreak within communities, particularly in cases that may have gone unreported. The observed results may be attributed to the increase in human-to-human transmission subsequent to the cessation of smallpox vaccination, which provided partial immunity against monkeypox. The occurrence of outbreaks beyond the African continent underscores the worldwide importance of the illness. The demographic that exhibits the highest vulnerability to infection is young males who engage in sexual activity with other males. While the existing understanding suggests that the clinical progression of the disease is very moderate, there remain several unresolved inquiries that necessitate additional investigation. These include the possibility of a genital reservoir of the virus in humans and the possibility of airborne transmission.
Keywords
Airborne; Endemic; Immunization; Reservoir; Susceptible; Zoonotics
Download this article as:| Copy the following to cite this article: Hossain M. M, Sarkar B. K, Sarkar A. P, Hasan M, Afroz M. S. Monkeypox Disease: An Updated Review. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Hossain M. M, Sarkar B. K, Sarkar A. P, Hasan M, Afroz M. S. Monkeypox Disease: An Updated Review. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/3LHCZzG |
Introduction
Monkeypox, a viral infection caused by the variola virus, belongs to the orthopoxvirus genus. Zoonosis, sometimes known as the transmission of diseases from animals to humans, include the occurrence of monkeypox1. Poxviruses represent a class of DNA viruses characterized by a substantial number of genes that undergo replication within the cytoplasm of the host cell, as opposed to the nucleus. Furthermore, these viruses exhibit remarkable stability in various environmental conditions2.
The 2022 monkeypox outbreak has garnered substantial international attention because to its impact on multiple nations, including both endemic and non-endemic regions. Over the course of the last two decades, the likelihood of this probable occurrence has increased as a result of various contributing causes. These reasons encompass deforestation, population growth, habitat degradation for animal reservoirs, escalated human mobility, and enhanced worldwide interconnection3. Based on genetic and geographic variation, the virus can be divided into two clades: the Central African (or Congo Basin) clade and the West African clade. The first clade appears to be more virulent, as evidenced by higher documented mortality rates. According to the World Health Organisation (WHO), both the United States and Nigeria have recorded a substantial proportion of infections ascribed to the West African clade. It is worth noting, however, that the Central African clade has a higher overall occurrence. The latter was also discovered during trips to Israel, Singapore, and the United Kingdom4 . One of the most widely recognized instances of a poxvirus outbreak pertains to the smallpox disease in human populations. This affliction was characterized by its highly lethal nature, extreme suffering endured by affected individuals, and profound impact on historical events. The Variola virus, responsible for a death toll exceeding 500 million individuals since 1880, was effectively managed through the utilization of an innovative “Jennerian” vaccine. This vaccine employed the principle of vaccination with a closely related virus known as vaccinia, derived from animals. This approach provided a degree of cross protection against the Variola virus while minimizing the likelihood of severe illness4.
History of outbreak
The monkeypox virus, a member of the orthopoxvirus family, is the causal agent of the very uncommon viral infection known as monkeypox. This particular species is only found in the rainforests of central and western Africa. The virus’s discovery can be traced back to 1958, when it was first discovered in laboratory animals. The presence of the virus was later confirmed in numerous African rodent species through blood sample analysis from animals endemic to the continent5. The first human cases of monkeypox were reported in 1970, when the virus was discovered in people living in isolated African locations. In the Democratic Republic of the Congo, 37 cases of monkeypox were officially registered between 1981 and 1986. From February 1996 to February 1997, a specific region saw a big monkeypox outbreak, raising queries about the storage of smallpox virus samples for comparative examination with closely related viruses such as monkeypox. Between February and August 1996, 71 clinical cases of monkeypox were identified in 13 communities in Zaire, including six deaths6.
Epidemiology
Cases from 10 distinct African states have been identified since 1970. A comprehensive overview of the epidemiological aspects pertaining to these instances during the period spanning from 1970 to 2022 is provided in a review7. The Democratic Republic of the Congo (DRC) experienced the highest number of incidents, with an increase from 38 cases in the period of 1970-1979 to 511 cases in 1990-1999, and a significant surge to 18,788 cases between 2010 and 2019. Nigeria, with a total of 181 cases, ranked as the second most affected country, trailing behind the Republic of Congo with 97 cases, and followed by the Central African Republic with 67 cases. A significant majority of these instances, over 90%, did not possess a history of smallpox immunization. The median age of those affected by the infection shown an increase from 4 years during the 1970s to 21 years during the 2010s. The case fatality rate (CFR), often known as the proportion of fatal cases, was found to be 8.7%. The case fatality rate (CFR) for clade-1 infections was 10.6%, whereas clade-2 infections had a CFR of 3.6%. In the 2010s, the proportion of mortality attributed to children under the age of 10 was 37.5%, in contrast to the collective mortality rate of individuals under the age of 10 during the period spanning from 1970 to 1990. Between the years 2018 and 2021, there were recorded cases in Israel, Singapore, and the UK that did not result in any fatalities. Notably, five of these cases involved individuals who had recently traveled from Nigeria. The identification of monkeypox occurred when an individual originating from the United Kingdom, who had recently completed a trip to Nigeria, was examined on May 6, 20228.
Aetiology
Given that the monkeypox virus is a DNA virus, it is expected that significant and frequent changes to its genetic composition will be less probable. Both humans and animals can transfer diseases to one another9. Natural reservoirs include monkeys, squirrels, Gambian pouched rats, dormice, nonhuman primates, and a variety of other creatures. Humans can become infected through a variety of mechanisms, including bites, scratches, direct physical contact, and the ingestion of raw animal meat. The main mechanisms of human-to-human transmission are the spread of large respiratory droplets, direct physical contact, and contact with infected surfaces or items. In comparison to smallpox, where the secondary attack rate among household contacts varied from 35% to 88%, the present secondary attack rate is under 10%. While the precise relevance of direct sexual transmission is unknown, it is worth mentioning that intimate skin-to-skin and mucosal contact during sexual activity facilitates transmission. Congenital monkeypox has been linked to vertical transmission from mother to foetus or infant10. Individuals frequently engage in outdoor sleeping practises in African locations characterised by endemicity, poor resources, and inadequate infrastructure, either directly on the ground or in close proximity to forested areas where the incidence of infected animals is significantly higher. As a result, exposure to faecal matter from infected animals emerges as a significant risk factor11 .
Structure of Monkeypox Virus
The Poxviridae family of viruses is characterized by its substantial dimensions, encapsulated structure, and possession of double-stranded DNA. A high prevalence of these viruses is reported in populations of rodents, rabbits, and non-human primates12. Entomopoxvirinae and Chordopoxvirinae are the two subfamilies that make up the Poxviridae family. Viruses that specifically affect insects are included in the subfamily Entomopoxvirinae, whereas viruses that specifically affect vertebrates are included in the subfamily Chordopoxvirinae. Each of the 18 genera that make up the subfamily Chordopoxvirinae contains several viruses having a zoonotic origin as their primary source of origin. Viruses that can spread from animals to people, like the ones that cause monkeypox, are examples of zoonotic transmission13.
This viral strain belongs to the family Poxviridae and the genus Orthopoxvirus. The virus has an enclosed structure with an irregular shape, with additional entities dispersed along its periphery14. Furthermore, the virus’s centre is described as dumbbell-shaped. Poxviruses have a different replication mechanism than other DNA viruses because they usually replicate in the cytoplasm of host cells rather than the nucleus. Poxviruses utilise a significant portion of their internally encoded proteins for replication rather than relying only on cellular proteins. The core region of the poxvirus genome contains genes required for important cellular activities like viral assembly and transcription. During this process, the genes at the viral genome’s termini participate in interactions between the virus and its host organism. The whole Poxviridae family has been sequenced, and 49 of the total 150 genes have been identified. The Chordopoxvirus subfamily, on the other hand, has 90 of these genes. The vast majority of conserved genes are found in the middle region of the genome. Poxviruses, such as Monkeypox, face difficulties in evading host immune responses and spreading rapidly due to their large physical dimensions.
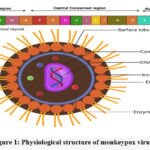 |
Figure 1: Physiological structure of monkeypox virus15. |
Because of their greater exposure to the host’s immune system, foxviruses are more likely to elicit an immune response. Poxviruses have evolved a suite of virulence genes that create chemicals with the ability to modulate the host’s immune response in order to avoid detection16. These proteins are classified as extracellular or intracellular based on how they function. Intracellular proteins include virotransducer proteins, which inhibit the cell’s response to infection, and virostealth proteins, which hide immune recognition molecules such as the Major Histocompatibility Complex.
Reservoirs and Transmission
Contrary to its nomenclature, monkeys do not serve as the reservoir for the monkeypox virus. In reality, humans and monkeys are seen as incidental hosts. It is widely thought that squirrels and Gambian rats constitute the primary reservoirs17. The transmission of MPX from animals to humans is a widely recognized phenomenon, occurring primarily through bites or direct exposure to bodily fluids of infected animals18. The study conducted on the 2003 US outbreak proposed a classification of exposure into two categories: “noninvasive” exposure, which involves coming into contact with an infected animal, and “complicated” exposure, which refers to being bitten by an ill animal. Individuals who experienced difficult exposures demonstrated a greater likelihood of acquiring systemic illness in comparison to those who were exposed noninvasively18. The primary modes of human-to-human transmission commonly observed include the transfer through large respiratory droplets, extended face-to-face contact, close proximity to infectious skin lesions, or direct exposure to bodily fluids. Residing in a shared household, using shared bedding, and ingesting sustenance and beverages from the utensils of an individual who is affected are all instances of contaminated surfaces and objects that have the potential to facilitate the transmission of viral pathogens19.
Demographic Characteristics and Risk Factors
According to a recent comprehensive investigation, it was observed that the weighted average median age of monkeypox infection in Africa exhibited an upward trend between 2010 and 20197. This age parameter experienced an increase from 4 years old in the 1970s to 5 years old in the 1980s. There is a greater degree of influence observed among males7. The prevailing proportion of monkeypox cases, ranging from 80% to 96%, has been observed in individuals who have not had immunization against smallpox. Notably, the epidemic in the United States exhibited the highest documented percentage of cases among vaccinated individuals, amounting to 21%20. The potential association between the incidence of monkeypox within this specific demographic and their absence of prior smallpox immunization has been suggested1. Indeed, a comprehensive examination of the epidemic in Nigeria spanning from September 2017 to April 2018 revealed that it was the most extensive recorded occurrence of monkeypox within the West African region. According to a recent systematic review21, all of the cases included in the analysis involved individuals who engaged in unprotected sexual activity with male partners. The review encompassed cases reported up until June 7, 2022. An alternative hypothesis posits that the transmission of the virus occurs via sexual intercourse. The notion is substantiated by the discovery that engaging in unprotected anal intercourse has a greater likelihood of transmitting sexually transmitted diseases (STDs) compared to engaging in unprotected vaginal intercourse, perhaps leading to a more rapid dissemination of the infection22. The literature search conducted by Zachary and Shenoy encompassed the period from 2000 to 2022, revealing a solitary occurrence of nosocomial transmission of monkeypox in the year 201823. A healthcare worker (HCW) in the United Kingdom (UK) contracted the virus due to a lack of appropriate personal protective equipment (PPE) when coming into contact with patient bedding that was believed to be contaminated24.
Clinical Symptoms
The clinical presentation can be categorized into two distinct periods. The initial stage of the disease is referred to as the invasion phase, alternatively recognized as the feverish stage. This stage often spans a duration of 0 to 5 days and is characterized by the presence of fever, a pronounced headache, lymphadenopathy, back pain, and myalgia, which denotes muscular discomfort. Monkeypox exhibits distinctive characteristics, such as lymphadenopathy, which sets it apart from other illnesses including chickenpox, measles, and smallpox25. The subsequent phase is characterized by the manifestation of skin eruptions, often occurring within a span of 1-3 days following the initial onset of fever. Rather of occurring on the trunk, the rash is commonly found on the face and limbs. According to World (2022), the facial region, including approximately 95% of the total surface area, together with the palms and soles of the hands and feet, exhibit manifestations. The progression of the rash occurs in distinct phases, beginning with the formation of macules, followed by the development of papules, vesicles, and pustules. Ultimately, the affected area forms crusts, which gradually desiccate and exfoliate26.
The number of lesions might vary from a small quantity to a large quantity, perhaps reaching several thousand. The most commonly documented sequelae include conjunctivitis and corneal scarring, nausea and diarrhea, encephalitis, sepsis, and bronchopneumonia26. Once the rashes have subsided, it may take several days to several weeks for a complete restoration of health27. The consequences and risk associated with infections in children. Severe cases of monkeypox primarily affect youngsters, pregnant women, individuals with comorbidities, and those with weakened immune systems, the disease generally follows a mild trajectory18.
Instances of miscarriages and fetal fatalities resulting from the transfer of monkeypox via the placenta have been documented. The demographic profile of individuals affected by monkeypox in Africa has exhibited a notable shift in median age over the course of time. Specifically, the median age of monkeypox cases was predominantly observed among children aged 4 to 5 years7. However, in more recent decades, namely the 2000s and 2010s, the median age range has witnessed an increase, with affected individuals falling within the age bracket of 10 to 21 years. The symptoms and indicators of monkeypox bear resemblance to those of smallpox, albeit often exhibiting less severity. Patients commonly describe a range of symptoms including fever, headache, muscular aches, backaches, enlarged lymph nodes, general malaise, and fatigue. The emergence of a rash resembling smallpox, characterized by the presence of vesicles and pustules, is commonly observed to initiate on the facial region. However, it may also manifest on various other regions of the body over a span of 1 to 3 days subsequent to the commencement of fever. The rash, which is initially popular, progresses through stages of vesiculation, pustulation, and crusting. These separate stages are observed simultaneously on the face, head, trunk, and extremities. The duration of the incubation phase typically spans approximately 12 days, with a range of 7 to 17 days. The typical duration of an illness is between two and four weeks30.
Diagnosis
In order to mitigate the impact of the ongoing monkeypox outbreak, it is crucial to prioritize early detection and the implementation of effective therapeutic interventions as transmission persists. The diagnosis of monkeypox is determined by considering the patient’s medical history, physical symptoms, and results of diagnostic tests. constructing a comprehensive travel and itinerary In light of evolving transmission patterns and the expansion of the illness beyond its endemic zone, it is crucial to emphasize the significance of individuals’ sexual histories31.
A pleomorphic skin rash with frequent umbilication is one of the defining signs of monkeypox. Prodromal symptoms including fever, malaise, chills, lymphadenopathy, myalgia, or headache are frequently present along with this rash. The lesions that have been seen have the ability to harm the skin on the palms and soles and are hard, deeply embedded, and well defined. The advancement of macules, papules, vesicles, and pustules results in the creation of scabs and desquamation32.
Until all skin lesions have completely re-epithelialized, a person is still contagious. In areas where earlier skin lesions were present, pitted scars or skin with uneven pigmentation may develop. Monkeypox is typically described as a self-limiting illness that lasts 2-4 weeks. Children are more likely to experience severe cases, and people with compromised immune systems are still vulnerable to even worse results. Severe pathological conditions are indicated by complications requiring hospitalization, such as encephalitis, sepsis, bleeding, coalescing cutaneous lesions, and others. Encephalopathy, ocular ulceration and scarring leading to vision impairment, subsequent bacterial infection of skin lesions, and respiratory tract infections such bronchopneumonia and sepsis are listed as possible side effects of monkeypox33.
 |
Figure 2: Six stages of monkeypox lesions: (1) early vesicle, (2) pustule, (3) umbilicated pustule, (4) ulcerated skin lesion with growing scab, (5) crusted, mature lesions, and (6) removed scab34 |
Various factors such as the intensity of the illness, age, history of prior smallpox vaccination, presence of medical comorbidities, use of immunosuppressive drugs, and other relevant considerations collectively influence the prognosis of the disease. There have been recent reports of asymptomatic monkeypox cases, the complete extent of which remains uncertain. In a study, it was observed that three male clinic patients from Belgium tested positive for MPV PCR in samples collected from the anorectal region35. However, it is important to note that the virus cleared spontaneously in these individuals. The clinical manifestations observed in cases of monkeypox in 2022 are characterized by the presence of dermal lesions affecting the external genitalia, anal region, and oral mucosa. These regions are the most likely sites of exposure and viral ingress. Monkeypox instances that have been confirmed using polymerase chain reaction (PCR) testing have been identified in patients residing in London, United Kingdom. These individuals have also reported symptoms such as sore throats, penile edema, and rectal pain. The researchers also observed a diverse temporal pattern in the occurrence of systemic and mucocutaneous symptoms throughout the cases. The 2022 cases reported novel clinical findings, including mucocutaneous ulcers, reduced incidence of skin lesions, decreased prevalence of disseminated disease, and proctitis leading to anorectal pain4.
Confirmatory laboratory tests are performed to validate the diagnosis and rule out other disorders. These techniques involve the examination of scab material, lesion fluid, vesicular fluid, or biopsy specimens acquired from people suspected of having monkeypox. Immunohistochemistry, enzyme-linked immunosorbent assay (ELISA), viral culture, and polymerase chain reaction (PCR) were all used in this investigation.
The utilization of gel-electrophoresis for evaluating MPV protein can serve as a means to confirm the diagnosis. However, it may not be deemed appropriate as an initial diagnostic tool in the clinical setting. Real-time polymerase chain reaction (PCR), a diagnostic technique that relies on nucleic acid amplification, is widely recognized as the preferred and most accurate method for immediate detection. In instances where polymerase chain reaction (PCR) findings indicate a negative outcome for a suspected case, it is recommended to pursue other diagnostic procedures36.
Table 1: Diagnostic test for monkeypox infected patient 37
|
Tests |
Description |
Sample used |
|
PCR |
It is based on NAAT; real-time PCR is presently the gold standard for detecting monkeypox DNA. |
Lesion fluid |
|
Viral culture |
A patient sample is cultured and separated to isolate the virus. |
Lesion fluid |
|
Electron microscopy |
An electron microscope is used to identify pox viruses morphologically. |
Biopsy specimen, scab material, vesicular fluid |
|
Immunohistochemistry |
Tests are conducted for the presence of Orthopoxvirus-specific antigens. |
Biopsy specimen |
|
Anti-Orthopoxvirus IgG and IgM tests |
These tests can be used to determine whether you have been exposed to Orthopoxvirus recently or in the past. |
Blood specimen |
B6R, which encodes the extracellular-envelope protein gene, DNA-dependent RNA polymerase subunit 18, Venereology 2022, 1 205 E9L, and F3L are among the genes targeted by RT-PCR testing. Nonetheless, whole-genome sequencing with Next-Generation Sequencing (NGS) is a thorough and informative testing approach that is frequently kept for downstream applications, owing to its labor-intensive, costly, and time-consuming nature. The turnaround time for RT-PCR test results can range from 24 to 72 hours, and only public health laboratories are authorised to perform these tests. Rajsri and his colleagues did a thorough examination of a patient in 2022. Contacting local or state public health authorities is the obligation of healthcare practitioners. If the orthopoxvirus testing by polymerase chain reaction (PCR) yields a positive result, the Centres for Disease Control and Prevention (CDC) is alerted about the characterisation of monkeypox virus (MPV).Persons who have been vaccinated against smallpox within the last three years should handle specimens suspected of being contaminated with monkeypox in a biosafety level 2 (BSL-2) containment facility while adhering to biosafety level 3 (BSL-3) standards. According to laboratory tests conducted by the MPV laboratory in the United States, the cases detected in the present outbreak may be related with the West African clade 38
Pathophysiology
B6R, which encodes the extracellular-envelope protein gene, DNA-dependent RNA polymerase subunit 18, Venereology 2022, 1 205 E9L, and F3L are among the genes targeted by RT-PCR testing. Nonetheless, whole-genome sequencing with Next-Generation Sequencing (NGS) is a thorough and informative testing approach that is frequently kept for downstream applications, owing to its labor-intensive, costly, and time-consuming nature. The turnaround time for RT-PCR test results can range from 24 to 72 hours, and only public health laboratories are authorised to perform these tests. Rajsri and his colleagues did a thorough examination of a patient in 2022. Contacting local or state public health authorities is the obligation of healthcare practitioners. If the orthopoxvirus testing by polymerase chain reaction (PCR) yields a positive result, the Centres for Disease Control and Prevention (CDC) is alerted about the characterisation of monkeypox virus (MPV).Persons who have been vaccinated against smallpox within the last three years should handle specimens suspected of being contaminated with monkeypox in a biosafety level 2 (BSL-2) containment facility while adhering to biosafety level 3 (BSL-3) standards. According to laboratory tests conducted by the MPV laboratory in the United States, the cases detected in the present outbreak may be related with the West African clade 38
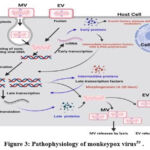 |
Figure 3: Pathophysiology of monkeypox virus39 . |
The viral factories expand and become more diverse in their morphology as the replication process advances. This is characterised by the creation of cavities within the factories that function as compartments for both the host translation machinery and viral mRNA. During the advanced stages of replication, a complex of viral gene products and membrane building proteins causes the membranes of the surrounding endoplasmic reticulum to burst. As a result, crescent-shaped structures emerge, which serve as a mechanism of assembling immature virions39. Collaboration between viral membrane building proteins and late gene products results in the development of immature virions (IVs) later in the replication cycle. The activity of this complicated system causes the membranes of the endoplasmic reticulum to degrade, resulting in the formation of crescent-shaped structures. The IVs are processed, resulting in the formation of mature virions (MVs), which are the virus’s primary infectious particles. When these MVs associate with the cytoplasmic membrane, they are liberated from the host cell.
Clinical Management
The provision of supportive care is considered to be the most important part of clinical management for persons with normal monkeypox infection18. Maintaining a proper fluid balance due to the potential escalation of insensible fluid losses from the skin, reduced oral consumption, and the development of vomiting or diarrhoea are all examples of supportive care18. When deemed necessary, supplemental interventions such as managing bacterial superinfections of cutaneous lesions and delivering hemodynamic support, enhanced oxygenation, or alternate kinds of breathing aid should be considered. Many alternative solutions in this particular case have been recommended40. Lubricants, topical antibiotics, and topical antivirals, such as trifluridine, are among these techniques. It is also advised that ophthalmology professionals be involved at an early stage.
Tecovirimat, cidofovir, and VIGIV are currently available under the Centres for Disease Control and Prevention’s (CDC) Expanded Access Investigational New Drug (EA-IND) processes. These drugs are provided by the Strategic National Stockpile and are meant to treat monkeypox virus infections. Cidofovir was approved by the Food and Drug Administration (FDA) in 1996 for the treatment of cytomegalovirus (CMV) retinitis in people with acquired immunodeficiency syndrome. Cidofovir exhibits antiviral action against a variety of virus families, including adenoviruses, orthopox viruses, herpes viruses, and others41. The FDA gave brincidofovir approval for the treatment of smallpox infection in June 2021. In previous research, this treatment approach was given to those who had OPXV, adenovirus, or CMV infections. The Food and Drug Administration (FDA) approved Tecovirimat as a therapeutic intervention for smallpox infection in 201842. Furthermore, the European Medicines Agency approved authorization for the use of the aforementioned medication in the management of cowpox and smallpox in January 2022 (EU, 2022). The patient’s sickness duration and virus shedding were reduced, and no adverse consequences were observed. Tecovirimat was given to a laboratory employee who had contracted the Vaccinia virus as a result of a needle stick injury in a research.
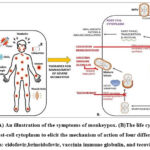 |
Figure 4: (A) An illustration of the symptoms of monkeypox. (B)The life cycle of MPV inside the host-cell cytoplasm to elicit the mechanism of action of four different antiviral therapies: |
The variola virus’s F13 gene encodes the viral protein p37, which is successfully inhibited by the antiviral drug tecovirimat. Tecovirimat is effective against numerous orthopoxviruses in vitro, including vaccinia, variola, cowpox, and monkeypox viruses. The protein’s extremely conserved properties have been observed in orthopoxviruses46.In the absence of a double membrane layer, the presence of the p37 protein is required for intracellular mature viral envelopment. This step results in the creation of the intracellular enveloped virus, which is important in the last phases of viral maturation. The intracellular enveloped virus (IEV) then forms a link with the cytoplasmic membrane to release virions that spread from the site of infection. Tecovirimat has a high selectivity since it specifically inhibits the replication of orthopoxviruses like smallpox by targeting the unique p37 protein47. Cidofovir is primarily utilized in the treatment of CMV retinitis, a commonly seen illness in persons with compromised immune systems, particularly those affected by HIV. Upon cellular entry, cidofovir necessitates activation by intracellular enzymes. The cidofovir molecule undergoes a conversion into its monophosphoryl form, which subsequently undergoes phosphorylation to produce the active form known as cidofovir diphosphoryl. The processes are catalyzed by nucleoside 5′-diphosphate kinase and pyrimidine nucleoside monophosphate kinase, respectively. The interaction between the DNA polymerase of the virus and cidofovir diphosphoryl leads to the incorporation of the latter into the viral DNA. CDVpp exhibits the capacity to function as a competitive inhibitor. Alternatively, it has the potential to substitute the substrate and become integrated, leading to the termination of the chain48.
After being absorbed, the lipid molecule undergoes degradation, resulting in the release of CDV. This CDV is then available for intracellular kinase phosphorylation, leading to the formation of CDV diphosphate, which is the active form of the medication. When doing a comparison between the two drugs, it can be shown that BCV exhibits a lower degree of renal toxicity in comparison to CDV. This distinction arises from the fact that BCV does not function as a substrate for the organic anion transporter 1. Consequently, BCV exhibits a lower degree of renal toxicity compared to CDV. In contrast to BCV, CDV has lower levels of tolerability. It should be noted that CDV is exclusively accessible in injection form, whereas BCV is available in both oral and suspension forms.
A reduction in serum bicarbonate levels, proteinuria, neutropenia, susceptibility to infections, hypotony of the eye, iritis, uveitis, nephrotoxicity, and fever are all signs of CDV. The use of BCV is not recommended for women who are pregnant or breastfeeding. It is critical to check the individual’s hepatic function both before and after the therapeutic intervention, as there is a risk of increased hepatic transaminases and blood bilirubin levels. CDV, on the other hand, has been shown to have the ability to affect the kidneys. As a result, in cases when renal function is impaired, the dosage must be adjusted49.
Vaccinia Immunoglobulin
A number of published publications on human orthopox virus infections have made reference to the utilization of the intravenous formulation of vaccinia immunoglobulin (VIGIV), which has received approval from the U.S. Food and Drug Administration (FDA). In the case reports, it was observed that a significant number of patients with OPXV infections were administered VIGIV together with antiviral medications as part of their treatment regimen43. Infection with OPXV, has the potential to provide immunological cross-protection against viruses of the same genus50. There is currently a scarcity of specialised vaccines that can successfully reduce the risk of monkeypox infection and associated illness. The Vaccinia virus-based vaccinations currently being tested for monkeypox prevention were originally intended to treat smallpox. A study conducted in the Democratic Republic of the Congo (DRC) in the late 1980s found that the secondary attack rate among unprotected household contacts of individuals with an illness was 9.28%, but 1.31% for vaccinated contacts. Previous smallpox immunisation provided an estimated 85% protection against monkeypox27.
Prior to 2019, the only orthopoxvirus (OPXV) vaccine available in the United States was ACAM2000. ACAM2000 is developed from a replication-competent strain of the Vaccinia virus (OPXV genus). The utilization of ACAM2000 presents a potential hazard for the occurrence of serious adverse effects due to its replication competent nature, such as progressive vaccinia, eczema vaccinatum, and myopericarditis51. Jynneos is considered to be a safer option for immunocompromised individuals compared to ACAM2000 due to its lack of live virus production in vaccinated individuals. Nevertheless, it is crucial to bear in mind that individuals with damaged immune systems may have a diminished immune reaction to the Jynneos vaccine, potentially leading to a reduced level of effectiveness in terms of protection compared to those with fully functional immune systems52.
Association Between Covid-19 and Monkeypox Outbreaks
The COVID-19 pandemic has increased the number of monkeypox cases, complicating the current situation. Infections induced by SARS-CoV-2 have been shown to cause a wide range of clinical symptoms, including both respiratory and extra-respiratory signs52. Dermatological manifestations associated with COVID-19 may include an erythematous maculopapular rash, erythema multiforme, vesicular rash, vascular livedo reticularis, figurate erythema, or a flexural rash. During the ongoing outbreak, healthcare practitioners should be on the lookout for COVID-19 and atypical monkeypox, as their clinical symptoms may be identical. Co-infection of SARS-CoV-2 with other viruses is a regular occurrence52. As a result, it is critical to consider the possibility of SARS-CoV-2 and monkeypox virus co-infection. Nonetheless, it is unclear whether changes in infectivity patterns, severity, management, or susceptibility to immunisation have occurred for one or both of the diseases. As a result, more research is needed to determine the link between COVID-19 outbreaks and monkeypox52.
Given that the majority of people diagnosed with monkeypox have fever, which is also a common symptom in COVID-19 patients, it is critical to investigate the potential overlap in clinical presentations between these two diseases. A rash can help distinguish between the two diseases; however, certain individuals with monkeypox may have atypical lesions that are not easily visible, while others may have pre-existing ailments that also manifest as a rash, complicating the accurate determination of a differential diagnosis54.
Discussion
The international community must cooperate in order to immediately close any knowledge gaps and contain the epidemic in order to effectively combat the spread of viruses, which know no national borders. It is essential to locate patients as soon as possible in order to control the disease because there are no widely available treatments or prophylactic measures. Monkeypox is regarded by the WHO as a reemerging illness and is capable of being turned into biological weapons. This is due to the possibility of it spreading quickly among those who are not immune to the virus. Although monkeypox is not as contagious or harmful as smallpox or even SARS-CoV-2, it still has the potential to be a deadly illness, especially in those with underlying illnesses. To avoid further issues, it is crucial to keep a close eye out for any disease-related symptoms.
Conclusion
The principal methods include disease surveillance, contact tracing, and ring vaccination with smallpox immunisations that have been approved for off-label use in monkeypox cases. There is rising concern about the possibility of cross-border transmission and subsequent global spread of monkeypox outbreaks, which might lead to a pandemic. More research is needed to fill existing information gaps, such as the precise method of transmission and the involvement of animal reservoirs. Consequently, the development of more effective remedies will be postponed until this additional information becomes available. Additional investigation is required in order to enhance comprehension of sexual transmission pathways, genetic modifications, waning smallpox immunity, and previously undetected instances of monkeypox that transpired earlier. Healthcare professionals are advised to promptly inform public health authorities and undertake further diagnostic procedures in the event of encountering a dermatological illness that exhibits symptoms associated with a likely orthopoxvirus infection.
Acknowledgement
We want to express our gratitude to Matrika Saha Roy, for her contribution to proofreading of this manuscript.
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- Beer E.M, Rao V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLOS Negl Trop Dis. 2019:13(10):e0007791.
- Roshchina V.V. Evolutionary considerations of neurotransmitters in microbial, plant, and animal cells. Microb Endocrinol Interkingdom Signal Infect Dis Health. 2010:17-52.
- Alakunle E., Moens U., Nchinda G., Okeke M.I. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12(11):1257.
- Thornhill J.P, Barkati S., Walmsley S., et al. Monkeypox virus infection in humans across 16 countries. N Engl J Med. 2022;387(8):679-691.
- Cohen J. Is an old virus up to new tricks? Science. 1997;277(5324):312-313.
- Centers for Disease Control and Prevention (US). Potential exposure to person with confirmed human monkeypox infection—United States.1997. https://emergency.cdc.gov/han/2021/han00446.asp.
- Bunge E.M., Hoet B., Chen L., et al. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLOS Negl Trop Dis. 2022;16(2):e0010141..
- Mahase E. Seven monkeypox cases are confirmed in England. BMJ. 2022;377:o1239.
- McCollum A.M., Damon I.K. Human monkeypox. Clin Infect Dis. 2014;58(2):260-267.
- Mbala P.K., Huggins J.W, Riu-Rovira T., et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216(7):824-828.
- Simpson K., Heymann D., Brown C.S, et al. Human monkeypox–After 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38(33):5077-5081.
- Joklik W.K. The poxviruses. Bacteriol Rev. 1966;30(1):33-66.
- Haller S.L., Peng C., McFadden G., Rothenburg S. Poxviruses and the evolution of host range and virulence. Infect Genet Evol. 2014;21:15-40.
- Moss B. Poxvirus cell entry: how many proteins does it take? Viruses. 2012;4(5):688-707.
- Miller J., Hachmann N.P., Collier A.Y., et al. Substantial neutralization escape by SARS-CoV-2 Omicron variants BQ. 1.1 and XBB. 1. N Engl J Med. 2023;388(7):662-664.
- Okyay R.A., Bayrak E., Kaya E., Şahin A.R., Koçyiğit B.F., Taşdoğan A.M., et al. Another epidemic in the shadow of Covid 19 pandemic: a review of monkeypox. EJMO. 2022;7(10):10.
- Nolen L.D., Osadebe L., Katomba J., et al. Introduction of monkeypox into a community and household: risk factors and zoonotic reservoirs in the Democratic Republic of the Congo. Am J Trop Med Hyg. 2015;93(2):410-415.
- Reynolds M.G., Davidson W.B., Curns A.T., et al. Spectrum of infection and risk factors for human monkeypox, United States, 2003. Emerg Infect Dis. 2007;13(9):1332-1339.
- Centers for Disease Control and Prevention (US). Potential exposure to person with confirmed human monkeypox infection—United States.2021. https://emergency.cdc.gov/han/2021/han00446.asp.
- Huhn G.D., Bauer A.M., Yorita K., et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41(12):1742-1751.
- Bragazzi N.L., Kong J.D., Mahroum N., et al. Epidemiological trends and clinical features of the ongoing monkeypox epidemic: A preliminary pooled data analysis and literature review. J Med Virol. 2023;95(1):e27931.
- Jenness S.M., Begier E.M., Neaigus A., Murrill C.S., Wendel T., Hagan H. Unprotected anal intercourse and sexually transmitted diseases in high-risk heterosexual women. Am J Public Health. 2011;101(4):745-750.
- Zachary K.C., Shenoy E.S. Monkeypox transmission following exposure in healthcare facilities in nonendemic settings: low risk but limited literature. Infect Control Hosp Epidemiol. 2022;43(7):920-924.
- Adler H., Gould S., Hine P., et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22(8):1153-1162.
- Ježek Z., Grab B., Szczeniowski M.V., Paluku K.M., Mutombo M. Human monkeypox: secondary attack rates. Bull World Health Organ. 1988;66(4):465-470.
- Hamdana A.H., Mohsin H., Habib Tharwani Z., et al. Monkeypox Virus and Other Emerging Outbreaks: An Overview and Future Perspective. Inquiry. 2023;60:469580231175437.
- Al-Mandhari A., Kodama C., Abubakar A., Hajjeh R., Brennan R. Monkeypox outbreak and response efforts in the eastern Mediterranean Region. East Mediterr Health J. 2022;28(7):465-468.
- Guarner J., Johnson B.J., Paddock C.D, et al. Monkeypox transmission and pathogenesis in prairie dogs. Emerg Infect Dis. 2004;10(3):426-431.
- MacNeil A., Reynolds M.G., Braden Z., et al. Transmission of atypical varicella-zoster virus infections involving palm and sole manifestations in an area with monkeypox endemicity. Clin Infect Dis. 2009;48(1):e6-e8.
- Hagan L.M., Beeson A., Hughes S., et al. Monkeypox case investigation—Cook County jail, Chicago, Illinois, July-August 2022. MMWR Morb Mortal Wkly Rep. 2022;71(40):1271-1277.
- Sah R., Padhi B.K., Siddiq A., et al. N, Rodriguez-Morales AJ. Public health emergency of international concern declared by the World Health Organization for Monkeypox. Glob Sec Health Sci Policy. 2022;7(1):51-56.
- Anderson D., Hostler C. Monkeypox: recognition and prevention in sports. Br J Sports Med. 2023;57(3):133-134.
- De Baetselier I., Van Dijck C., Kenyon C., et al. Retrospective detection of asymptomatic monkeypox virus infections among male sexual health clinic attendees in Belgium. Nat Med. 2022;28(11):2288-2292.
- Girometti N., Byrne R., Bracchi M., et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis. 2022;22(9):1321-1328.
- Cheema A.Y., Ogedegbe O.J., Munir M., Alugba G., Ojo T.K. Monkeypox: a review of clinical features, diagnosis, and treatment. Cureus. 2022;14(7):e26756.
- Rajsri K.S., Rao M. A review of monkeypox: the new global health emergency. Venereology. 2022;1(2):199-211.
- Kumar N., Acharya A., Gendelman H.E., Byrareddy S.N. The 2022 outbreak and the pathobiology of the monkeypox virus. J Autoimmun. 2022;131:102855.
- Katsafanas G.C., Moss B. Colocalization of transcription and translation within cytoplasmic poxvirus factories coordinates viral expression and subjugates host functions. Cell Host Microbe. 2007;2(4):221-228.
- Hughes C., McCollum A., Pukuta E., et al. Ocular complications associated with acute monkeypox virus infection, DRC. Int J Infect Dis. 2014;21:276-277.
- Vora S., Damon I., Fulginiti V., et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46(10):1555-1561.
- Hoy S.M. Patisiran: first global approval. Drugs. 2018;78(15):1625-1631.
- Ivanov D.T., Slabakova Y.A., Argirova R.M., Valkov T.K. Antivirals for the treatment of Monkeypox: utilization in the general and HIV-positive population and gaps for research. A short narrative review. Infez Med. 2023;31(2):186-194.
- Hsu C.H., Farland J., Winters T., et al. Laboratory-acquired vaccinia virus infection in a recently immunized person–Massachusetts. Morb Mortal Wkly Rep. 2015;64(16):435-438
- Russo A.T., Grosenbach D.W., Chinsangaram J., et al. An overview of tecovirimat for smallpox treatment and expanded anti-Orthopoxvirus applications. Expert Rev Anti-Infect Ther. 2021;19(3):331-344.
- Yang G, Pevear DC, Davies MH, et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal Orthopoxvirus challenge. J Virol. 2005 October 15;79(20):13139-13149.
- Andrei G., Snoeck R. Cidofovir activity against poxvirus infections. Viruses. 2010;2(12):2803-2830.
- Rizk J.G., Lippi G., Henry B.M., Forthal D.N., Rizk Y. Prevention and treatment of monkeypox. Drugs. 2022;82(9):957-963.
- Oliveira G.P., Rodrigues R.A.L., Lima M.T, Drumond B.P., Abrahão J.S. Poxvirus host range genes and virus-host spectrum: a critical review. Viruses. 2017;9(11):331.
- Reed J.L., Scott D.E., Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54(6):832-840.
- Keckler M.S., Salzer J.S., Patel N., et al. IMVAMUNE® and ACAM2000® provide different protection against disease when administered postexposure in an intranasal monkeypox challenge prairie dog model. Vaccines. 2020;8(3):396.
- Lai C.C., Ko W.C., Lee P.I., Jean S.S., Hsueh P.R. Extra-respiratory manifestations of COVID-19. Int J Antimicrob Agents. 2020;56(2):106024.
- Mohammed G.F., Al-Dhubaibi M.S., Atef L. Cutaneous manifestations of coronavirus disease 2019: skin narratives and dialogues. J Clin Aesthet Dermatol. 2022;15(5):E77-E81.
- El‐Qushayri A.E., Kamel A.M.A., Reda A., Ghozy S. Does dengue and COVID‐19 co‐infection have worse outcomes? A systematic review of current evidence. Rev Med Virol. 2022;32(5):e2339.







