Manuscript accepted on :16-04-2023
Published online on: 18-08-2023
Plagiarism Check: Yes
Reviewed by: Dr. Deepak Jain
Second Review by: Dr. Rajkishor Gogoi
Final Approval by: Dr. Ayush Dogra
Zeba Siddiqui , Mohammad Irfan Khan
, Mohammad Irfan Khan , Badruddeen
, Badruddeen , Juber Akhtar
, Juber Akhtar and Mohammad Ahmed
and Mohammad Ahmed
Faculty of Pharmacy, Integral University, Lucknow, India.
Corresponding Author E-mail: irfanrndgp@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2716
Abstract
Background: For safe and efficacious use of natural products, quality control of the crude drug is of paramount importance. Due to lack of scientific investigations pertaining to quality control phytochemical and pharmacological properties of Phyllanthus acidus (L.) Skeels, the present study was undertaken. Objective: The aim of the study is to establish various pharmacognostical and phytochemical parameters, including both macro and microscopic studies, along with HPTLC and FTIR fingerprinting analysis, total phenolic and flavonoid content of stem bark of Phyllanthus acidus, followed by exploring its anti-oxidant potential. Method: The determination of total phenolic and flavonoid content was done by various colorimetric assays. DPPH assay was used to establish antioxidant activity. Physiochemical analysis was carried out and presence of various functional groups was determined using various techniques like HPTLC, FTIR analysis and colorimetric assays. Result: The phytochemical screening showed the presence of various phytoconstituents like phenols, carbohydrates, flavonoid, tannins and terpenoids. in various solvent systems. Total phenolic was established as 189.74±0.52mg GAE/g and flavonoid content was found to be38.92±0.47 mg QE/g. Concentration of heavy metal was within acceptable limits. The stem bark showed comparable antioxidant activity in methanolic and aqueous extract showed IC50 values of 26.92 and 26.52 respectively compared to ascorbic acid having IC50 value of 31.82. HPTLC fingerprinting envisaged the presence of several phytoconstituents in Phyllanthus acidus. The FTIR analysis established the presence of phenolic functional groups. Conclusion: The referential information provided by this study will be useful to determine and manage adulterations in raw material. The study also provides insight into antioxidant property of this plant validating its ethno pharmacological use as a natural antioxidant.
Keywords
DPPH; FTIR; HPTLC; Pharmacognostical; Quality control
Download this article as:| Copy the following to cite this article: Siddiqui Z, Khan M. I, Badruddeen B, Akhtar J, Ahmed M. In Vitro Antioxidant Activity, Pharmacognostical Evaluation, HPTLC and FTIR Fingerprinting of Phyllanthus Acidus L. Stem Bark Extract for Better Application in Phytotherapy. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Siddiqui Z, Khan M. I, Badruddeen B, Akhtar J, Ahmed M. In Vitro Antioxidant Activity, Pharmacognostical Evaluation, HPTLC and FTIR Fingerprinting of Phyllanthus Acidus L. Stem Bark Extract for Better Application in Phytotherapy. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/3KJV7IF |
Introduction
Post covid pandemic phase has caused a paradigm shift into how herbal medicines are perceived all around world. With more and more people turning to alternate system of medicine, there is a dire need to provide referential information regarding the safe and authentic use of these herbal drugs. Due to lower cost, appreciable patience endurance, accessibility and availability of herbal products from local healers or from the market and the perception that herbal products do not contain detrimental chemicals and have no side effects has led to a surge in the demand of plant based pharmaceuticals. The use of plants for medicinal treatment is also based on long established historical usage of that plant which is mostly indigenous and has deep roots in cultural heritage of a particular community. This has led to an invigorating interest in plant based products and lead molecules as source of unconventional medicament for treating various ailments. Ground breaking work in the fields like ethnopharmacology, molecular biology, medicinal chemistry and studies related to clinical trials of these herbal medicines have substantiated the clinical usage of these plant based products.1 Plant secondary metabolites like phenols and flavonoids have been reported to possess effective antioxidants , antidiabetic, cardioprotective, anti-inflammatory, immune-boosting activity among several other pharmacological activities and hence have emerge as an interesting candidates for pharmaceutical and medicinal research.2,3,4 High performance thin-layer chromatography (HPTLC) has evolved as an effective tool for chromatographic profiling of herbal drugs to determine the various phytoconstituents present in the plant extract. Because of its lucidity, and negligible sample concoction, and the ease to analyse more than one sample at the same time has also increased the popularity of this technique. Used along with mass spectroscopy it is used to determine the phytoconstituents present on the plate.5 Fourier-transform infrared spectroscop (FTIR) is a sustainable technology which requires no solvent and negligible preparation of sample and provides spectrum with high resolution over a short period of time. Hence an extensive method used to elucidate phytoconstituents or determine their structure.6 The need to provide appropriate documentation, quality control measures and standardisation of the crude, as well as finished herbal products has gained importance.
Phyllanyhus acidus also known as ‘arinellikka’, ‘nellikkapuli’ in Malayalam ‘hariful’ in Bangla is also known by many other names like Star gooseberry, Otaheite gooseberry, and Malay gooseberry.7 Although the plant initially originated in Madagasgar, is now spread across south-east Asia including Malaysia, Philippines and Indian Subcontinent.8An ornamental plant with edible yellowish green berry fruits Phyllanthus.acidus has a long standing gastronomic use due to sour and tart taste of its fruit and is part of many culinary preparations like syrups, chutneys jellies and beverages.9 Different parts of these plants have well established ethno-pharmacological and folklore uses. According to Ayurveda practices the plant is said to increase ‘Vata’.10 The roots of the plant are used as inhalations for headaches and congestion relief11, and an infusion is said to reduce asthma attacks.12 It is also used to manage psoriasis, treat various skin diseases, antipyretic and relieve chronic constipation.13-14 The leaves are use as vegetable in some parts of India, Malaysia and Thailand.15 It is a common remedy for treating headaches, blood pressure and fever.16-18 The bark is useful to treat catarrh and leaves are utilized to treat utricaria.19 The fruit is acidic in nature and a rich source of vitamin C, it used to treat hepatic disorders by some tribal healers, and as a blood purifier, tonic for liver, remedy for hepatopathy and as an laxative.12,15,19,20, It is also used to relieve cough, diabetes, asthma and also used as a cathartic.21-22
Compositional analyses of fruits of Phyllanthus acidus shows that the plant is rich in mineral source of both macro and micro elements like calcium, potassium, phosphorous, zinc iron etc., it is also a rich source of ascorbic acid, carotenoids, fructose and glucose and therefore is advocated as a potential dietary supplement.23-26 Phytochemical analysis has shown that various phytoconstituents have been isolated and characterised from various parts of this plant like leaf, fruits, roots and bark. Most of the constituents isolated are terpenoids, flavonoids, volatile oil component and phenolic compounds.27A plethora of pharmacological activities of bioactive components as well as extract of different parts of plants like antidiabetic cytotoxic, hepatoprotective antioxidant, anti-inflammatory etc., have been reported in both in-vivo and in-vitro experimental studies.28
Material and Methods
Material
The authenticated sample of dried bark of P acidus was collected from the hills of Kerala. The collected sample was identified with the help of a botanist at the National Botanical Research Institute, Lucknow, India.Chemicals that were used in the work were of analytical grade and procured from SD Fine Chemicals Ltd., Mumbai, India. Lead, arsenic, cadmium, and mercury were obtained from Sigma Aldrich, Bangalore, India.
Microscopical studies
Morphological and organoleptic evaluation of the powdered stem bark of P.acidus was carries out using magnifying lens and naked eyes.29 Fine powder was taken to observe microscopical features of the bark of P.acidus.
Physiochemical study
Evaluation of physiochemical parameters was evaluated based on WHO guidelines.30 Parameters such as loss on drying, extractive value, ash value, and moisture content by Karl Fischer titration was determined.
Preliminary phytochemical screening
Various chromophoric reagents were used to determine the existence of various classes of phytochemicals such carbohydrates alkaloids, phenolic and flavonoidal compounds, saponnins, lipids, tannins and steroids. The petroleum ether, chloroform and methanolic extracts were treated with chemical reagents in test tubes for the screening of various phytoconstituents.31-33
Preparation of extracts
The bark of P.acidus was shade dried at 370C and powdered. Powdered crude drug (10gm) each was soaked in 100ml of methanol, water, and chloroform and petroleum ether respectively for 24 hours. The extracts were filtered followed by evaporation to dryness under vacuum.
DPPH radical scavenging assay
Estimation of scavenging of unbound DPPH radicals by the methanolic and aqueous extract of P.acidus forms the basis for establishing its antioxidant potential. The in-vitro antioxidant assay as stipulated by Nithianantham et al with few alterations has been used.34 Different concentrations of methanolic and aqueous extract were added to 0.4mM DPPH solution (0.1ml) respectively. The mixture after vigorous shaken was placed in an unlit area for 15 min at 37oC and UV-Vis microplate reader was used determine the absorbance at 517 nm. Ascorbic acid was taken as the positive control. The degree of decolourisation of purple coloured DPPH into yellowish solution indicates the scavenging activity of the extracts.35 From the linear regression line of concentration versus inhibition (%) graph concentration that indicated 50% free radical scavenging (IC50 value) was extrapolate.36 Radical scavenging is expressed as percentage of inhibition which is deliberated by employing the formula:

Where, A0 is absorbance of control (DPPHsolution minus the extract) and A1 is absorbance of extract along with DPPH solution. The IC50 values are used to express scavenging potential of the plant extracts. All the data were represented as mean values ± Standard deviation (n=3).
Determination of total phenolics compounds
Folin–Ciocalteu (FC) method has been employed to establish the total phenolic value of various concentrations of plant methanolic extracts using gallic acid as standard as stipulated by Singleton et al.37 A calibration curve was obtained by using different concentrations from 10 to 1000µg/mL of gallic acid in methanol. Different concentrations of extract (10-1000µg/mL) were prepared and thirty microlitre of standard and extract respectively was put in 150µL of FC reagent (10%) and after 10min, 120 µL of Na2CO3 (7%) was added. After 2 hours absorbance were observed at 760nm compared to blank. The phenols present in P.acidus extract are oxidised by FC reagent which changes the colour of the extract to bluish green that in turn is determined by UV-Vis spectrometer. The sample was prepared thrice for every examination. The mean value of absorbance was employed to mark calibration curve to obtain total phenols in extract. The total phenolic concentration is expressed as mg gallic acid equivalents (GAE) per gram of dry samples (mg/g). The formula used to calculate total phenolic content was:
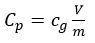
Whereby, Cp = total phenolic concentration mg GAE/g dried sample, cg= gallic acid concentration evaluated from calibration curve in mg/mL, V= volume (mL) of extract, and m= mass of dried extract in grams.
Estimation of Total Flavonoid Content
Aluminium chloride colorimetric assay is employed to evaluate overall flavonoid concentration as stipulated by Chang et al.38 Standard quercetin for calibration curve was prepared by preparing stock solution of quercetin (4mg/ml) and diluting serially to make concentrations from 0.1-1.0mg/mL. Similarly the plant extract was also serially diluted to make concentration ranging from 0.1- 1mg/mL. Quercetin (1mL) from each concentration was diluted with 4mL distilled water and then 0.3mL of NaNO2 (5%) and 0.3 mL of AlCl3 (10%) was mixed after 5 minutes. Volume was made up to 10mL by adding distilled water. Same procedure was followed with different concentrations of extract. Absorbance was measure at 510 nm using spectrophotometer. Triplicate readings of extract were used to calculate average absorbance. Quercetin Equivalent (mg QE/g) was determined by employing linear equation based on standard calibration graph was employed to express total flavonoid concentration.
Heavy metal analysis
Atomic absorption spectrophotometer was utilised to carry out the Heavy metal analysis of bark extracts of P.acidus. Standards of Mercury (Hg), Lead (Pb), Arsenic (As) and Cadmium (Cd) were prepared and calibration plot was developed. The drug samples were analysed using calibration plot.39
HPTLC Screening
HPTLC fingerprinting profile of P.acidus (bark) methanolic extract was determined to establish presence of various phytoconstituents. 15mg bark extract was dissolved in 5mL of methanol and filtered using nylon membrane filter. Various combinations of solvents of varying polarity were combines to obtain a solvent system that gave excellent resolution and acute peaks for scanning. The solvent system that gave satisfactory resolution of phytoconstituents present in methanolic extract of P.acidus was chloroform: methanol (19:1v/v). HPTLC was performed on pre-coated aluminium sheet TLC Silica gel 60 F254 (20 ×20 cm, Merck, Germany) using a Camag syringe(100µl). Samples (5µL) were spotted as 6mm bands, 10mm from bottom and 15 mm from left edge. The plates were developed in Twin Trough chamber were initially saturated with mobile phase (chloroform: methanol: 19:1) and then the plated were developed with distance of solvent migration of 80 mm. The developed plates were placed in hot air to dry.
Phytoconstituents like, terpenes, flavonoids, phenols terpenoids, and steroids, were ascertained succeeding chemical derivatization by anisaldehyde/sulfuric acid reagent (p-anisaldehyde(0.5mL) in methanol (85mL), acetic acid (10mL) and concentrated sulfuric acid(5mL).40 Chromatograms were recorded at 366 nm and 540 nm using CAMAG LINOMAT 5 spectro- densitometer. The retention factor (Rf value) and peak area of each band were recorded. The plates were photo documented.41
FTIR fingerprinting
FTIR (PerkinElmer Version 10.03.06) was used to analyse the functional moieties existing in the etanolic extract in the range of 400-4000 cm-1.
Statistical analysis
Inhibitory concentration (IC50) i.e. the concentration of the extract which effectively inhibited 50 % of free radicals, was estimated by regression analysis between % inhibition and different concentrations of extract. All assays were performed in triplicates and presented as ±SEM or ±SD, n=3.
Result
Organoleptic and Microscopical studies
The powdered stem bark of P.acidus is pale brown in colour. The stem bark powder had a stimulant odour and an astringent taste. The various diagnostic features observed during powder microscopy were elongated cork cells, cortex with sclereids, parenchyma cells, stone cell brown tannin masses, lignified fibres and non lignified fibres, and prismatic calcium oxylate crystals as shown in Figure 1.
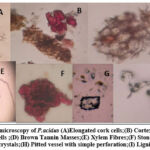 |
Figure 1: Powder microscopy of P.acidus (A)Elongated cork cells;(B) Cortex with Sclereids;(C) Parenchymatous cells ;(D) Brown Tannin Masses;(E) Xylem Fibres; |
Physiochemical parameters
The various physiochemical parameters that were evaluated to establish the purity and quality of the plant drug were, ash value, loss on drying, extractive value and moisture content. The parameters evaluate are represented in Table 1 along with standard deviation. The results obtained were within limits, corresponding with pharmacopeal standards.42, 43
Table 1: Summary of phytochemical analysis of P.acidus.
|
Parameters |
%w/w(Mean ±SD) |
|
LOD |
25.23±0.33 |
|
Ash Values |
|
|
Total Ash |
7.54±0.45 |
|
Water soluble Ash |
6.41±0.36 |
|
Acid insoluble Ash |
0.59±0.074 |
|
Extractive values |
|
|
Petroleum ether |
3.4±0.2 |
|
Chloroform |
12.81±0.19 |
|
Methanol |
27.27±0.35 |
|
Water: alcohol (50:50) |
31.37±1.15 |
|
Moisture content |
22.36±0.27 |
Preliminary phytochemical screening
Exploratory phytochemical analysis of petroleum ether, chloroform and methanolic extracts respectively showed the existence of carbohydrates, glycosides, phenolic compounds, flavonoids, tannins, lipids, saponins and steroids. Alkaloids and protein were absent in all the extract, while lipids was positive only in petroleum ether extract. The conclusions are given in Table 2.
Table 2: Qualitative Phytochemical analysis of P.acidus stem bark extracts
|
Phytochemicals |
Pet. ether extract |
Chloroform extract |
Methanolic extract |
|
Alkaloid |
– |
– |
– |
|
Flavanoids |
+ |
++ |
+++ |
|
Phenolic substances |
+ |
+++ |
+++ |
|
Proteins |
– |
– |
– |
|
Tannins |
– |
++ |
+++ |
|
Glycosides |
++ |
+++ |
+++ |
|
Carbohydrates |
– |
++ |
+++ |
|
Saponins |
++ |
+++ |
+++ |
|
Fixed oil & Fats |
+++ |
++ |
+ |
Key:+++: strong; ++: medium; +: weak; – : absent
Heavy metal evaluation
The presence of heavy metals like arsenic (As), cadmium (Cd), and lead (Pb), in P.acidus was found to be within permissible limits. Level of Mercury (Hg) was found to be slightly higher than the permissible limit(i.e. over limit ratio of 2%) therefore carcinogenic and non carcinogenic risk assessment is requires before determining the dose of the drug for human consumption.(Table 3).
Table 3: Heavy metals analysis of P.acidus stem bark
|
Metal |
Concentration(ppm) |
Limit(ppm) |
|
Lead |
0.80±0.04 |
10 |
|
Cadmium |
0.01±.001 |
0.3 |
|
Mercury |
0.52±0.03 |
0.5 |
|
Arsenic |
1.50±0.23 |
3.0 |
n = 3
Antioxidant activity of extracts
Folin-Ciocalteu assay was employed to establish the Total Phenolic Content (TPC) of P.acidus methanolic extract. Serial dilution of gallic acid was used to establish a calibration graph. Calibration curve was used to establish regression equation (Y=0.012x; R2=0.894) which was used to calculate total phenolic concentration and indicated as mg gallic acid equivalents (GAE) per gram of dry extract (mg/g). The total phenolic content was found to be 189.74±0.52mg GAE/g. This is in conformity with the findings of Sulaiman and Ooi44, where the phenolic content of the fruit juice extract was found to be 204.75±4.99mg GAE/g. Comparably another research reported the total phenolic concentration of 41.801±0.815mg GAE/gm of the leaf extract which is much lower than the phenolic content of the stem bark.45 Phenolic compounds are hydrogen donors and act as reducing agents. Therefore, the phenolic content is an indication of antioxidant property of the plant. The present study showed a considerable existence of phenolic compounds in P.acidus and may contribute to its antioxidant activity and also provides a validation to the ethno pharmacological use of this plant to treat various ailments.
Aluminium chloride colorimetric assay was used to establish the Total Flavonoid Content (TFC) present in the extracts. Serial dilution of quercetin was utilised as standard to establish the calibration curve and regression equation(Y=0.031x; R2=0.865) was established to determine the flavonoid concentration which was stated as mg quercetin equivalents(QE) per gram of dry extract weight(mg/g).TFC was determined as to be 38.92±0.47 mg QE/g. This value is similar to the one obtained by Habib et al which showed flavonoid content of fruit as 30.05 mg QE/g.46 The antioxidant potential of flavonoids is associated with the number hydroxyl groups and their position in the phytoconstituent.47 It has been shown that flavonoid content may vary significantly due to genetic diversity, seasonal and biological differences.48
Methanolic and aqueous extract of P.acidus bark was screened for radical scavenging activity against DPPH radicals. The conversion of DPPH to DPPH2 on receiving a hydrogen ion from the plant extract was responsible for changing of colour from purple to yellow.49 This change brought about change in absorbance which was measured spectrophotometrically to determine the concentration of antioxidants. The DPPH radical scavenging assay of methanolic and aqueous extract showed IC50 values of 26.92 and 26.52 respectively. As shown in Table 4 ascorbic acid a well known antioxidant was used as standard with IC50 value of 31.82 to measure the in-vitro antioxidant activity of the extracts. Lower IC50 value is indicative of higher free radical scavenging and therefore high antioxidant potential. The methanolic extract showed marginally higher anti oxidant activity than aqueous extract and both were close when compared with the standard ascorbic acid as shown in Figure 2. Study of literature has showed that antioxidant activity of different parts of P. acidus has been studied previously. Habib et al reported antioxidant capacity of chloroform extract of fruit of P.acidus withIC50 value of 2745.86 μg mL−1and that of standard ascorbic acid as 13.37μg/mL.46 However the IC50 value reported is more than 100 and therefore cannot be considered relevant.50 High phenolic content and presence of known antioxidants like gallic acid, ellagic acid rutin, quercetin and luteolin by HPLC analysis supports the present data.51 The study also demonstrated that high phenolic and flavonoid content in extracts scientifically validates the ethnological use of P.acidus as natural antioxidant.
Table 4: Mean absorbance and IC50 determination of extracts and ascorbic acid
|
% Inhibition (scavenging capacity) |
|||
|
Concentration µg/ml |
Ascorbic acid |
Ethanolic extract |
Aqueous extract |
|
0 |
0 |
0 |
0 |
|
25 |
49 |
42 |
45 |
|
50 |
67 |
56 |
58 |
|
75 |
80 |
62 |
67 |
|
100 |
89 |
68 |
71 |
|
IC50(µg/ml) |
31.82 |
26.92 |
26.52 |
 |
Figure 2: A.DPPH scavenging assay of P.acidus extracts. B.IC50 values for different extracts in DPPH radical scavenging assay
|
HPTLC Fingerprinting
In order to get a general idea of the various phytoconstituents found in the methanolic extract of P.acidus HPTLC screening was instrumental. Mobile phase comprising of chloroform: methanol (96:4) v/v gave satisfactory separation of compounds. After spotting of samples the chromatograms were scanned at 250nm, 360nm and 540nm (after derivatization with anisaldehyde in sulphuric acid). The chromatogram at 360nm and 540nm showed 9 and 6 peaks respectively (Figure 3). Table 5 summarises Rf value of the various separated constituents along with peak areas. Literature survey of P.acidus revealed that this study provides the first data pertaining to HPTLC fingerprinting of this plant.
 |
Figure 3: A. HPTLC chromatogram image of methanolic extract of P.acidus at 360nm and 540nm respectively. B and C. HPTLC chromatograms of P.acidus methanolc extract at 360nm and 540 nm showing various peaks (bands) of phytoconstituents. |
Table 5: HPTLC data of different extracts of P.acidus
|
Wavelength |
Solvent System |
No. of Peaks |
Rf values |
Peak Area (%) |
|
366 nm |
Chloroform: Methanol (96:4 v/v) |
9 |
0.06,0.16,0.22,0.28, 0.35,0.42,0.49,0.66, 0.79 |
4.81,3.44,37.85,9.55, 17.68,8.74,3.07, 9.83,5.04 |
|
540nm (derivatized in anisaldehyde sulphuric acid reagent ) |
Chloroform: Methanol (96:4 v/v) |
6 |
0.29,0.35,0.38,0.47, 0.82,0.93 |
6.11,4.02,9.98, 7.28,10.74,61.86 |
Fourier Transform InfraRed (FTIR) Fingerprinting
FTIR being one of the most extensively employed methods for the determination of the various functional groups present in the plant extract. This spectroscopic method was used to elucidate the functional groups present in the methanolic extract of P.acidus. The FTIR spectra showing characteristic peaks are shown in Figure 4. The broad peak at 3372cm-1 is an indicative of the presence of phenolic compounds. The absorption bands at 2924cm-1 and 2857cm-1are attributed to C-H symmetrical and asymmetrical stretching of methylene functional group. Sharp peak at 1722cm-1 is indicative of stretching vibrations of carbonyl group (C=O). The bands at 1519-1453 cm-1 may be assigned to C=C aromatic stretching both symmetrical and asymmetrical related to unsaturated linkages and aromatic groups.52 The bands at 1264-1225 cm-1 may be allocated to C-O-C group. The band at 1380 cm-1 is indicative of bending vibration of O-H group. The bands at 800- 627 cm1 is due to the substitutions at Ar-H group.53 Thus FTIR was used to determine the various functional groups present in the plant extract. Comparing the present spectra to the spectra of some well known antioxidants like gallic acid, rutin, quercetin and tannic acid we see similarity in the region of 3400-2500 cm-1 representing O-H and C-H stretching vibrations of phenolic groups present in these compounds.54 which is similar to the FTIR spectra of P.acidus , which also shows similar broad band at 3372cm-1. Since OH group plays a major role in the antioxidant activity of these standard compounds, it can be stipulated that the extract of P.acidus contains similar phytoconstituents like phenols and flavonoids thus making it an effective anti-oxidant which has been further confirmed by DPPH assay in the above studies.
 |
Figure 4: a. FTIR spectra of stem bark of P.acidus ethanolic extract.b. FTIR spectra of pure standard compound of 1) tannic acid 2) quercetin 3) rutin 4) gallic acid from literature data54 |
Discussion
An increase in use of herbal drugs as alternative or complimentary treatment of various diseases requires well established quality control parameters for their safe and efficacious use. Bioassays play a pivotal role in establishing these quality control measures for the standardisation of herbal drugs. As per WHO Guideline the primary step towards establishing the authenticity and purity of herbal drugs is macroscopic and microscopic examination before taking any further tests or assays. Morphological study is one of the elementary and economic means of evaluation of crude drug and involves organoleptic and sensory observations along with microscopical observations. It is a vital tool in authentication of herbal drugs.48The present study provides an anatomical and morphological description of bark of P.acidus as reference for quality control.
Presence of moisture or volatile components in crude drug was analysed using Moisture content and loss on drying. High moisture content is an indicator of poor quality and efficacy of the drug as moisture may hydrolyse the active components.55 Presence of any possible foreign contamination like sand, water soluble salts etc in the crude drug is evaluated by ash value or ash content. The residue left behind after ignition of crude drug and usually consists of inorganic salts present in the drug or adulterants. Total ash value consists of two types of ash ‘physiological ash’ comprising of plant tissues and ‘non-physiological ash’ which contains any adulterants present in the crude drug. Acid insoluble ash is an indication of the amount of silica present in the form of soil and earth materials, and is a component of total ash value. Components of total ash which are water soluble are determined by water soluble ash, also a part of total ash value.42,43 Total ash, water-soluble ash, and acid-insoluble ash of P.acidus were found to be 7.54 ± 0.45, 6.41 ± 0.36, and 0.59± 0.74 respectively; the value of total ash indicates that the inorganic contents of the crude drug are below the limits. Acid-insoluble ash value of P.acidus (0.59 ± 0.74) shows that a very small amount of the inorganic component is insoluble in acid. It indicates that adulteration by substances, such as silica etc., is very less, and a low acid-insoluble ash value may also affect the amount of the component absorbed in the gastrointestinal canal when taken orally.56 Crude drugs are sometimes contaminated with metals like Cd, As, Hg and Pb which are toxic to humans even in very minute quantities.57,58 Gestational exposure to cadmium may cause ovo-toxicity, hepato-toxicity and renal disorders and therefore they should be within acceptable limits in crude drug, in order for it to be safe and efficacious for consumption.59 The extractive value is mostly used for the determination of exhausted drug. It also gives a general idea about the chemical nature of different phytoconstituents found in each extract. For example petroleum ether extract contains non polar compounds like fatty acids etc, while methanolic extract contains polar constituents like glycosides alkaloid, steroids, phenols etc. In recent times plants used in ethno medicine and folk medicine have been backed by scientific investigations and data. The therapeutic properties exhibited by the herbal drugs are mostly due to the phytoconstituents present in the plants. Therefore, a screening of phytochemicals present in the plant is of paramount importance and gives an insight into the various chemical moieties present in the extract. In order to create a profile of phytoconstituents present in the P.acidus a preliminary phytochemical screening was performed on methanolic extract. The preliminary screening also suggested that the polarity of the solvent also perform a paramount part in the extraction of these phytoconstituents. The result shown in Table 2 showed that maximum constituents were extracted when ethanol was use as a solvent because of its higher polarity which enables the polar molecules present in the plant to dissolve in it. However, the chloroform and petroleum ether extract showed lesser amount of phytoconstituents as compared to the etanolic extract. Therefore, polar solvents were found to be more effective for extraction as compared to semi-polar and non polar solvents.60 This may be instrumental in further identification and quantitative estimation of pharmacologically active phytoconstituents. HPTLC is an instrumental quantitative tool to determine the phytochemical of herbal drugs.61 HPTLC helped in creating a standard phytochemical fingerprint of P.acidus for future reference for quality control and standardisation.
FTIR was used to determine the various functional moieties that exist in the etanolic extract. The presence of O-H functional group in plant extract has been associated to a plethora of pharmacological activities like antioxidant, anti-inflammatory, antidiabetic activity etc. the presence of OH- group also demonstrates the phenols and flavonoid content of the plant, also associated with above pharmacological effects.62
Plants consisting of phytochemical like phenolic and flavonoids have been established to diminish the threat of neoplastic disorder, hepatic disorders, diabetes, inflammation, bacterial and viral diseases. This attribute of natural compounds comprising of phenols and flavonoids might be ascribed to the free radical scavenging, diminishing oxidative stress and antioxidant potential of various polyphenolic bioactive components.63 Therefore analysis of the extract for total phenol and flavonoid content gives an insight into the mechanism of potential antioxidant and other pharmacological activities associated with this plant. The DPPH assay which showed reasonable free radical scavenging may also be attributed to these polyhydroxy compounds present in P.acidus extracts. The data pertaining to antioxidant activity may be instrumental in determining the compounds responsible for this action as well as advocating the use of this plant to combat oxidative stress associated with chronic and acute disorders like cancer, diabetes, neurodegenerative, liver disorders etc normally associated with free radicals or reactive oxygen species (ROS).
Conclusion
The plant-based pharmacologically active compounds are a valuable alternative resource for the treatment of various ailments. The pharmacognostical tools used in this study have positive implications in providing valuable information regarding the standardization parameters and pharmacological use of P.acidus. The study reveals that P.acidus can be used as a potential antioxidant due to its higher phenolic and flavonoid content. To the best of our knowledge this is the first study that has reported the pharmacognostical parameters for quality control of P. acidus, including HPTLC and FTIR fingerprinting. We recommend further screening and isolation of the phytochemical constituents responsible for the high antioxidant activity of P.acidus , especially in in vivo free radical scavenging studies to establish a relationship between the antioxidant activity and isolated phenolic and flavonoid compounds and authenticate their possible use as natural antioxidants
References
- Pamunuwa G, Karunaratne D.N and Waisundara V.Y. Antidiabetic Properties, Bioactive Constituents, and Other Therapeutic Effects of Scoparia dulcis. Evid Based Complement Alternat Med., 2016; 8243215. doi: 10.1155/2016/8243215.
CrossRef - Ahmed S.I, Hayat M.Q, Tahir M, Mansoor Q, Ismail M, Keck K and Bates R.B. Pharmacologically active flavonoids from the anticancer, antioxidant and antimicrobial extracts of Cassia angustifolia Vahl. BMC Complement. Altern. Med., 2016; 16: 460. doi: 10.1186/s12906-016-1443-z.
CrossRef - Andreu L, Nuncio-Jáuregui N, Carbonell-Barrachina Á.A, Legua P and Hernández F. Antioxidant properties and chemical characterization of Spanish Opuntia ficus-indica Mill. cladodes and fruits. J. Sci. Food Agric.,2018; 98: 1566–1573.
CrossRef - Meng X.H, Liu C, Fan R, Zhu L.F, Yang S.X, Zhu H.T. Wang D, Yang C.R and Zhang Y.J. Antioxidative flavan-3-ol dimers from the leaves of Camellia fangchengensis. J. Agric. Food Chem.,2018; 66:247–254. doi: 10.1021/acs.jafc.7b04572.
CrossRef - Krüger S, Bergin A and Morlock G.E. Effect-directed analysis of ginger (Zingiber officinale) and its food products, and quantification of bioactive compounds via high-performance thin-layer chromatography and mass spectrometry.Food Chem., 2018; 243: 258–268.
CrossRef - Agatonovic-Kustrin S, Gegechkori V, Petrovich D.S, Ilinichna K.T and Morton D.W. HPTLC and FTIR Fingerprinting of Olive Leaves Extracts and ATR-FTIR Characterisation of Major Flavonoids and Polyphenolics. Molecules, 2021;26: 6892. https://doi.org/10.3390/molecules26226892
CrossRef - Tarafdar R G, Nath S, Talukdar A D, Choudhury M.D. Cicca acida L.: Phytochemistry and Pharmacological studies. J. Pharm. Pharmacol. 2016; 68: 148-158.
CrossRef - Peixoto Araujo N.M, Arruda H.S, de Paulo Farias D, Molina G, Pereira G.A and Pastore G.M. Plants from the genus Eugenia as promising therapeutic agents for the management of diabetes mellitus: A review. Food Res Int., 2021; 142: 110182.
CrossRef - Unander D W, Venkateswaran P.S, Millman I, Bryan H.H, Blumberg B.S. Phyllanthus species: sources of new antiviral compounds. In Advances in New Crops, Janick J, Simon JE (eds). Timber Press: Portland, 1990:518–521.
- Kirtikar K.R and Basu B.D. Indian medicinal plants. Allahabad: Lalit Mohan Basu., 1987: 2227.
- Morton J, Morton J.F, Miami F.L. Otaheite Gooseberry. In fruits of warm climates.1987: 217-219.
- Orwa C, Mutua A, Kindt R, Jamnadass R, Anthony S. Agroforestree Database: a tree reference and selection guide version 4.0. 2009. (http://www.worldagroforestry.org/sites/treedbs/treedatabases.asp).
- Tropical Plants Database, Ken Fern, 2014. tropical.theferns.info. <tropical. theferns.info/ viewtropical. php?id=Phyllanthus+acidus> (accessed 2022 January 31st).
- Kittikajorn S. Thai traditional herbal medicines. Thai Capital Publisher: Bangkok. 1983:195.
- Prasad D. Edible fruits and vegetables of the English-speaking Caribbean food and nutrition institute. Kingston, Jamaica. 1986:75
- Pongboonrod S. Mai Thet Muang Thai Kasembunnakich Press, Bangkok.1959: 148.
- Subhadrabandhu S. Under-utilized tropical fruits in Thailand. RAP Publication: Bangkok. 2001:37-38.
- Teingburanathum V. Thai Medicinal Plant Dictionary, 5th ed. Roumsarn Press, Bangkok.1999: 633-643.
- Hazarika R, Abujam S.S, Neog B. Ethno Medicinal Studies of Common Plants of Assam and Manipur. International Journal of Pharmaceutical & Biological Archives., 2012; 3(4): 809
- Chowdhary Z, Alamgir A N M, Alauddin M, Islan M S, Chakma K, Hogue M.R, Kabir M.G. Traditional knowledge related to medicinal and aromatic plants in tribal societies and the quantitative study of alkaloids in medicinal plants of the hill tracts in Bangladesh. Pharma. Mag. 2008; 4(15(Suppl.)): S137–S144
- Unander D.W, Webster D.W, Blumberg,B.S. Uses and bioassays in Phyllanthus (Euphobiaceae): a compilation I. Subgenera Isocladus, Kirganelia, Cicca and Emblica. J. Ethnopharmacol. 1991; 30: 233-264
CrossRef - Lemmens R.H, Bunyapraphatsara M.I and De Padua L.S. Plant resources of south-East Asia. Medicinal and poisonous plants I. Prosea Foundation, Bogor, Indonesia, 1999;12(1):386
- Leterme P, Buldgen A, Estrada F, Londońo A M. Mineral content of tropical fruits and unconventional foods of the Andes and the rain forest of Columbia. Food Chem.2006; 95:644-652.
CrossRef - Mahapatra A K, Mishra S, Basak U C, Panda P C. Nutrient analysis of some selected wild edible fruits of deciduous forests of India: an explorative study towards non-conventional bio-nutrition. Adv. J. Food Sc. Tech.2012; 4: 15-21.
- Shajib M.T.I, Kawser M, Miah Begum M.N, Bhattacharjee P.L, Hossain A, Fomsgaard I.S and Islam S.N. Nutritional composition of minor indigenous fruits: C.heapest nutritional source for the rural people of Bangladesh. Food Chem., 2013; 140:466
CrossRef - Rahman S.M.M and Mosihuzzaman, M. Free sugars and dietary fibre in some fruits of Bangladesh. Food Chem., 1991;42:19
CrossRef - Tan S.P, Tan E.N.Y, Lim Q.Y and Nafiah M.A. Phyllanthus acidus (L.) Skeels: A review of its traditional uses, phytochemistry, and pharmacological properties. Journal of Ethnopharmacology, 2020; 253 : 112610
CrossRef - Siddiqui Z. Khan M. I, Badruddeen B, Akhtar J, Ahmad M. Multifunctional Role of Phyllanthus Acidus L. As a Therapeutic Agent for Management of Diabetes and Associated Complications: A Review. Biomed Pharmacol J 2022;15(4).
CrossRef - Khandelwal K.R. Practical pharmacognosy. 19th ed. Pune, India: Nirali Prakashan; 2008: 49–70
- Anonymous. General guidelines for methodologies on research and evaluation of traditional medicineWHO/EDM/TRM/2000. Geneva: World Health Organization, 2000
- Yadav R.N.S and Agrawala M. Phytochemical analysis of some medicinal plants. J. Phytol., 2011; 3: 10–14.
- Sasidharan S, Chen Y, Saravanan D, Sundram K.M and Latha Y.L. Extraction, isolation and characterization of bioactive compounds from plants extract. Afr. J. Tradit. Complement. Altern. Med., 2011; 8: 1–10.
CrossRef - Sheikh N, Kumar Y, Mishra A.K and Pfoze L. Phytochemical screening to validate the ethanobotanical importance of root tubers of Dioscorea species of Meghalaya, north east India. J. Med. Plants Stud., 2013; 1: 62–69.
- Nithianantham K, Shyamala M, Chen Y, Latha L.Y, Jothy S.L and Sasidharan S. Hepatoprotective potential of Clitoria ternatea leaf extract against paracetamol induced damage in mice. Molecules, 2011; 16(12): 10134–10145.
CrossRef - Galvez M, Martin-Cordero C, Houghton P.J and Ayuso M.J. Antioxidant activity of methanol extracts obtained from Plantago species. J Agric Food Chem., 2005, 53(6), 1927-33.
CrossRef - Chen Z, Bertin R and Froldi G. EC50 estimation of antioxidant activity in DPPH assay using several statistical programs. Food Chemistry, 2013; 138(1): 414-20.
CrossRef - Singleton V.L, Orthofer R and Lamuela-Ravent´os R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Oxidants and Antioxidants Part A, 1999; 299:152–178,
CrossRef - Chang C.C, Yang M.H, Wen H.M and Chern J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis, 2002;10(3):178-82.
CrossRef - Association of Official Analytical Chemists. Official methods of analysis of the AOAC. 18th Ed. In: Horwitiz W, editor. Washington DC: Association of Official Analytical Chemists, 2006.
- Agatonovic-Kustrin S, Gegechkori V and Morton D.W. The effect of extractive lacto-fermentation on the bioactivity and natural products content of Pittosporum angustifolium (gumbi gumbi) extracts. J. Chromatogr. A, 2021; 1647: 462153
CrossRef - Amir M, Mujeeb M, Ahmad S, Akhtar M and Ashraf, K. Design expert-supported development and validation of HPTLC method: an application in simultaneous estimation of quercetin and rutin in Punica granatum, Tamarindus indica and Prunus domestica. Pharm Methods, 2013; 4(2):62-67.
CrossRef - Anonymous. Indian Pharmacopoeia, Ministry of Health and Family Welfare, Govt. of India, New Delhi. 2007; 1:78.
CrossRef - Evans W.C. Trease and Evans Pharmacognosy. 15th ed. Edinburgh: W.B. Saunders. 2002: 86
CrossRef - Sulaiman S.F and Ooi K.L. Antioxidant and α-glucosidase inhibitory activities of 40 tropical juices from Malaysia and identification of phenolics from the bioactive fruit juices of Barringtonia racemosa and Phyllanthus acidus. J. Agric. Food Chem., 2014; 62: 9576-9585.
CrossRef - Khabiya R, Choudhary G and Darwhekar G.N. Spectrophotometric determination of total phenolic content for standardization of various phyllanthus species. Asian J Pharm Clin Res., 2019;12(8): 297-301
CrossRef - Habib M.R, Rahman M.M, Mannan A, Zulfiker A.H.M, Uddin M.R and Sayeed M.A. Evaluation of antioxidant, cytotoxic, antibacterial potential and phytochemical screening of chloroform extract of Phyllanthus acidus. Int. J. App. Bio. Pharma. Tech., 2011; 2: 420-427.
- Panche A.N, Diwan A.D and Chandra S.R. Flavonoids: An overview. J. Nutr. Sci., 2016;5:e47
CrossRef - Kumar V and Roy B.K. Population authentication of the traditional medicinal plant Cassia tora L. based on ISSR markers and FTIR analysis. Sci. Rep., 2018;8:10714
CrossRef - Dudonné S, Vitrac X, Coutiere P, Woillez M and Mérillon J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem.,2009; 57(5):1768-74.
CrossRef - Kukić J, Petrović S and Niketić M. Antioxidant activity of four endemic Stachystaxa. Biol. Pharm. Bull. , 2006; 29: 725–729.
CrossRef - Shilali K, Ramachandra Y.L, Rajesh K.P and Kumara Swamy, B.E. Assessing the Antioxidant Potential of Phyllanthus acidus Bark Extracts. Int. J. Pharma. Sci., 2014; 6:522-531.
- Kubovský I, Kačíková D and Kačík F. Structural changes of oak wood main components caused by thermal modification. Polymers, 2020; 12: 485.
CrossRef - Vihakas M. Flavonoid and Other Phenolic Compounds: Characterization and Interactions With Lepidopteran and Sawfly Larvae, Turun Yliopiston Julkaisuja. Annales Universitatis Turkuensis, 2014:53
- Patle T.K, Shrivas K, Kurrey R, Upadhyay S, Jangde R, Chauhan R. Phytochemical screening and determination of phenolics and flavonoids in Dillenia pentagyna using UV–vis and FTIR spectroscopy. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2020;242:118717.
CrossRef - Rashid S, Zafar M, Ahmad M, Lone FA, Shaheen S, Sultana S, Ashfaq S, Shinwari MI. Microscopic investigations and pharmacognostic techniques used for the standardization of herbal drug Nigella sativa L. Microscopy research and technique. 2018;81(12):1443-50.
CrossRef - Ajazuddin S S. Evaluation of physicochemical and phytochemical properties of Safoof-E-Sana, a Unani polyherbal formulation. Pharmacognosy Res. 2010 Sep;2(5):318-22
CrossRef - Rai S, Sharma D.K, Arora S.S, Sharma M and Chopra A.K. Concentration of the heavy metals in Aloe vera L. (Aloe barbadensis Miller) leaves collected from different geographical locations of India. Ann. Biol. Res., 2011; 2(6): 575-579.
- Haider S, Naithani V, Barthwal J and Kakkar P. Heavy metal content in some therapeutically important medicinal plants. Bull Environ Contam Toxicol.,2004; 72(1): 119-127.
CrossRef - Samuel J.B, Stanley J.A, Princess R.A, Shanthi P and Sebastian M.S. Gestational cadmium exposure-induced ovotoxicity delays puberty through oxidative stress and impaired steroid hormone levels. J Med Toxicol., 2011;7(3):195-204.
CrossRef - Venkatachalam R, Kalimuthu K, Chinnadurai V, Saravanan M, Radhakrishnan R, Shanmuganathan R and Pugazhendhi A. Various solvent effects on phytochemical constituent profiles, analysis of antioxidant and antidiabetic activities of Hopea parviflora. Process Biochem.,2019;89:227-232
CrossRef - Ahmad A, Husain A, Mujeeb M, Siddiqui N.A, Damanhouri Z.A and Bhandari A. Physicochemical and phytochemical standardization with HPTLC fingerprinting of Nigella sativa L. seeds. Pak J Pharm Sci., 2014; 27(5): 1175-1182.
- Sabandar C.W, Jalil J, Ahmat N and Aladdin N.A. Medicinal uses, chemistry and pharmacology of Dillenia species (Dilleniaceae). Phytochem. , 2017; 134: 6–25.
CrossRef - Shahidi F and Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects – A review. Journal of Functional Foods 2015; 18: 820–897
CrossRef







